Portal hypertension is an important cause of
morbidity and mortality in cirrhotic patients, which can lead to
severe complications, including esophageal variceal bleeding
(1,2), ascites, hepatic encephalopathy,
hepatorenal syndrome, bacteremia and hypersplenism. To date,
non-selective β-blockers (NSBBs) are the drugs of choice for the
treatment of esophageal variceal bleeding in cirrhotic patients.
However, it has been revealed that only 30–40% of the patients
under long-term therapy with NSBBs demonstrate a good hemodynamic
response (reduction in HVPG to ≤12 mmHg or at least a 20% reduction
from the baseline), and another 15% do not tolerate NSBBs (3). In the past few years, studies have
revealed that the renin-angiotensin-aldosterone system (RAAS) is
important in chronic hepatic diseases and portal hypertension.
Angiotensin II was found to stimulate hepatic stellate cells in
order to increase intrahepatic resistance and promote fibrosis
(4). Moreover, in addition to its
established effect of increasing portal vein blood flow via
water-sodium retention (5),
aldosterone has been shown to increase inflammation, endothelial
dysfunction, oxidative stress and insulin resistance (6). In addition, a previous systematic
review and meta-analysis confirmed that antagonists of the RAAS
appear to be able to decrease HVPG in patients with compensated
cirrhosis (7). Theoretically, as
RAAS inhibitors and β-blockers function via different mechanisms to
decrease the pressure of portal veins, it is possible that the RAAS
inhibitor and β-blocker combination therapy may lead to a more
significant reduction in portal venous pressure. Several studies
(8–10) have previously compared the RAAS
inhibitor and β-blocker combination therapy vs. β-blocker
monotherapy. However, the results remain inconsistent.
The aim of the present study was to assess the
efficacy of RAAS inhibitor and β-blocker combination therapy vs.
β-blocker monotherapy on HVPG reduction in patients with
cirrhosis.
Studies were included using the following criteria:
i) Full-text article; ii) randomized controlled trial; iii)
cirrhosis; iv) clinically significant portal hypertension; v) if
the study was a clinical trial comparing the effects of RAAS
inhibitor and β-blocker combination therapy with β-blocker
monotherapy on portal pressure; and vi) HVPG measurement before and
after treatment. Moreover, studies were excluded if TIPS or a
surgical shunt were present.
PubMed, Embase, Medline and the Cochrane Library
were searched up to July 2015 to retrieve pertinent studies
(11,12). We searched (losartan OR candesartan
OR irbesartan OR valsartan OR telmisartan OR olmesartan OR
enalapril OR quinapril OR ramipril OR lisinopril OR captopril OR
fosinopril OR perindopril OR RASS inhibitor OR ACEI OR ATII blocker
OR angiotensin inhibitor OR renin angiotensin OR eplerenone OR
spironolactone OR aldactone OR canrenone) AND (adrenergic
β-antagonists OR β blockers OR propranolol OR nadolol OR timolol)
AND (portal hypertension OR cirrhosis) AND controlled trials. A
manual search of the reference lists of related articles and
reviews was also performed. Moreover, related congresses were
hand-searched.
Two authors (Dr Jianrong Wang and Dr Wenxia Lu)
extracted data independently. Discrepancies were resolved through
discussion before the analyses. The following data were extracted
from each trial: i) Trial characteristics such as study population
demographics, intervention and control and time of outcome
measured; ii) patient characteristics such as the number of
patients, age, gender ratio, Child-Pugh class, number of patients
with previous variceal hemorrhage and ascites; iii) outcome such as
the reduction in HVPG, number of patients achieving a hemodynamic
response, change in heart rate and mean arterial pressure (MAP) as
adverse events.
Methodological quality of the articles included was
assessed using the Jadad scale and Schulz hidden grouping (13,14). A
Jadad score of 1 to 2 was considered low quality, and a Jadad score
of 3 to 5 was considered high quality. Moreover, the Schulz hidden
grouping was described as ‘adequate’ ‘inadequate’ and
‘unclear’.
Results of the studies included are reported as the
number of observations, ratio or mean ± standard deviation. When
the result was reported as the standard error, the standard
deviation was calculated from the standard error.
Data analysis and graph synthesis were performed by
RevMan (version 5.2; The Cochrane Collaboration, Oxford, UK).
Continuous outcomes, including the reduction in HVPG between the
control and experimental groups were reported as a weighted mean
difference (WMD) with a 95% confidence interval (CI). Moreover,
heterogeneity was assessed using the χ2 test and
I2-values (15).
χ2 statistics P>0.1 were considered to have no
heterogeneity. Moreover, I2-values <25% were
considered to have a low risk, 25–50% was considered a moderate
risk and values >50% were considered to have a high risk of
heterogeneity. If there was significant heterogeneity, potential
reasons for the heterogeneity were explored and combinability of
trials was reassessed, respectively.
The WMD in the heart rate and MAP between the
treatment and control groups was also assessed as a measure of an
adverse effect.
