Introduction
Diabetic nephropathy (DN), a major microvascular
complication of diabetes mellitus, is the leading cause of
end-stage renal disease (ESRD) in the western world and the second
most common cause of renal failure with increasing morbidity in
China (1). Although maintenance of
normoglycemia and therapeutic intervention in the
renin-angiotensin-aldosterone system are effective treatments
(2,3), these interventions only partially delay
the progression of DN (4);
therefore, identifying new therapeutic strategies remains of great
importance. The pathogenesis of DN is complicated; renal fibrosis
is the primary pathophysiologic process associated with chronic
renal failure caused by DN (5).
Epithelial-to-mesenchymal transition (EMT) and extracellular matrix
(ECM) accumulation under pathological conditions also occur in
mature tubular epithelial cells of the adult kidney, and have been
implicated in renal interstitial fibrosis (6,7). It has
previously been indicated that a large proportion of interstitial
fibrosis cases originate from tubular epithelial cells via EMT in
diseased kidneys (8). However, the
underlying mechanisms for this remain to be elucidated.
RhoA, which is a member of the Rho guanosine
triphosphatases (GTPases) family, is essential in the regulation of
cellular functions (9). It cycles
between an inactive GDP-bound and an active GTP-bound form, and its
intrinsic hydrolytic activity is affected by various Rho regulators
(10). Membrane localization via
post-translational modification is required for RhoA activation
(9). Previous studies have
demonstrated that RhoA and its downstream kinase Rho-kinase (ROCK)
are able to mediate matrix elaboration via mesangial cells (MCs) in
hyperglycemic and DN conditions (4,11), and
the RhoA/ROCK signaling pathway has been demonstrated to be
associated with fibrosis progression in multiple organs including
the kidneys, liver and lungs (4,9,12). Furthermore, RhoA/ROCK was previously
reported to be associated with EMT (7,13), which
was prevented by inhibition of RhoA activation in rat peritoneal
cells (13). A previous study by the
present authors also revealed that transforming growth factor
(TGF)-β1-mediated RhoA/ROCK activation contributed to the
dissolution of tight junctions during EMT in HK-2 cell in
vitro (14).
In recent years, the protective effect of vitamin D
(VitD)/VitD receptor (VDR) in patients with DN has been illustrated
(15,16). Clinical treatment with active VitD is
typically accompanied by hypercalcemic side effects (17), therefore, VitD analogs with fewer
side effects may provide therapeutic benefits. A previous clinical
study demonstrated that treatment with paricalcitol, which is a
VitD analog, was able to safely reduce residual albuminuria in
patients with DN (18). Treatment
with 1,25-(OH)2D3 (also known as calcitriol)
or its analogs is able to repress the expression of α-SMA and
collagen I in a unilateral ureteral occlusion model and cultured
HK-2 cells (19,20). The renoprotective role of
1,25-(OH)2D3 and its analogs have been
demonstrated to be associated with anti-inflammation,
renin-angiotensin system (RAS) inhibition and prevention of EMT
(17,20–22). By
binding to the VDR, 1,25-(OH)2D3 recruits
cofactors to form a transcriptional complex which subsequently
binds to VitD response elements in the promoter region of target
genes, altering transcriptional events within target cells
(17,20,23).
However, the underlying mechanism of this renoprotective effect
remains largely unknown at present.
Morelli et al (24) illustrated that treatment with
elocalcitol (BXL-628), which is a VitD analog and VDR agonist,
impaired RhoA membrane translocation and inhibited RhoA/ROCK
activation in bladder smooth muscle cells, repressing RhoA-mediated
cell migration and cytoskeleton remodeling (24,25).
However, whether the anti-EMT and anti-fibrosis role of
1,25-(OH)2D3 and analogs is associated with
the RhoA/ROCK signaling pathway remains to be elucidated.
The aim of the present study was to investigate the
effect of the endogenous VDR ligand
1,25-(OH)2D3 and its analog BXL-628 on high
glucose-induced activation of the RhoA/ROCK pathway in human renal
proximal tubular cells. The results of the preset study may
potentially elucidate a mechanism of the protective effect of VitD
in DN.
