Introduction
Perforator flaps are in frequent use in
reconstructive and plastic surgery to cover soft-tissue defects
caused by tumor ablation or trauma (1,2).
However, due to critical disruption of the blood supply, flaps
often present with partial necrosis (3). Choke vessels have an important role in
skin flap survival (4,5). They are part of the venous as well as
arterial skin circulation, and under normal physiological
conditions, they are small-caliber vessels extending between the
tips of the branches of adjacent vascular trees (6). An angiographic study reported that the
difference in the survival area of flaps is attributable to the
behavior of the choke zone (7). One
perforator vessel can safely perfuse an adjacent territory via
dilation of a choke vessel. According to a detailed study on choke
vessels by Zhuang et al (8),
the diameter of choke vessels increased at 3 days after flap
elevation and further increased to reach a maximum at day 5.
Subsequent to flap elevation, their tortuosity increased as well,
leading to increased vascular length, with the greatest change
occurring from day 5 until day 7. However, the underlying
mechanisms of choke vessel dilation remain elusive.
Dhar and Taylor (9)
postulated that the physical effects of blood flow and hypoxia are
two major factors that result in choke vessel dilation. Miyamoto
et al (7) also suggested that
flow-mediated dilation is a factor contributing to the dilation of
choke vessels. If a flap is transferred with anastomosis to a
recipient artery with higher blood flow, shear stress even showed
sufficient increases to dilate the second choke vessels in an
extended flap. Of note, in heart disorders and limb ischemic
models, the important role of the inflammatory response has been
extensively illustrated with regard to its association with
hypoxia-dependent as well as mechanical stress-dependent vessel
growth (10–12). The present study therefore
hypothesized that the inflammatory response was associated with
choke vessel changes in the extended perforator flap.
Inflammatory responses contribute to vascular
remodeling during tissue repair or ischemia (13,14).
Several studies have linked mechanical stress with production of
various pro-inflammatory molecules, such as interleukin (IL)-8,
IL-6, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis
factor-α (TNF-α) (15,16). Of these, MCP-1 and TNF-α are widely
recognized as major components of chronic inflammation associated
with a variety of ischemic events (17,18).
Chemokines are potent mediators of cell migration and adhesion via
interacting with a family of G-protein-coupled receptors expressed
on leukocytes. MCP-1 is most extensively studied chemokine