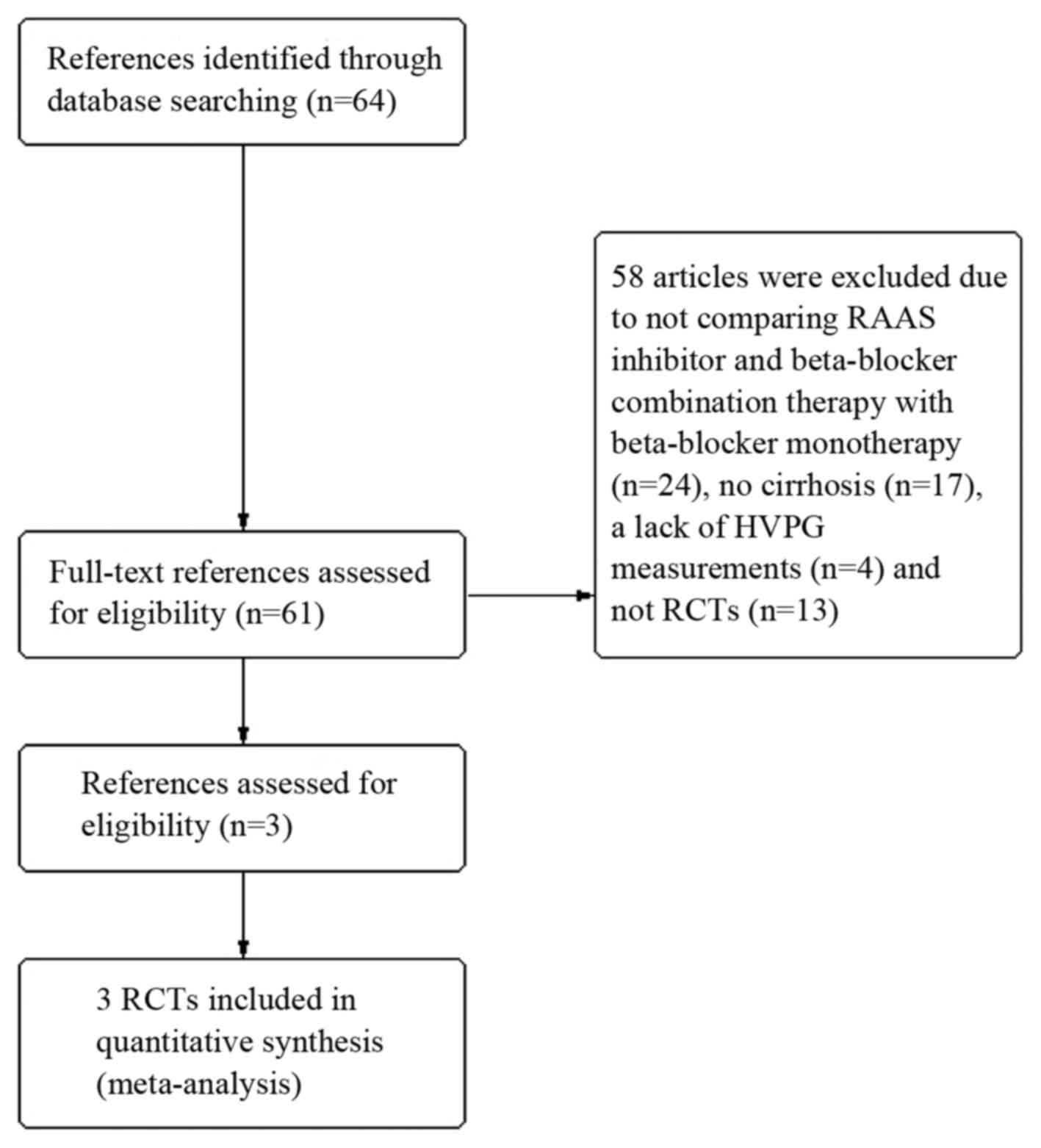
The search was conducted in July 2015, and a total
of 64 abstracts were identified. The full-text of 61 of these
pertinent reports were reviewed, respectively. In total, 58
articles were excluded for the following reasons: i) They did not
compare the RAAS inhibitor and β-blocker combination therapy with
β-blocker monotherapy (24/61) (16–39); ii)
there was no cirrhosis (17/61) (40–56);
iii) there was a lack of HVPG measurements (4/61) (57–60); and
iv) there were no RCTs (13/61) (61–73). The
remaining three articles (8–10) met the inclusion criteria (Fig. 1).
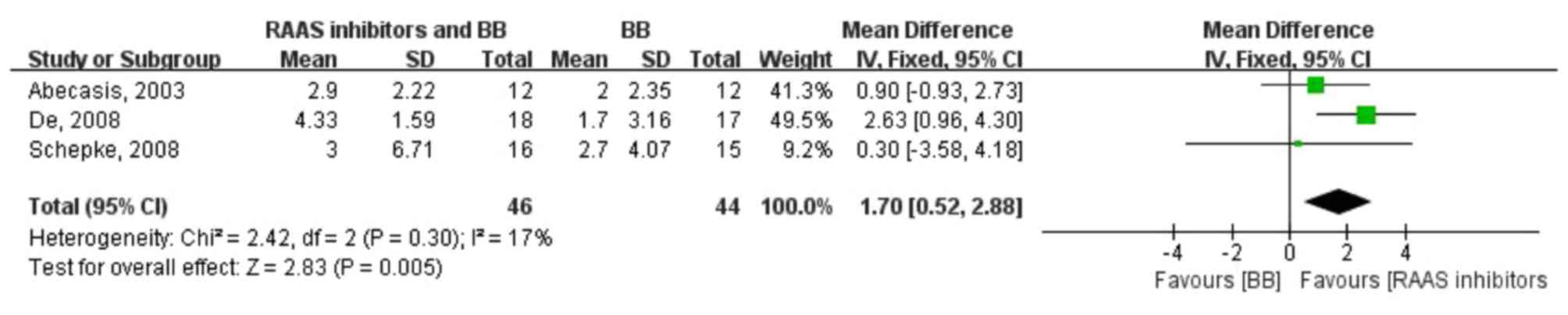
The pooled WMD of HVPG reductions in the two groups
was 1.70 (0.52, 2.88; fixed-effect model), test for overall effect:
Z=2.83 (P=0.005), indicating a significantly higher HVPG reduction
with the RAAS inhibitor and β-blocker combination therapy compared
to β-blocker monotherapy. Moreover, no heterogeneity was observed