Materials and methods
Cell culture and treatment
Human renal proximal tubular epithelial cells, HK-2
(ATCC, Manassas, VA, US), were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), streptomycin (100
µg/ml) and penicillin (100 U/ml) in a humidified atmosphere
containing 5% CO2 at 37°C. When cells reached 80%
confluence, they were treated with high glucose (HG group; 30
mmol/l; Henyang Kaixin Chemical Reagent Company, Ltd., Hunan,
China) for 0, 0.5, 1, 2, 4, 8 and 12 h to verify whether high
glucose conditions activated RhoA. To determine the time at which
high glucose induced EMT, HK-2 cells were treated with high glucose
for 0, 12, 24, 48 and 72 h at 37°C respectively; mannitol (30
mmol/l; Henyang Kaixin Chemical Reagent Company, Ltd.) was used as
negative control. For further analysis, HK-2 cells were cultured in
high glucose medium (supplemented DMEM with 30 mmol/l glucose) at
37°C, and co-treated with 1,25-(OH)2D3 (VD3
group; 100 nM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), or
BXL-628 (BXL group; 10 nM; Molecular Devices, LLC, Sunnyvale, CA,
USA) at 2 or 48 h, respectively, according to the results of the
previous experiment. Low glucose (5.6 mmol/l) was used as the
normal control (NC) group.
Western blotting
Total protein was extracted from indicated cells
using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China), and membrane protein
was extracted using a Plasma Membrane Protein Extraction kit
(BioVision, Inc., Milpitas, CA, USA) according to the
manufacturer's protocol. A total of 50 µg total protein or membrane
protein was separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. Membranes were blocked for 1 h
at room temperature with 5% non-fat milk, subsequently rinsed and
incubated overnight at 4°C with anti-RhoA (1:1,000; #2117; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-α-SMA (1:3,000;
sc-32251; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-epithelial (E)-cadherin (1:3,000; #3195; Cell Signaling
Technology, Inc.), anti-ATPase Na+/K+
(1:4,000; ab7671; Abcam, Cambridge, MA, USA),
anti-phosphorylated-myosin-binding subunit (MBS) (1:1,000;
sc-514261; Santa Cruz Biotechnology, Inc.), anti-ROCK (1:1,000;
sc-17794; Santa Cruz Biotechnology, Inc.) and anti-β-actin
antibodies (1:5,000; PA1-183; Invitrogen; Thermo Fisher Scientific,
Inc.). Membranes were subsequently washed with TBST and incubated
with the appropriate secondary antibody (goat anti-rabbit or goat
anti-mouse; CW0114S and CW0102S, respectively; 1:3,000; Kangwei
Biotech Company, Beijing, China) for 2 h at room temperature.
Enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.) was used to detect the signal on the membrane.
Data was analyzed using densitometry with Image-Pro plus software
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) and normalized to
β-actin or ATPase Na+/K+ expression.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde (pH 7.4)
at room temperature for 30 min, permeabilized for 10 min with PBS
containing 0.1% Triton X-100, rinsed with PBS and subsequently
incubated with blocking buffer (normal goat serum; CW0130S; Kangwei
Biotech Company) at room temperature for 30 min. Immunostaining was
performed as previously described (24) using anti-pan-cadherin (1:100;
ab195203; Abcam) and anti-RhoA antibodies (1:100; ab54835; Abcam),
followed by tetramethylrhodamine-conjugated AffiniPure (red) goat
anti-mouse (1:200; SA00007-1; Proteintech Group, Inc., Chicago, IL,
USA) and fluorescein isothiocyanate-conjugated AffiniPure (green)
goat anti-rabbit (1:200; SA00003-2; Proteintech Group, Inc.)
antibodies, respectively. A fluorescent microscope (BX61 Automated
Fluorescent Microscope; Olympus Corporation, Tokyo, Japan) was use
to observe the cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 2 µg RNA was used for cDNA
synthesis with a ReverTra Ace qPCR RT kit (Toyobo Co., Ltd., Osaka,
Japan) according to the manufacturer's protocol. Total RNA was
treated with DNase I prior to transcription according the kit
manual. A total reaction volume of 20 µl was used for PCR with a
Taq DNA polymerase-based 2x master mix for real-time PCR
(SYBR®-Green Real-time PCR Master Mix, Toyobo Co., Ltd.)
using the iQ5 real-time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The sequence detector was programmed for the
following PCR conditions: 50°C for 2 min, 95°C for 10 min, 40
cycles at 95°C for 15 sec and 60°C for 1 min. The relative
expressions of α-SMA, collagen I, fibronectin and E-cadherin mRNA
were expressed relative to β-actin as an internal control, using
the 2−ΔΔCq method (26).