contributing to neovascularization. Numerous studies have
demonstrated the marked enhancement of MCP-1 at sites of collateral
growth (19,20). TNF-α is cytokine with multiple
functions, which regulates diverse physiological and
pathophysiological events, including cell growth, differentiation,
angiogenesis, survival, apoptosis and inflammation (21). Therefore, MCP-1 and TNF-α have been
evaluated as inflammatory markers to evidence inflammatory
responses.
The purpose of the present study was to investigate
the possible association between inflammatory responses and choke
vessel remodeling in the extended perforator flap model. The
inflammatory markers MCP-1 and TNF-α were also investigated in
order to elucidate the underlying molecular events in this
process.
Materials and methods
Animals
A total of 24 male Sprague-Dawley rats (Department
of Laboratory Animals, Central South University, Changsha, China,
10 weeks old, weight 250–300 g) were used in the present study. The
rats were housed in the Animal Care Center of Xiangya Hospital of
Central South of University and provided with free access to food
and water. Rats were kept at a regulated temperature (21±3°C) and
humidity (55±5%), and a 12-h light/dark cycle. All manipulations
and surgical procedures were performed in accordance with the
guidelines of the China Council of Animal Care and with approval of
the Central South University Committee on Laboratory Animals. The
animal protocol was reviewed and approved by the Ethical Committee
of XiangYa School of Medicine, Central South University. Following
flap elevation, the animals were randomly divided into three groups
(n=6 in each group) for tissue analysis at three, five or seven
days after flap surgery. Six additional rats served as a control
group (no flap elevation).
Flap design
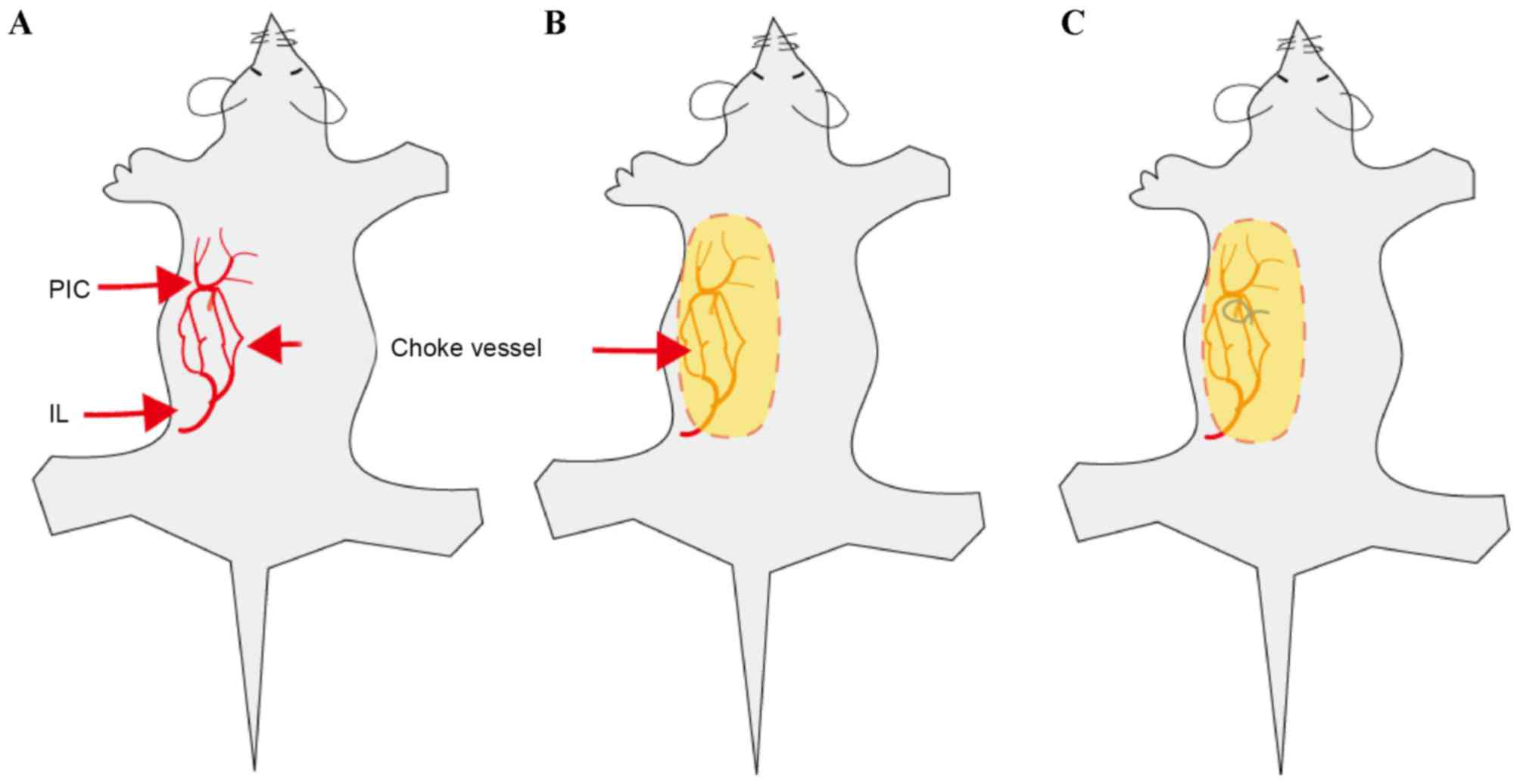
In the present study, an extended dorsal skin
perforator flap model was used. The perforator flap was marked on
the dorsolateral side. Based on previous studies and an
angiographic study by our group (8),
this flap contains two even vascular territories, the iliolumbar
artery perforator vessel and the posterior interior intercostal
artery perforator vessel. The size of the flap was ~3×8 cm
(Fig. 1).
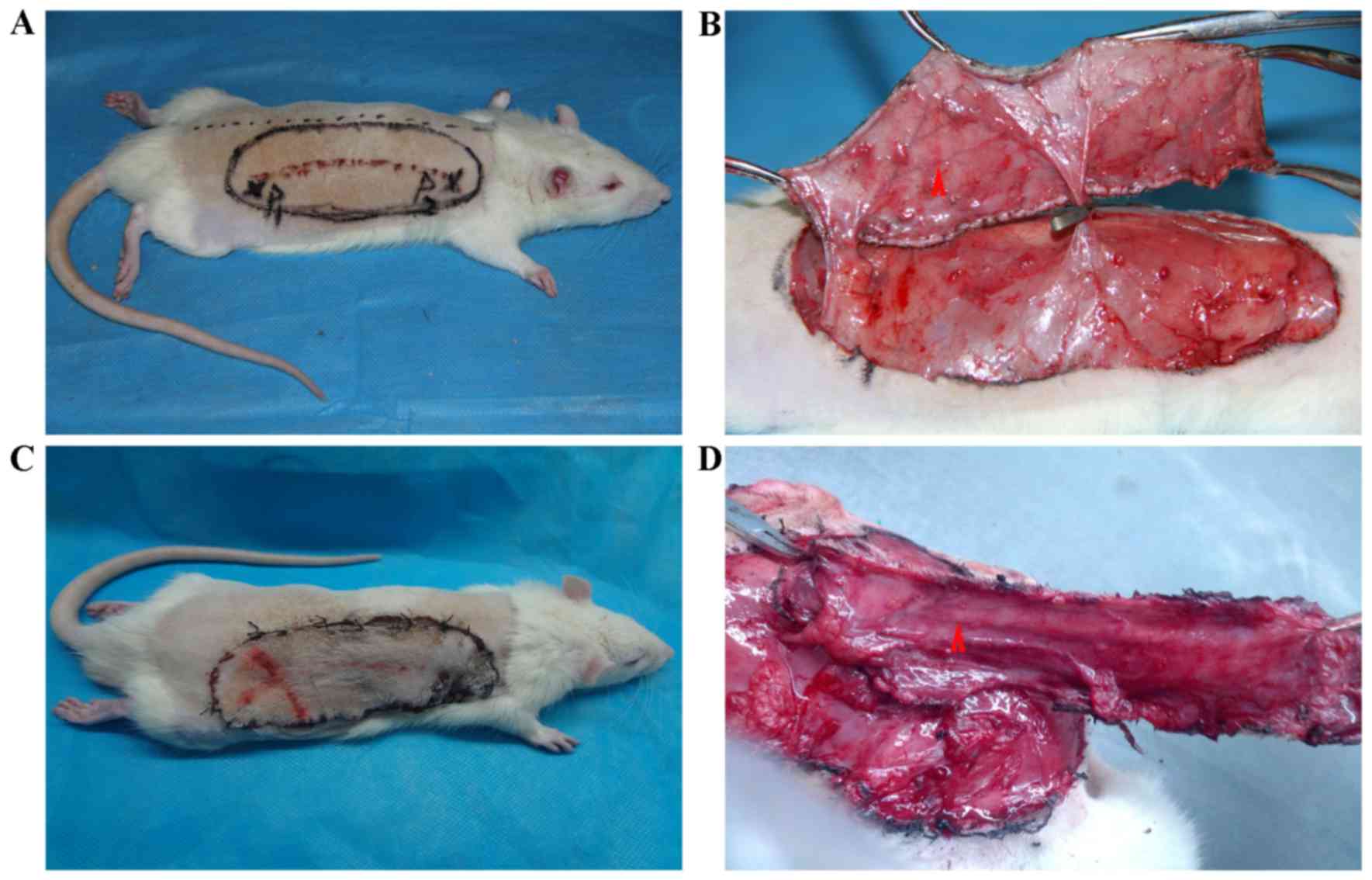
Flap elevation
All animals were anesthetized with pentobarbital
sodium (30 mg/kg, intraperitoneal; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). Fur was shaved from the dorsal surface and the
skin was washed with hibitane, 75% alcohol and iodine Shandong
Ruitai Odd Washing Disinfection Technology Co., Ltd., Shandong,
China). The flap was outlined with a felt-tipped surgical marker.