in the analysis of combination therapy vs. monotherapy (P=0.30;
I2=17%) (Fig. 2). As
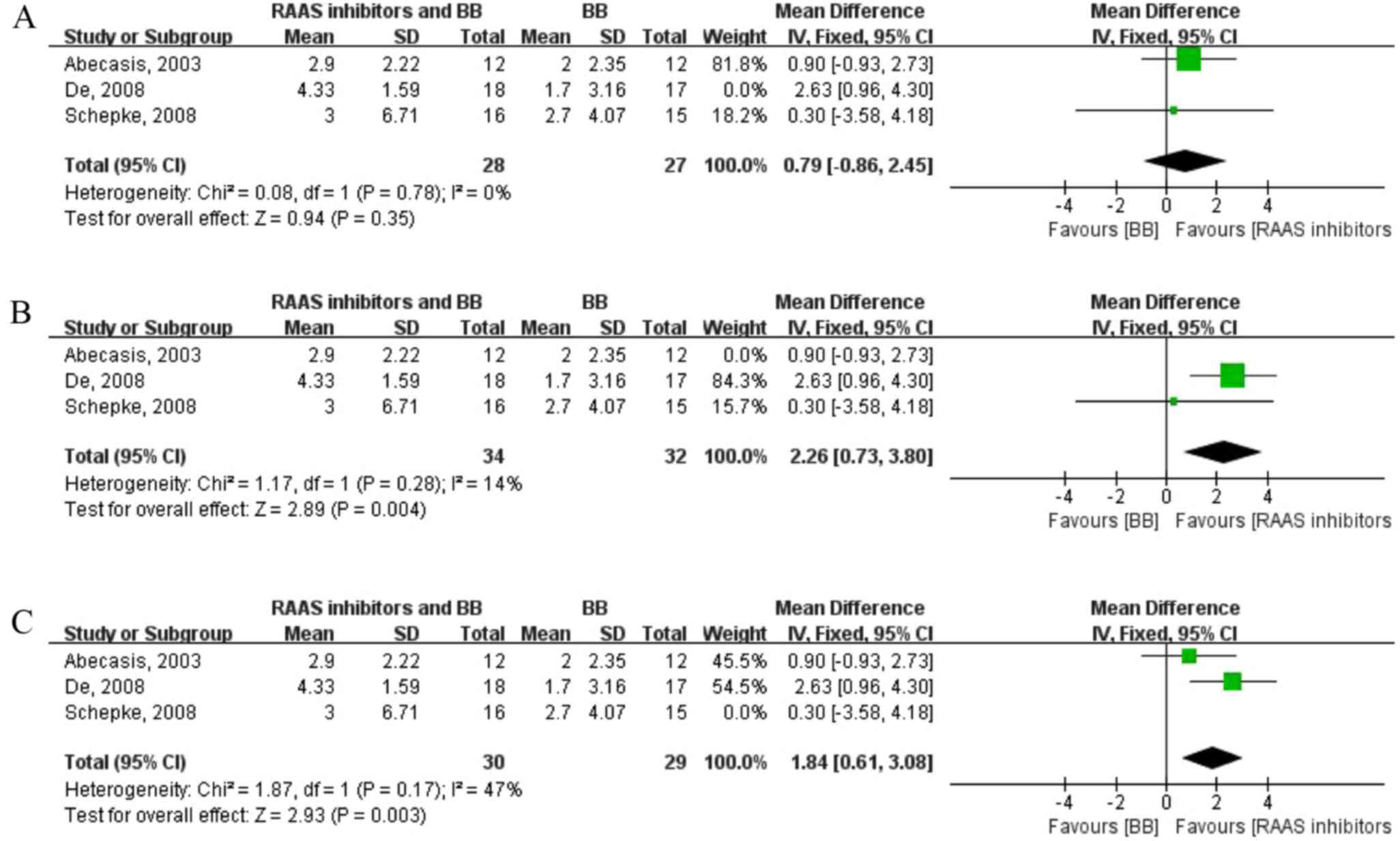
there were only three articles included, influence analysis was
performed in order to study the effect of individual research on
the total combined effect quantity. The result revealed that the
second article (8) has a large
influence on the total consolidation effect quantity (Fig. 3).
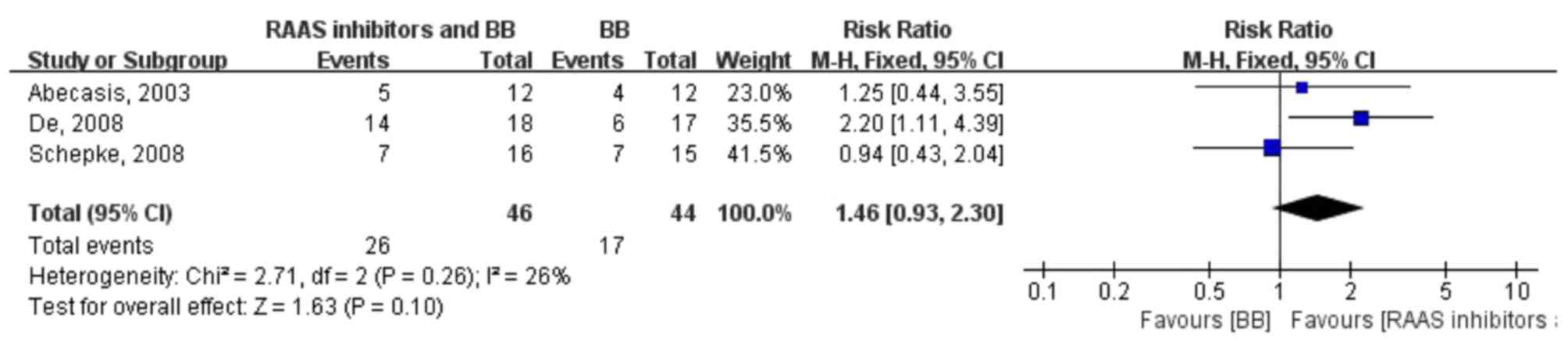
A total of 26 of 46 patients (56.52%) in the RAAS
inhibitor and β-blocker combination therapy group and 17 of 44
patients (38.64%) in the β-blocker monotherapy group revealed a
hemodynamic response. The pooled relative risk of achieving a
hemodynamic response in the two groups was 1.46 (0.93, 2.30;
fixed-effect model), indicating that there was no significant
difference between the hemodynamic response with RAAS inhibitor
plus β-blocker combination therapy and β-blocker monotherapy.