Oligo 6.0 software (Molecular Biology Insights, Inc., Cascade, CO,
USA) was used to design PCR primers (Table I), which were synthesized by Shanghai
Sangong Pharmaceutical Co., Ltd. (Shanghai, China).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| mRNA | Primer
sequences | Primer length
(bp) |
|---|
| α-SMA | Forward:
5′-TTCAATGTCCCAGCCATGTA-3′ | 125 |
|
| Reverse:
5′-GAAGGAATAGCCACGCTCAG-3′ |
|
| E-cadherin | Forward:
5′-TGCCCAGAAAATGAAAAAGG-3′ | 120 |
|
| Reverse:
5′-GTGTATGTGGCAATGCGTTC-3′ |
|
| Collagen I | Forward:
5′-CCAAATCTGTCTCCCCAGAA-3′ | 136 |
|
| Reverse:
5′-TCAAAAACGAAGGGGAGATG-3′ |
|
| Fibronectin | Forward:
5′-GCTTCCTGGCACTTCTGGTC-3′ | 136 |
|
| Reverse:
5′-CTACATTCGGCGGGTATGGT-3′ |
|
| β-actin | Forward:
5′-CATCCTGCGTCTGGACCTGG-3′ | 107 |
|
| Reverse:
5′-TAATGTCACGCACGATTTCC-3′ |
|
ELISA assay
The concentrations of collagen I and fibronectin
secreted from cultured cells in medium were determined using an
ELISA kits (CSB-E13445 h and CSB-EL005721HU; Cusabio Biotech Co.,
Ltd., Wuhan, China) according to the manufacturer's protocol. The
final absorbance at 450 nm was measured using TECAN Infinite M200
microplate reader (Thermo Fisher Scientific, Inc.). Concentrations
of collagen I and fibronectin were calculated using a standard
curve constructed in the same plate, and expressed relative to the
cell protein concentration.
Statistical analysis
All experiments were performed at least 3 times.
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used for the analysis and graphics presentation. One-way
analysis of variance followed by Tukey's multiple comparisons test
or Student's t-test was used depending on the experimental
condition. The data were expressed as mean ± standard error of the
mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
1,25-(OH)2D3 and
BXL-628 decreases RhoA activation and ROCK activity in HK-2
cells
Using western blot analysis, it was demonstrated
that high glucose conditions induced RhoA activation in a
time-dependent manner, which was significantly increased from 1 to
8 h (P<0.01) compared with the control group, reaching a peak at
2 h and returning to the control level at 12 h post-administration
(Fig. 1A). The effects of
1,25-(OH)2D3 and its analog BXL-628 on HK-2
cells treated with high glucose for 2 h were also investigated. As
illustrated in Fig. 1B, active RhoA
protein expression in the HG group was significantly upregulated
compared with the NC group (P<0.05), and treatment with
1,25-(OH)2D3 or BXL-628 significantly
decreased the expression of active RhoA protein (both P<0.05).
Additionally, ROCK activity was evaluated by measuring the
phospho-MBS level (Fig. 1C). It was
revealed that ROCK activity was significantly lower in the VD3 and
BXL groups compared with the high glucose group (P<0.01).