Flap elevation was started with an incision at the medial border.
The periphery of the flap was incised and hemostasis was achieved.
The surgical flap was then raised by sharp dissection in the plane
between the panniculus carnosus and the deep fascia and the two
blood vessels of interest were confirmed. Cutaneous blood vessels
were cauterized as they were encountered, except for the planned
perforator pedicle. The posterior intercostal artery perforator was
ligated and severed, creating an island flap that relied solely on
the iliolumbar artery perforator for its blood supply. After flap
elevation, the anastomotic line between the iliolumbar artery
perforator and the posterior intercostal artery perforator (choke
vessel zone) was marked on the flap's surface (Fig. 2). The flap was then replaced at the
surgical site and secured with 4–0 monofilament sutures and wound
clips. Following anesthetic recovery, the rats were placed in a
clean cage. Animals were monitored daily for signs of dehiscence
and self-mutilation.
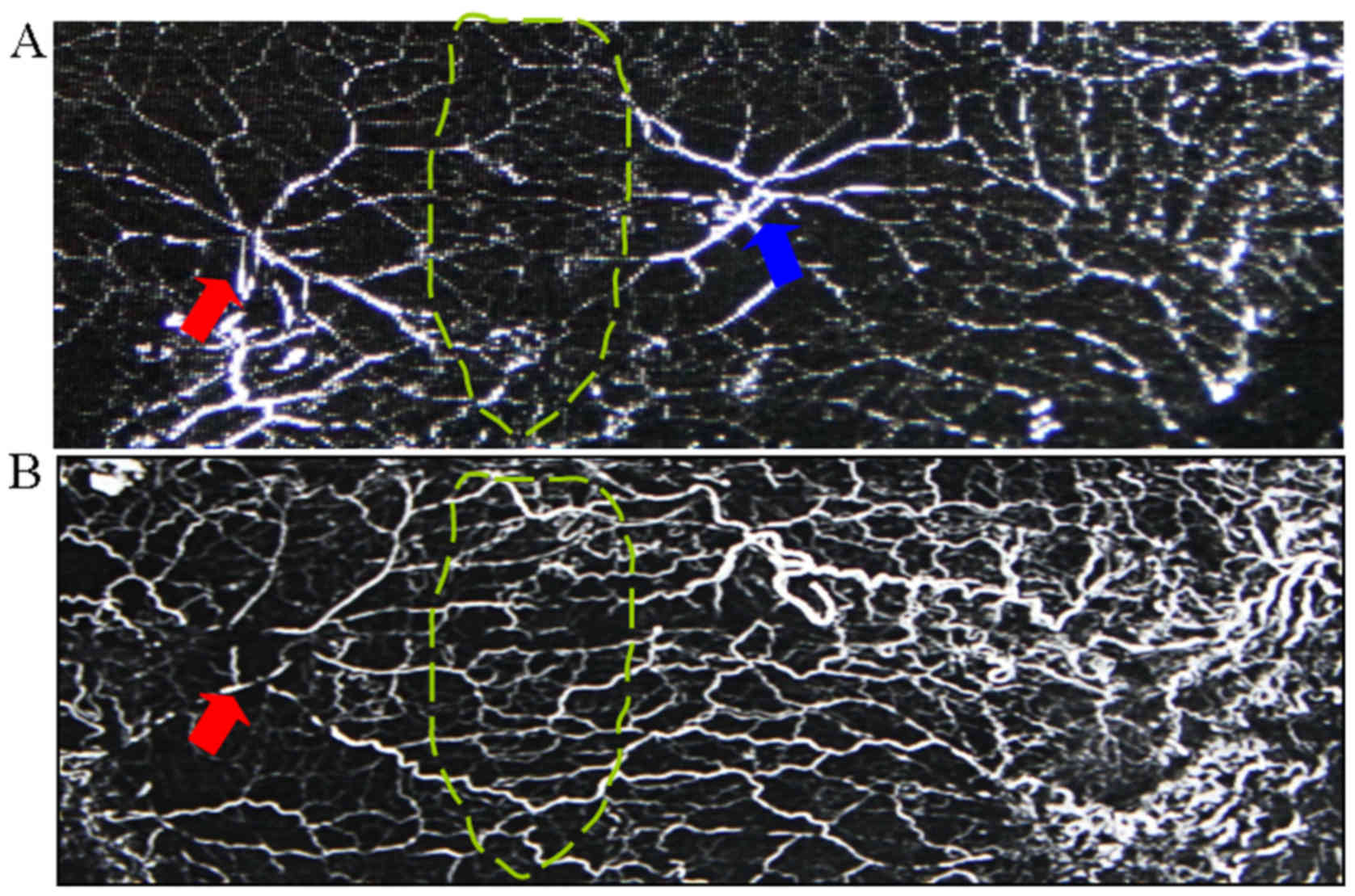
Angiography
To visualize vascular networks in the skin flap,
following exsanguination, one control rat and one rat were injected
with gelatin/lead oxide seven days after flap surgery, following
the method described by Zhuang et al (8). In brief, 5 g gelatin was diluted in 100
ml tap water, heated to 40°C and followed by the addition of 100 mg
water-soluble red lead oxide (Pb3O4, Jining
Hengtai Chemical Co., Ltd., Jining, China). This mixture was
injected into the rat's carotid artery until the rat's limbs turned
red. After injection, the integument was carefully dissected in the
plane between the panniculus carnosus and the deep fascia. The
integument was then fixed for 24 h at 4°C. The flaps were obtained
and radiographed (55 kVp, 25 mA, 20 sec exposure) with a soft X-ray
machine (Fuji Computerized Radiography XG-1; Fujifilm, Tokyo,
Japan).