Moreover, no heterogeneity was identified in the pooled analysis of
the trials (P=0.26; I2=26%) (Fig. 4).
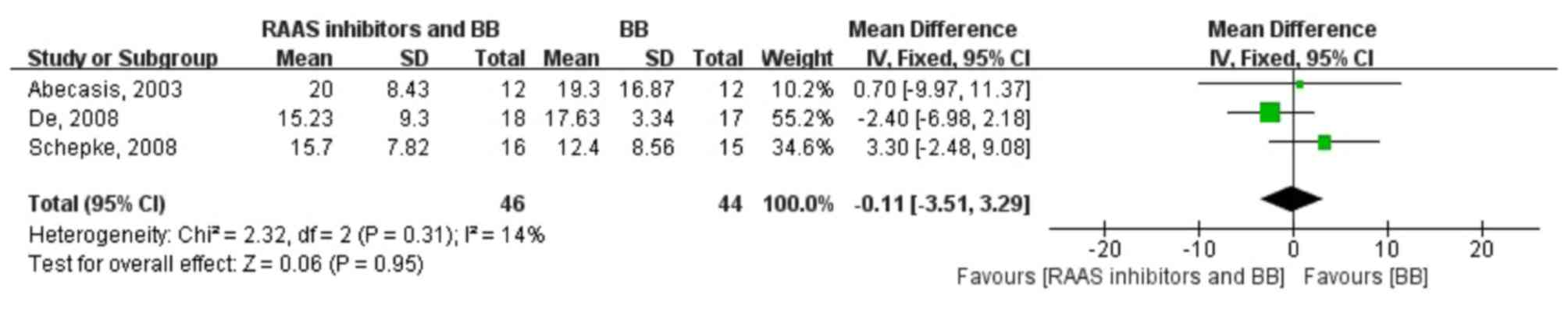
The pooled WMD in the heart rate change in the two
groups was −0.11 (−3.51, 3.29; fixed-effect model), test for
overall effect: Z=0.06 (P=0.95), indicating that there was no
significant heart rate change with the RAAS inhibitor plus
β-blocker combination therapy and β-blocker monotherapy. Moreover,
no heterogeneity was identified (P=0.31; I2=14%)
(Fig. 5).
Currently, β-blockers have become the recommended
medicine for the therapy of portal hypertension, which decrease
portal pressure in two main ways. Firstly, they block β-1 cardiac
receptors, which results in decreased cardiac output and MAP
(75). Secondly, β-blockers function
by blocking β-2 vascular receptors, leading to splanchnic
vasoconstriction results from the unopposed effect of alpha-1
receptors (76). In recent years,
studies (4,6,24,77) have
increasingly revealed that the RAAS system is important in the
pathophysiology of portal hypertension.
Angiotensin II is a vasoconstrictor, which has an
elevated serum concentration in patients with cirrhosis. A prior
study (4), which investigated the
effect of angiotensin II on activated human hepatic stellate cells,
demonstrated that angiotensin II can increase cell contraction and
proliferation, which were rarely detected in resting cells. These
results indicate that angiotensin II induces hepatic stellate cell
activation in order to increase intrahepatic resistance. In
addition, angiotensin type1 (AT1) receptor antagonists were
reported to reduce the progression of hepatic fibrosis and decrease
portal pressure in rats (77). A
previous study investigated the long-term effects of the AT1
receptor on portal hypertension and demonstrated that 25% of
patients achieved a reduction >20%. Moreover, HVPG significantly
decreased in the treated group (−8.4%±2.4) vs. (+5.6%±2.9) in the
controlled group (21). In addition
to the effect of decreasing portal vein pressure by reducing the
plasma volume and the vascular relaxing activity (24), aldosterone antagonist has also been
reported to suppress inflammation, improve endothelial dysfunction,
reduce oxidative stress, decrease insulin resistance and slow down
the progress of liver fibrosis (6).
Since only 30–40% of the patients under long-term
therapy with β-blockers achieve a good hemodynamic response
(3), it is hypothesized that the
RAAS inhibitor and β-blocker combination therapy may achieve a
better effect.
The present meta-analysis aimed to assess the
efficacy of the RAAS inhibitor and β-blocker combination therapy
compared with β-blocker monotherapy on HVPG reduction in patients
with cirrhosis. The results demonstrated that the RAAS inhibitor
and β-blocker combination therapy reduced HVPG to a more
significant extent compared to β-blocker monotherapy. In addition,
the pooled WMD between HVPG reduction with RAAS inhibitor plus
β-blocker combination therapy and β-blocker monotherapy was 1.70
(95% CI: 0.52–2.88), and no heterogeneity was identified.
The number of patients achieving a hemodynamic
response was reported in all studies included and was higher with
the RAAS inhibitor and β-blocker combination therapy (26/46 vs.
17/44). However, there were no significant differences in the
pooled relative risk of achieving a hemodynamic response with RAAS
inhibitor and β-blocker combination therapy compared with β-blocker
monotherapy (1.46; 95% CI: 0.93–2.30).
Nevertheless, there are many limitations in the
present study. Firstly, the number of available studies and
patients included was too small, which mitigated the achievement of
satisfactory results. Secondly, the course of the selected trials
was not the same, and the span was large. In addition, angiotensin
I-converting enzyme inhibitors, angiotensin receptor blockers and
aldosterone antagonists were regarded as the same drugs to be
analyzed, while they function through different targets, which may
result in a different hemodynamic response. However, despite these
limitations, to the best of our knowledge the present study is the
first meta-analysis comparing the RAAS inhibitor and β-blocker
combination therapy with the β-blocker monotherapy effect on portal
pressure, and included all high quality randomized controlled
trials.
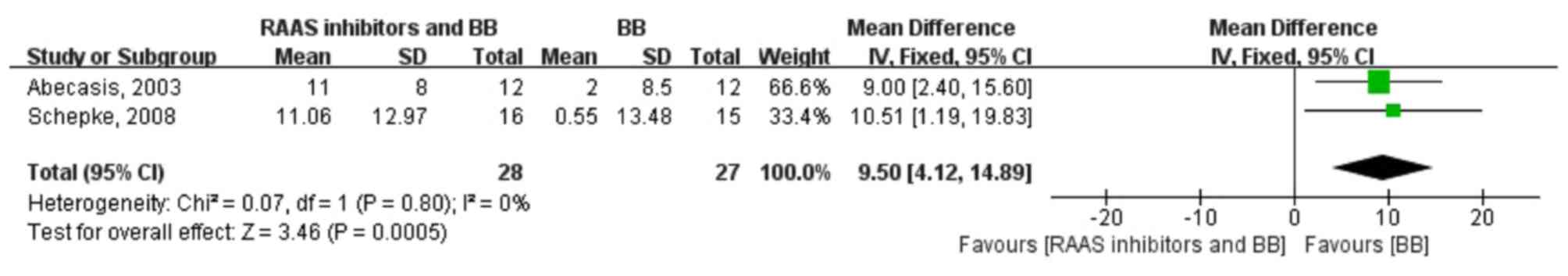
In conclusion, the RAAS inhibitor and β-blocker
combination therapy reduces portal hypertension to a more
significant extent than β-blocker monotherapy. Although both
therapies reduced the heart rate to similar levels, the RAAS
inhibitor and β-blocker combination therapy reduced the MAP to a
greater extent compared to β-blocker monotherapy. Further
larger-scale trials are required in order to determine the efficacy
and safety of the RAAS inhibitor and β-blocker combination therapy
for the reduction of HVPG in patients with cirrhosis.
The present study was supported by the Shanghai
Health System Important Disease Joint Research Project (grant no.
2014ZYJB0201).
|
1
|
Feu F, García-Pagín JC, Bosch J, Luca A,
Terés J, Escorsell A and Rodés J: Relation between portal pressure
response to pharmacotherapy and risk of recurrent variceal
haemorrhage in patients with cirrhosis. Lancet. 346:1056–1059.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Groszmann RJ, Bosch J, Grace ND, Conn HO,
Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M,
et al: Haemodynamic events in a prospective randomized trial of
propranolol versus placebo in the prevention of a first variceal
hemorrhage. Gastroenterology. 99:1401–1407. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia-Tsao G and Bosch J: Management of
varices and variceal hemorrhage in cirrhosis. N Engl J Med.
362:823–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bataller R, Ginès P, Nicolás JM, Görbig
MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V and Rodés J:
Angiotensin II induces contraction and proliferation of human
hepatic stellate cells. Gastroenterology. 118:1149–1156. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia-Pagán JC, Salmerón JM, Feu F, Luca
A, Ginés P, Pizcueta P, Claria J, Piera C, Arroyo V, Bosch J, et
al: Effects of low-sodium diet and spironolactone on portal
pressure in patients with compensated cirrhosis. Hepatology.
19:1095–1099. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sowers JR, Whaley-Connell A and Epstein M:
Narrative review: The emerging clinical implications of the role of
aldosterone in the metabolic syndrome and resistant hypertension.
Ann Intern Med. 150:776–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tandon P, Abraldes JG, Berzigotti A,
Garcia-Pagan JC and Bosch J: Renin-angiotensin-aldosterone
inhibitors in the reduction of portal pressure: A systematic review
and meta-analysis. J Hepatol. 53:273–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De BK, Dutta D, Som R, Biswas PK, Pal SK
and Biswas A: Haemodynamic effects of propranolol with
spironolactone in patients with variceal bleeds: A randomized
controlled trial. World J Gastroenterol. 14:1908–1913. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schepke M, Wiest R, Flacke S, Heller J,
Stoffel-Wagner B, Herold T, Ghauri M and Sauerbruch T: Irbesartan
plus low-dose propranolol versus low-dose propranolol alone in
cirrhosis: A placebo-controlled, double-blind study. Am J
Gastroenterol. 103:1152–1158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abecasis R, Kravetz D, Fassio E,
Ameigeiras B, Garcia D, Isla R, Landeira G, Dominguez N, Romero G,
Argonz J and Terg R: Nadolol plus spironolactone in the prophylaxis
of first variceal bleed in nonascitic cirrhotic patients: A
preliminary study. Hepatology. 37:359–365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Yang J, Zhu R, Zheng Y, Zhou Y,
Dai W, Wang F, Chen K, Li J, Wang C, et al: Combination therapy of
ursodeoxycholic acid and budesonide for PBC-AIH overlap syndrome: A
meta-analysis. Drug Des Devel Ther. 9:567–574. 2015.PubMed/NCBI
|
|
12
|
Zhang Y, Li S, He L, Wang F, Chen K, Li J,
Liu T, Zheng Y, Wang J, Lu W, et al: Combination therapy of
fenofibrate and ursodeoxycholic acid in patients with primary
biliary cirrhosis who respond incompletely to UDCA monotherapy: A
meta-analysis. Drug Des Devel Ther. 9:2757–2766. 2015.PubMed/NCBI
|
|
13
|
Kjaergard LL, Villumsen J and Gluud C:
Reported methodologic quality and discrepancies between large and
small randomized trials in meta-analyses. Ann Intern Med.