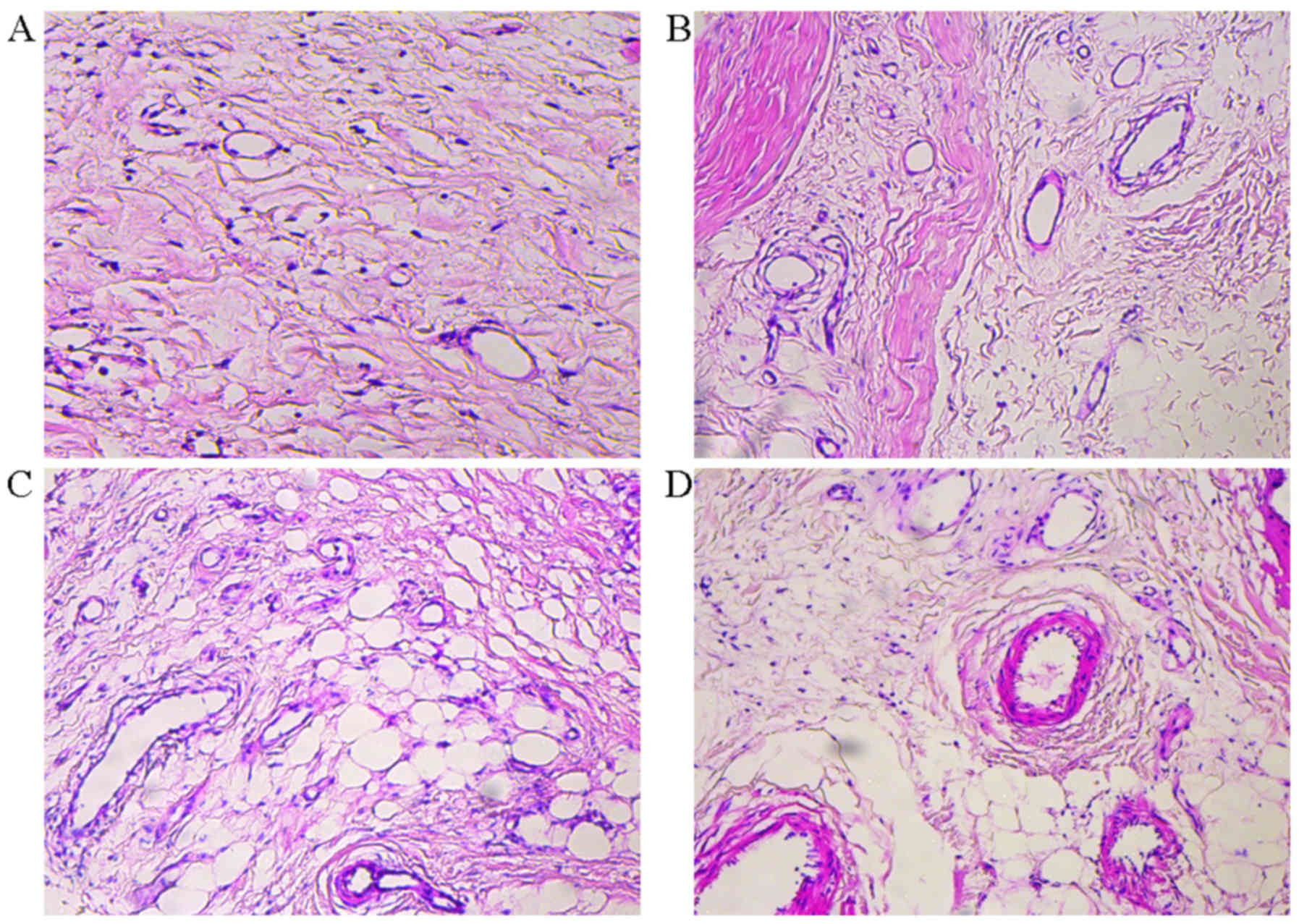
Histological analysis
A sample from the first choke zone was excised and
stored in 4.5% buffered formaldehyde solution. Formaldehyde-fixed
samples were processed in paraffin and stained with
hematoxylin/eosin using standard histology protocols. Samples were
examined for infiltration of polymorphonuclear leukocytes,
inflammatory cells and interstitial edema.