135:982–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gluud LL: Bias in clinical intervention
research. Am J Epidemiol. 163:493–501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sen S, De BK, Biswas PK, Biswas J, Das D
and Maity AK: Haemodynamic effect of spironolactone in liver
cirrhosis and propranolol-resistant portal hypertension. Indian J
Gastroenterol. 21:145–148. 2002.PubMed/NCBI
|
|
17
|
Winkler C, Hobolth L, Krag A, Bendtsen F
and Møller S: Effects of treatment with β-blocker and aldosterone
antagonist on central and peripheral haemodynamics and oxygenation
in cirrhosis. Eur J Gastroenterol Hepatol. 23:334–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De BK, Bandyopadhyay K, Das TK, Das D,
Biswas PK, Majumdar D, Mandal SK, Ray S and Dasgupta S: Portal
pressure response to losartan compared with propranolol in patients
with cirrhosis. Am J Gastroenterol. 98:1371–1376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castano G, Viudez P, Riccitelli M and
Sookoian S: A randomized study of losartan vs propranolol: Effects
on hepatic and systemic haemodynamics in cirrhotic patients. Ann
Hepatol. 2:36–40. 2003.PubMed/NCBI
|
|
20
|
Agasti AK, Mahajan AU, Phadke AY, Nathani
PJ and Sawant P: Comparative randomized study on efficacy of
losartan versus propranolol in lowering portal pressure in
decompensated chronic liver disease. J Dig Dis. 14:266–271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Debernardi-Venon W, Martini S, Biasi F,
Vizio B, Termine A, Poli G, Brunello F, Alessandria C, Bonardi R,
Saracco G, et al: AT1 receptor antagonist Candesartan in selected
cirrhotic patients: Effect on portal pressure and liver fibrosis
markers. J Hepatol. 46:1026–1033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonzalez-Abraldes J, Albillos A, Bañares
R, Del Arbol LR, Moitinho E, Rodríguez C, González M, Escorsell A,
García-Pagán JC and Bosch J: Randomized comparison of long-term
losartan versus propranolol in lowering portal pressure in
cirrhosis. Gastroenterology. 121:382–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venon WD, Baronio M, Leone N, Rolfo E,
Fadda M, Barletti C, Todros L, Saracco G and Rizzetto M: Effects of
long-term Irbesartan in reducing portal pressure in cirrhotic
patients: Comparison with propranolol in a randomised controlled
study. J Hepatol. 38:455–460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nevens F, Lijnen P, VanBilloen H and
Fevery J: The effect of long-term treatment with spironolactone on
variceal pressure in patients with portal hypertension without
ascites. Hepatology. 23:1047–1052. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vilas-Boas WW, Ribeiro-Oliveira A Jr, Rda
Ribeiro C, Vieira RL, Almeida J, Nadu AP, Simões e Silva AC and
Santos RA: Effect of propranolol on the splanchnic and peripheral
renin angiotensin system in cirrhotic patients. World J
Gastroenterol. 14:6824–6830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pagliaro L: Lebrec D, Poynard T, Hillon P,
Benhamou J-P. Propranolol for prevention of recurrent
gastrointestinal bleeding in patients with cirrhosis. A controlled
study [N Engl J Med 1981;305:1371-1374]. J Hepatol. 36:148–150.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah HA, Azam Z, Rauf J, Abid S, Hamid S,
Jafri W, Khalid A, Ismail FW, Parkash O, Subhan A and Munir SM:
Carvedilol vs. esophageal variceal band ligation in the primary
prophylaxis of variceal hemorrhage: A multicentre randomized
controlled trial. J Hepatol. 60:757–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazebnik LB, Mikheeva OM, Toporkov AS,
Komissarenko IA, Vasnev OS and Fedulenkova LV: Correction of portal
hypertension by beta-adrenoblockers (atenolol and metoprolol) and
inhibitors of ACE (lisinopril and enalapril) in liver cirrhosis.
Eksp Klin Gastroenterol. 57–66. 2007.PubMed/NCBI
|
|
29
|
Wagatsuma Y, Naritaka Y, Shimakawa T,
Kanako H, Keiichiro I, Shunichi S, Konno S, Katsube T and Ogawa K:
Clinical usefulness of the angiotensin II receptor antagonist
losartan in patients with portal hypertensive gastropathy.
Hepatogastroenterology. 53:171–174. 2006.PubMed/NCBI
|
|
30
|
Baik SK, Park DH, Kim MY, Choi YJ, Kim HS,
Lee DK, Kwon SO, Kim YJ, Park JW and Chang SJ: Captopril reduces
portal pressure effectively in portal hypertensive patients with
low portal venous velocity. J Gastroenterol. 38:1150–1154. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klein CG: Medicinal influence on portal
vein haemodynamics by cirrhosis of the liver-comparison between
angiotensin II receptor antagonists and β-blockers in combination
with spironolactone. J Zeitschrift fur Gastroenterologie.
39:6692001.
|
|
32
|
Alessandria C, Elia C, Mezzabotta L, Risso
A, Andrealli A, Spandre M, Morgando A, Marzano A and Rizzetto M:
Prevention of paracentesis-induced circulatory dysfunction in
cirrhosis: Standard vs half albumin doses. A prospective,
randomized, unblinded pilot study. Dig Liver Dis. 43:881–886.
2011.PubMed/NCBI
|
|
33
|
Block JP: Rifaximin improves the treatment
of hepatic encephalopathy. J Clin Outcomes Manage. 17:202–204.
2010.
|
|
34
|
Bass NM, Mullen KD, Sanyal A, Poordad F,
Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et
al: Rifaximin treatment in hepatic encephalopathy. N Engl J Med.
362:1071–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van den Velde S, Nevens F, Van hee P, van
Steenberghe D and Quirynen M: GC-MS analysis of breath odor
compounds in liver patients. J Chromatogr B Analyt Technol Biomed
Life Sci. 875:344–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsien L, Breddemann A, Frobel AK, Heusch
A, Schmidt KG and Läer S: Off-label drug use among hospitalised
children: Identifying areas with the highest need for research.
Pharm World Sci. 30:497–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Revill P, Serradell N, Bolós J and Bayes
M: Satavaptan. Drug Fut. 32:26–36. 2007. View Article : Google Scholar
|
|
38
|
Merkel C: Nonselective beta-blockers plus
nitrates in portal hypertension: An open question. Hepatology.
37:1254–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Merkel C, Sacerdoti D, Bolognesi M, et al:
Pharmacological associations in the primary/prophylaxis of variceal
bleeding in cirrhosis. Giornale Italiano di Endoscopia Digestiva.
21:1–5. 1998.
|
|
40
|
Fox K, Ford I, Steg PG, Tardif JC, Tendera
M and Ferrari R: SIGNIFY Investigators: Ivabradine in stable
coronary artery disease without clinical heart failure. N Engl J
Med. 371:1091–1099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rupnik Trošt A, Pajek J, Guček A, Osredkar
J, Kovač D, Bren A, Klančič D, Saksida S, Rus I, Globokar M, et al:
Influence of renin-angiotensin-aldosterone system-blocking drugs on
peritoneal membrane in peritoneal dialysis patients. Ther Apher
Dial. 17:425–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fallahzadeh MK, Dormanesh B, Sagheb MM,
Roozbeh J, Vessal G, Pakfetrat M, Daneshbod Y, Kamali-Sarvestani E
and Lankarani KB: Effect of addition of silymarin to
renin-angiotensin system inhibitors on proteinuria in type 2
diabetic patients with overt nephropathy: A randomized,
double-blind, placebo-controlled trial. Am J Kidney Dis.
60:896–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kosmala W, Przewlocka-Kosmala M,
Szczepanik-Osadnik H, Mysiak A, O'Moore-Sullivan T and Marwick TH:
A randomized study of the beneficial effects of aldosterone
antagonism on LV function, structure, and fibrosis markers in
metabolic syndrome. JACC Cardiovasc Imaging. 4:1239–1249. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spinar J, Vitovec J and Spinarová L:
Zastoupení resitelů studie FARIM: FARIM-FARmakoterapie po Infarktu
Myokardu (Post-Myocardial Infarction Pharmacotherapy Study).
Vnitrni lekarstvi. 57:778–784. 2011.(In Czech). PubMed/NCBI
|
|
45
|
Guney I, Selcuk NY, Altintepe L, Atalay H,
Başarali MK and Büyükbaş S: Antifibrotic effects of aldosterone
receptor blocker (spironolactone) in patients with chronic kidney
disease. Ren Fail. 31:779–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tylicki L, Renke M, Rutkowski P,
Larczyński W, Aleksandrowicz E, Lysiak-Szydlowska W and Rutkowski
B: Dual blockade of the renin-angiotensin-aldosterone system with
high-dose angiotensin-converting enzyme inhibitor for
nephroprotection: An open, controlled, randomized study. Scand J
Urol Nephrol. 42:381–388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tylicki L, Rutkowski P, Renke M,
Larczyński W, Aleksandrowicz E, Lysiak-Szydlowska W and Rutkowski
B: Triple pharmacological blockade of the
renin-angiotensin-aldosterone system in nondiabetic CKD: An
open-label crossover randomized controlled trial. Am J Kidney Dis.
52:486–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muller-Brunotte R, Kahan T, López B, Edner
M, González A, Díez J and Malmqvist K: Myocardial fibrosis and
diastolic dysfunction in patients with hypertension: Results from
the Swedish Irbesartan Left Ventricular Hypertrophy Investigation
versus Atenolol (SILVHIA). J Hypertens. 25:1958–1966. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Orea-Tejeda A, Colín-Ramírez E,
Castillo-Martínez L, Asensio-Lafuente E, Corzo-León D,
González-Toledo R, Rebollar-González V, Narváez-David R and
Dorantes-García J: Aldosterone receptor antagonists induce
favorable cardiac remodeling in diastolic heart failure patients.
Rev Invest Clin. 59:103–107. 2007.PubMed/NCBI
|
|
50
|
Cavallari LH, Momary KM, Groo VL, Viana
MA, Camp JR and Stamos TD: Association of beta-blocker dose with
serum procollagen concentrations and cardiac response to
spironolactone in patients with heart failure. Pharmacotherapy.
27:801–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tylicki L, Biedunkiewicz B, Chamienia A,
Wojnarowski K, Zdrojewski Z, Aleksandrowicz E, Lysiak-Szydlowska W
and Rutkowski B: Renal allograft protection with angiotensin II
type 1 receptor antagonists. Am J Transplant. 7:243–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Christensen MK, Olsen MH, Wachtell K,
Tuxen C, Fossum E, Bang LE, Wiinberg N, Devereux RB, Kjeldsen SE,
Hildebrandt P, et al: Does long-term losartan- vs atenolol-based
antihypertensive treatment influence collagen markers differently
in hypertensive patients? A LIFE substudy. Blood Press. 15:198–206.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ciulla MM, Paliotti R, Esposito A, Dìez J,
López B, Dahlöf B, Nicholls MG, Smith RD, Gilles L, Magrini F and
Zanchetti A: Different effects of antihypertensive therapies based
on losartan or atenolol on ultrasound and biochemical markers of
myocardial fibrosis: Results of a randomized trial. Circulation.
110:552–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hallberg P, Lind L, Billberger K,
Michaelsson K, Karlsson J, Kurland L, Kahan T, Malmqvist K, Ohman
KP, Nyström F, et al: Transforming growth factor beta1 genotype and
change in left ventricular mass during antihypertensive
treatment-results from the Swedish Irbesartan Left Ventricular
Hypertrophy Investigation versus Atenolol (SILVHIA). Clin Cardiol.