Western blot analysis
Tissue samples were harvested from the first choke
zones. These samples were homogenized in lysis buffer containing 20
mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l
EGTA, 1% Triton X-100, 2.5 mmol/l sodium orthovanadate, 1 µg/ml
leupeptin and 1 mmol/l phenylmethyl sulfonyl fluoride. The samples
were centrifuged to pellet the debris and the supernatants were
analyzed using Bradford protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A volume of each extract corresponding to 25 µg
of total protein was resolved on 10% sodium dodecyl
sulfate-polyacrylamide gels and electrotransferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked in phosphate-buffered saline with 0.1%
Tween-20 (PBS-T) containing 5% milk powder for 30 min at room
temperature and then incubated overnight at 4°C with one of the
following primary antibodies: Anti-TNF-α antibody (sc-1349, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at a dilution of
1:1,000, anti-MCP-1 antibody (sc-1785, Santa Cruz Biotechnology,
Inc.) at a dilution of 1:1,000 and anti-β-actin polyclonal antibody
(A5441, Sigma-Aldrich; Merck Millipore) at a dilution of 1:1,000 as
a loading control. The membranes were subsequently incubated with
horseradish peroxidase-conjugated anti-mouse immunoglobulin G
(1:1,000; sc-2748, Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. Immunoreactivity signals were visualized by
3,3′-Diaminobenzidine tetrahydrochloride (AR1000; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and a Tocan 240 Tanon
Gel Imaging System (Tanon Science & Technology Co., Ltd.,
Shanghai, China) and analyzed using Image-Pro plus Software 6.0
(Media Cybernetics, Rockville, MD, USA).
Total RNA extraction and reverse-transcription
quantitative polymerase chain reaction (RT-qPCR). RT-qPCR analysis
was performed to examine the expression of MCP-1 and TNF-α. Total
RNA was isolated from skin flap tissues using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
DNA was removed using DNase I (Invitrogen; Thermo Fisher
Scientific, Inc.). These RNA samples were then reverse-transcribed
into single-stranded complementary (c)DNA using the first-strand
cDNA synthesis kit (Fermentas, Vilnius, Lithuania). These cDNA
products were further amplified using qPCR by using the SYBR-Green
RT-PCR kit (Bioteke, Beijing, China). The primers were from
Invitrogen (Thermo Fisher Scientific, Inc.). Amplification was
performed with a real-time qPCR machine (Stratagene, La Jolla, CA,
USA). GAPDH was used as an internal control. The sequences of the
PCR primers used in this study are listed in Table I. Relative expression of PCR products
was determined using the ΔΔCq method (22) with normalization to GAPDH mRNA
expression.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Primer name | Primer sequence |
|---|
| GADPH | F:
5′-TGCCCCATGTTTGTGATG-3′ |
| GADPH | R:
5′-TTACGTAGGACGTGGTGGT-3′ |
| TNF-α | F:
5′-CCAGGAGAAAGTCAGCCTCCT-3′ |
| TNF-α | R:
5′-TCATACCAGGGCTTGAGCTCA-3′ |
| MCP-1 | F:
5′-AGCACCTTTGAATGTGAACT-3′ |
| MCP-1 | R:
5′-AGAAGTGCTTGAGGTGGTT-3′ |
Statistical analysis
SPSS version 17.0 for Windows (SPSS Inc., Chicago,
IL, USA) was used for data management and statistical analysis.
Values are expressed as the mean ± standard error of the mean.
Statistical analyses were performed using one-way analysis of
variance followed by post-hoc multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Choke vessel remodeling of flap post
operation
At 7 days post operation, the choke vessels at the
choke zone were clearly dilated. Arteriography showed that choke
vessels showed dilation and tortuous paths, and that the iliolumbar
artery had increased its territory to supply adjacent vascular
territories (Fig. 3).
Histopathological changes in flap post
operation
Histopathological changes of flaps were seen between
the groups. No marked inflammation was present in the control
group. However, in the experimental group, clearances in the
tissues indicated edema, and inflammatory cell infiltration as well
as vessel dilation were present at 3 days post operation. Dilation
of the choke vessels and increasing vessel wall thickness were
obvious over the following days until 7 days (Fig. 4). These results indicated that
inflammation was involved in choke vessel remodeling.