27:169–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
el-Agroudy AE, Hassan NA, Foda MA, Ismail
AM, el-Sawy EA, Mousa O and Ghoneim MA: Effect of angiotensin II
receptor blocker on plasma levels of TGF-beta 1 and interstitial
fibrosis in hypertensive kidney transplant patients. Am J Nephrol.
23:300–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hayashi M, Tsutamoto T, Wada A, Tsutsui T,
Ishii C, Ohno K, Fujii M, Taniguchi A, Hamatani T, Nozato Y, et al:
Immediate administration of mineralocorticoid receptor antagonist
spironolactone prevents post-infarct left ventricular remodeling
associated with suppression of a marker of myocardial collagen
synthesis in patients with first anterior acute myocardial
infarction. Circulation. 107:2559–2565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang YY, Lin HC, Lee WP, Chu CJ, Lin MW,
Lee FY, Hou MC, Jap JS and Lee SD: Association of the G-protein and
α2-adrenergic receptor gene and plasma norepinephrine level with
clonidine improvement of the effects of diuretics in patients with
cirrhosis with refractory ascites: A randomised clinical trial.
Gut. 59:1545–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ljubicić N, Bilić A and Plavsić V: Effect
of propranolol on urinary prostaglandin E2 excretion and renal
interlobar arterial blood flow after furosemide administration in
patients with hepatic cirrhosis. Eur J Clin Pharmacol. 43:555–558.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mikheeva OM, Drozdov VN and Komisarenko
IA: Pharmacokinetic and pharmacodynamic characteristics of
antihypertensive drugs in the treatment of hypertensive patients
with chronic diseases of the liver. Ter Arkh. 83:49–55.
2011.PubMed/NCBI
|
|
60
|
Mikheeva OM: Pharmacokinetic and
pharmacodynamic features of hypertensive drugs in treatment of
patients with arterial hypertension associated with liver disease.
Eksp Klin Gastroenterol. 95–101. 2010.(In Russian). PubMed/NCBI
|
|
61
|
Nevens F: Non-invasive variceal pressure
measurements: Validation and clinical implications. Verh K Acad
Geneeskd Belg. 58:413–437. 1996.PubMed/NCBI
|
|
62
|
Talwalkar JA and Kamath PS: Influence of
recent advances in medical management on clinical outcomes of
cirrhosis. Mayo Clin Proc. 80:1501–1508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang JD and Kim WR: Surveillance for
hepatocellular carcinoma in patients with cirrhosis. Clin
Gastroenterol Hepatol. 10:16–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lubel JS, Herath CB, Burrell LM and Angus
PW: Liver disease and the renin-angiotensin system: Recent
discoveries and clinical implications. J Gastroenterol Hepatol.
23:1327–1338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Albillos A: Preventing first variceal
hemorrhage in cirrhosis. J Clin Gastroenterol. 41:(Suppl 3).
S305–S311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Garcia-Tsao G, Sanyal AJ, Grace ND and
Carey W: Practice Guidelines Committee of the American Association
for the Study of Liver Diseases; Practice Parameters Committee of
the American College of Gastroenterology: Prevention and management
of gastroesophageal varices and variceal hemorrhage in cirrhosis.
Hepatology. 46:922–938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Samonakis DN, Triantos CK, Thalheimer U,
Patch DW and Burroughs AK: Management of portal hypertension.
Postgrad Med J. 80:634–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
D'Amico G: The role of vasoactive drugs in
the treatment of oesophageal varices. Expert Opin Pharmacother.
5:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rosado B and Kamath PS: Transjugular
intrahepatic portosystemic shunts: An update. Liver Transpl.
9:207–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bosch J: The sixth Carlos E. Rubio
memorial lecture. Prevention and treatment of variceal hemorrhage.
P R Health Sci J. 19:57–67. 2000.PubMed/NCBI
|
|
71
|
Patch D and Burroughs AK: Pharmacological
treatment of portal hypertension. Prog Liver Dis. 13:269–292.
1995.PubMed/NCBI
|
|
72
|
D'Amico G, Pagliaro L and Bosch J: The
treatment of portal hypertension: A meta-analytic review.
Hepatology. 22:332–354. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Forns X, Ginès A, Ginès P and Arroyo V:
Management of ascites and renal failure in cirrhosis. Semin Liver
Dis. 14:82–96. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Iwakiri Y: Pathophysiology of portal
hypertension. Clin Liver Dis. 18:281–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ohnishi K, Nakayama T, Saito M, Hatano H,
Tsukamoto T, Terabayashi H, Sugita S, Wada K, Nomura F, Koen H, et
al: Effects of propranolol on portal haemodynamics in patients with
chronic liver disease. Am J Gastroenterol. 80:132–135.
1985.PubMed/NCBI
|
|
76
|
Mastai R, Bosch J, Navasa M, Kravetz D,
Bruix J, Viola C and Rodés J: Effects of alpha-adrenergic
stimulation and beta-adrenergic blockade on azygos blood flow and
splanchnic haemodynamics in patients with cirrhosis. J Hepatol.
4:71–79. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka
Y, Noguchi R, Nakatani T, Tsujinoue H and Fukui H: Angiotensin-II
type 1 receptor interaction is a major regulator for liver fibrosis
development in rats. Hepatology. 34:745–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schepke M, Werner E, Biecker E,
Schiedermaier P, Heller J, Neef M, Stoffel-Wagner B, Hofer U,
Caselmann WH and Sauerbruch T: Haemodynamic effects of the
angiotensin II receptor antagonist irbesartan in patients with
cirrhosis and portal hypertension. Gastroenterology. 121:389–395.
2001. View Article : Google Scholar : PubMed/NCBI
|