Inflammatory response-associated
biomarker levels in the flap
To assess the effects of inflammatory factors on
choke vessel remodeling, a fraction of the flap was excised from
the choke zone and the expression levels of MCP-1 and TNF-α as
inflammatory markers were examined by using RT-qPCR and western
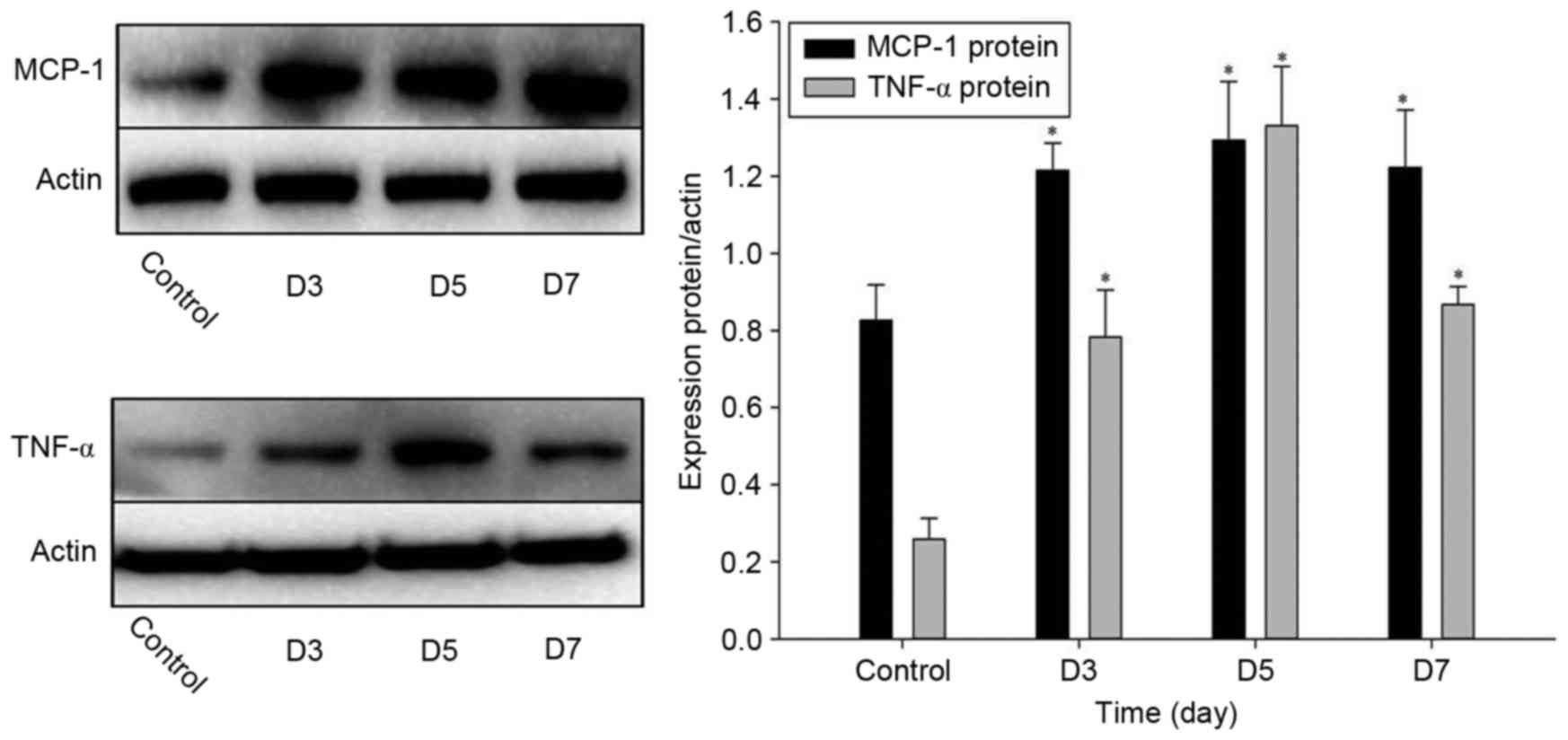
blot analysis. As indicated in Fig.
5, western blot analysis revealed changes in the protein
expression of MCP-1 and TNF-α in the flap choke zone at various
time-points after operation. The protein levels of MCP-1 and TNF-α
were significantly elevated at 3 and 5 days after operation, and
had slightly declined at 7 days (Fig.
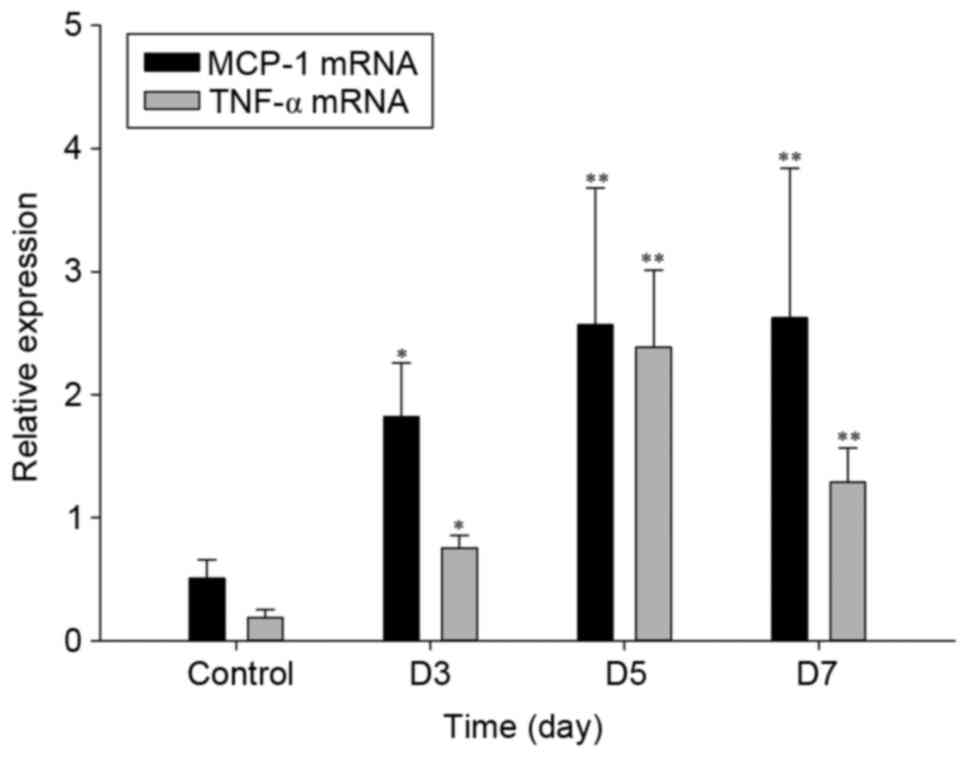
5). Similarly, the mRNA expression of MCP-1 and TNF-α were also
significantly upregulated at 3–7 days after the operation with a
maximum at 5 days (Fig. 6). These
results suggested that these two inflammatory factors were involved
in inflammation in the flap.
Discussion
Ischemic necrosis of the surgical skin flap is a
common complication and may result in significant cosmetic and
functional defects (23). Vascular
supply to the integument is crucial for the survival of surgical
perforator flaps. Angiosomes are the areas of skin perfused by
blood vessels (24). Adjacent
angiosomes are linked through choke vessels. When blood flow to
angiosomes is disrupted, adjacent angiosomes can expand via choke
vessel remodeling to compensate for the decreased blood flow
(25,26). In the present study,
histopathological analysis and arteriography showed that the
dilation of the choke vessel caliber and increasing vessel wall
thickness was obvious at 7 days post operation. The iliolumbar
artery had increased its territory to supply adjacent vascular
territories.
Choke vessel remodeling occurs via the process of
arteriogenesis (27), which is the
sprouting of microvessels from a preexisting capillary network. It
requires an inflammatory environment and numerous cytokines are
commonly involved in general inflammatory responses and
arteriogenesis. The inflammatory response has been considered as
the major factor involved in neovascularization and vascular
remodeling (26). To determine
whether inflammatory responses are involved in postoperative choke
vessel remodeling, MCP-1 and TNF-α were examined in the present
study as an inflammatory cytokines in the choke zone. The results
showed that the expression levels of TNF-α and MCP-1 in the choke
zone were obviously increased in tissues showing postoperative
choke vessel growth. The results of the present study confirmed the
hypothesis that the inflammatory response may have an important
role in choke vessels remodeling. This result was similar to that
of a study by Williams et al (26), which supported that ischemia may not
have a role in choke-vessel changes, whereas an inflammatory
environment was shown to be involved in the growth of choke
vessels.
In the present study, TNF-α increased at day 3 after
flap elevation and was further increased at day 5, while showing a
decline at day 7. MCP-1 increased at day 3 after flap elevation,
reached a maximum at day 5 and then plateaued. It is commonly
accepted that hypoxia/ischemia is involved in the extended
perforator flap prior to choke vessel remodeling (7,9) TNF-α is
well known to be involved in the inflammatory response elicited in
regions of cerebral ischemia. Subsequent to an ischemic insult, the
levels of TNF-α may indeed remain elevated in the affected brain
tissue for at least 24 h (28).
TNF-α mediates remodeling and repair through activating collagen
formation and matrix metalloproteinases, and regulates integrins,
progenitor cell mobilization and angiogenesis (21). MCP-1 also has an important role in
ischemia-induced angiogenesis by promoting early inflammatory
mononuclear cell infiltration (13,29).
MCP-1 was found to be upregulated in the venular endothelium of
ischemic myocardial segments (18).
Transforming growth factor (TGF)-β1 was reported to significantly
contribute to this chemotactic activity, and monocyte chemotactic
activity in lymph was largely dependent on the concerted action of
MCP-1 and TGF-β1 (30).
After establishment of the extended perforator flap
model, the blood flow through the choke vessels may be elevated
(7,9). Physical forces generated within the
collateral arterioles after an increase of blood flow trigger
vessel growth. Increases in physical forces stimulate the
production of chemokines and chemokine receptors. Chemokines
regulate the accumulation of leukocytes at inflammatory sites
(10). MCP-1 is one of the key
chemokines that regulate migration and infiltration of
monocytes/macrophages (31).
Macrophages are important in the induction of new blood vessel
growth during wound repair, tumor growth and inflammation. In the
present study, histological analysis revealed edema and
inflammatory cell aggregation at 3, 5 and 7 days post operation.
Several studies also suggested that TNF-α is responsible for
macrophage-derived angiogenic activity (11,17,32). The
angiogenic activity produced by activated murine macrophages was
reported to be neutralized by a polyclonal antibody to TNF-α.
Although it has been illustrated that TNF-α induces capillary blood
vessel formation during tumor development in inflammation and wound
repair, TNF-α also augmented repair by stimulating the growth of
new blood vessels (15,33). In the present study, the mRNA levels
of the inflammatory markers showed obvious changes in flap tissues
with postoperative choke vessel growth. The mRNA levels of MCP-1
and TNF-α were upregulated post operation. These results showed
that MCP-1 and TNF-α are potent inducers of choke vessel
remodeling.
In conclusion, the findings of the present study
indicated that the inflammatory response may have an important role
in choke vessel remodeling. Furthermore, molecular evidence of the
involvement of an inflammatory environment in vessel development in
the choke zone was provided. MCP-1 and TNF-α may become possible
target molecules to modulate the behavior of choke vessels.
References
|
1
|
Tang J, Fang T, Song D, Liang J, Yu F and
Wang C: Free deep inferior epigastric artery perforator flap for
reconstruction of soft-tissue defects in extremities of children.
Microsurgery. 11:221272013.
|
|
2
|
Zeltzer AA and Van Landuyt K:
Reconstruction of a massive lower limb soft-tissue defect by giant
free DIEAP flap. J Plast Reconstr Aesthet Surg. 65:e42–e45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill PS, Hunt JP, Guerra AB, Dellacroce
FJ, Sullivan SK, Boraski J, Metzinger SE, Dupin CL and Allen RJ: A
10-year retrospective review of 758 DIEP flaps for breast
reconstruction. Plast Reconstr Surg. 113:1153–1160. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor GI, Chubb DP and Ashton MW: True
and ‘choke’ anastomoses between perforator angiosomes: Part i.
anatomical location. Plast Reconstr Surg. 132:1447–1456.
2013.PubMed/NCBI
|
|
5
|
Chubb DP, Taylor GI and Ashton MW: True
and ‘choke’ anastomoses between perforator angiosomes: Part II.
dynamic thermographic identification. Plast Reconstr Surg.
132:1457–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taylor GI and Pan WR: Angiosomes of the
leg: Anatomic study and clinical implications. Plast Reconstr Surg.
102:599–618. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyamoto S, Minabe T and Harii K: Effect
of recipient arterial blood inflow on free flap survival area.
Plast Reconstr Surg. 121:505–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuang Y, Hu S, Wu D, Tang M and da Xu C:
A novel in vivo technique for observations of choke vessels in a
rat skin flap model. Plast Reconstr Surg. 130:308–317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhar SC and Taylor GI: The delay
phenomenon: The story unfolds. Plast Reconstr Surg. 104:2079–2091.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smits AI, Ballotta V, Driessen-Mol A,
Bouten CV and Baaijens FP: Shear flow affects selective monocyte
recruitment into MCP-1-loaded scaffolds. J Cell Mol Med.
18:2176–2188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cochain C, Channon KM and Silvestre JS:
Angiogenesis in the infarcted myocardium. Antioxid Redox Signal.
18:1100–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szabó C and Papapetropoulos A: Hydrogen
sulphide and angiogenesis: Mechanisms and applications. Br J
Pharmacol. 164:853–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsui H, Motooka M, Koike H, Inoue M,
Iwasaki T, Suzuki T, Kurabayashi M and Yokoyama T:
Ischemia/reperfusion in rat heart induces leptin and leptin
receptor gene expression. Life Sci. 80:672–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chalothorn D, Clayton JA, Zhang H, Pomp D
and Faber JE: Collateral density, remodeling, and VEGF-A expression
differ widely between mouse strains. Physiol Genomics. 30:179–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida S, Yoshida A and Ishibashi T:
Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial
cells: Implications for retinal neovascularization during
post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol.
242:409–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Chopp M, Teng H, Bolz M, Francisco
MA, Aluigi DM, Wang XL, Zhang RL, Chrsitensen S, Sager TN, et al:
Tumor necrosis factor a primes cerebral endothelial cells for
erythropoietin-induced angiogenesis. J Cereb Blood Flow Metab.
31:640–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stowe AM, Wacker BK, Cravens PD, Perfater
JL, Li MK, Hu R, Freie AB, Stüve O and Gidday JM: CCL2 upregulation
triggers hypoxic preconditioning-induced protection from stroke. J
Neuroinflammation. 9:332012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts TK, Eugenin EA, Lopez L, Romero
IA, Weksler BB, Couraud PO and Berman JW: CCL2 disrupts the
adherens junction: Implications for neuroinflammation. Lab Invest.
92:1213–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vinores SA, Xiao WH, Shen J and
Campochiaro PA: TNF-alpha is critical for ischemia-induced
leukostasis, but not retinal neovascularization nor VEGF-induced
leakage. J Neuroimmunol. 182:73–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Li QF, Zhang Y, Hu R and Jiang H:
Isoflurane preconditioning increases survival of rat skin
random-pattern flaps by induction of HIF-1alpha expression. Cell
Physiol Biochem. 31:579–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton K, Wolfswinkel EM, Weathers WM,
Xue AS, Hatef DA, Izaddoost S and Hollier LH Jr: The delay
phenomenon: A compilation of knowledge across specialties.
Craniomaxillofac Trauma Reconstr. 7:112–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gigliofiorito P, Iacob S, Pendolino AL,
Piombino L, Segreto F and Persichetti P: True and ‘choke’
anastomoses between perforator angiosomes: Part I. Anatomical
location. Plast Reconstr Surg. 133:890e–891e. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams BA, Currie RW and Morris SF:
Impact of arteriogenesis in plastic surgery: Choke vessel growth
proceeds via arteriogenic mechanisms in the rat dorsal island skin
flap. Microcirculation. 16:235–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghali S, Butler PE, Tepper OM and Gurtner
GC: Vascular delay revisited. Plast Reconstr Surg. 119:1735–1744.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goukassian DA, Qin G, Dolan C, Murayama T,
Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, et al:
Tumor necrosis factor-alpha receptor p75 is required in
ischemia-induced neovascularization. Circulation. 115:752–762.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stevens CR, Williams RB, Farrell AJ and
Blake DR: Hypoxia and inflammatory synovitis: Observations and
speculation. Ann Rheum Dis. 50:124–132. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouchentouf M, Paradis P, Forner KA,
Cuerquis J, Boivin MN, Zheng J, Boulassel MR, Routy JP, Schiffrin
EL and Galipeau J: Monocyte derivatives promote angiogenesis and
myocyte survival in a model of myocardial infarction. Cell
Transplant. 19:369–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rangasamy S, McGuire PG, Nitta Franco C,
Monickaraj F, Oruganti SR and Das A: Chemokine mediated monocyte
trafficking into the retina: Role of inflammation in alteration of
the blood-retinal barrier in diabetic retinopathy. PLoS One.
9:e1085082014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM,
Lee MJ, Jang IH, Kwon SM, Lee JS and Kim JH: Tumor necrosis
factor-a-activated mesenchymal stem cells promote endothelial
progenitor cell homing and angiogenesis. Biochim Biophys Acta.
1832:2136–2144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gardiner TA, Gibson DS, de Gooyer TE, de
la Cruz VF, McDonald DM and Stitt AW: Inhibition of tumor necrosis
factor-alpha improves physiological angiogenesis and reduces
pathological neovascularization in ischemic retinopathy. Am J
Pathol. 166:637–644. 2005. View Article : Google Scholar : PubMed/NCBI
|