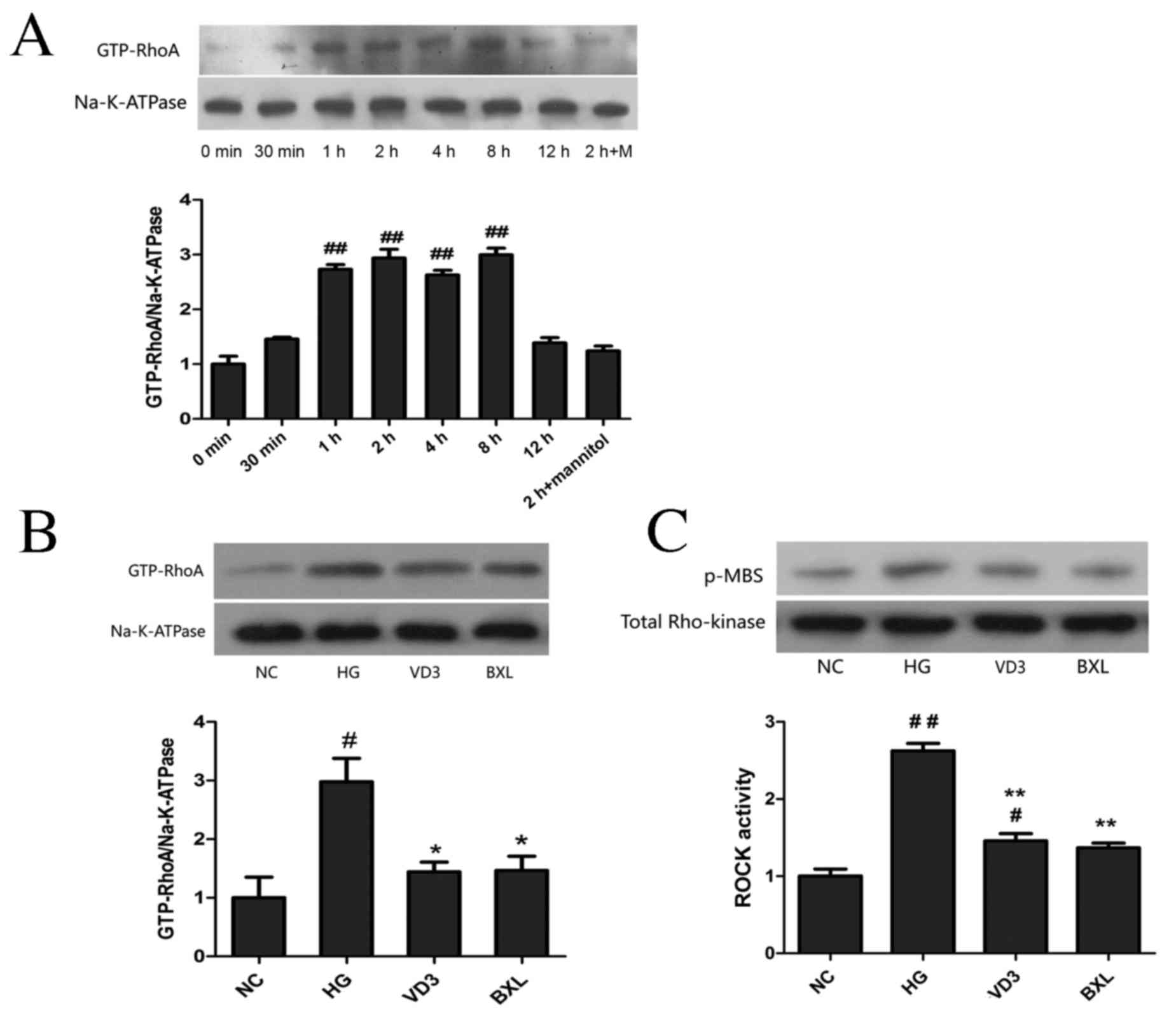 | Figure 1.Effect of
1,25-(OH)2D3 and BXL-628 on GTP-RhoA and
active ROCK expression. (A) GTP-RhoA expression in HK-2 cells
exposed to high glucose for 0, 0.5, 1, 2, 4, 8 and 12 h. (B)
Expression of GTP-RhoA protein in HK-2 cells from NC, HG, VD3 and
BXL groups following 2 h treatment. (C) ROCK activity in HK-2 cells
from NC, HG, VD3 and BXL groups following 2 h treatment. ROCK
activity was assessed p-MBS. Data are expressed as the mean ±
standard error of the mean (n=3). #P<0.05,
##P<0.01 vs. NC group; *P<0.05, **P<0.01 vs. HG
group. GTP-RhoA, activated RhoA; ROCK, Rho associated protein
kinase; NC, normal control group; HG, high glucose group; VD3,
1,25-(OH)2D3 group; BXL, BXL-628 group;
p-MBS, phosphorylated myosin-binding subunit. |
Indirect immunofluorescence also revealed that
expression of active RhoA protein was markedly reduced in the VD3
and BXL groups compared with the NC group (Fig. 2). The intracellular localization of
RhoA was monitored by staining with a specific monoclonal antibody
and compared with pan-cadherin immune reactivity as a cell membrane
marker. The merged images obtained by dual labeling of RhoA and
pan-cadherin immune staining confirmed that high glucose
stimulation increased RhoA localization at the plasma membrane,
whereas treatment with 1,25-(OH)2D3 or
BXL-628 treatment reduced RhoA membrane expression.
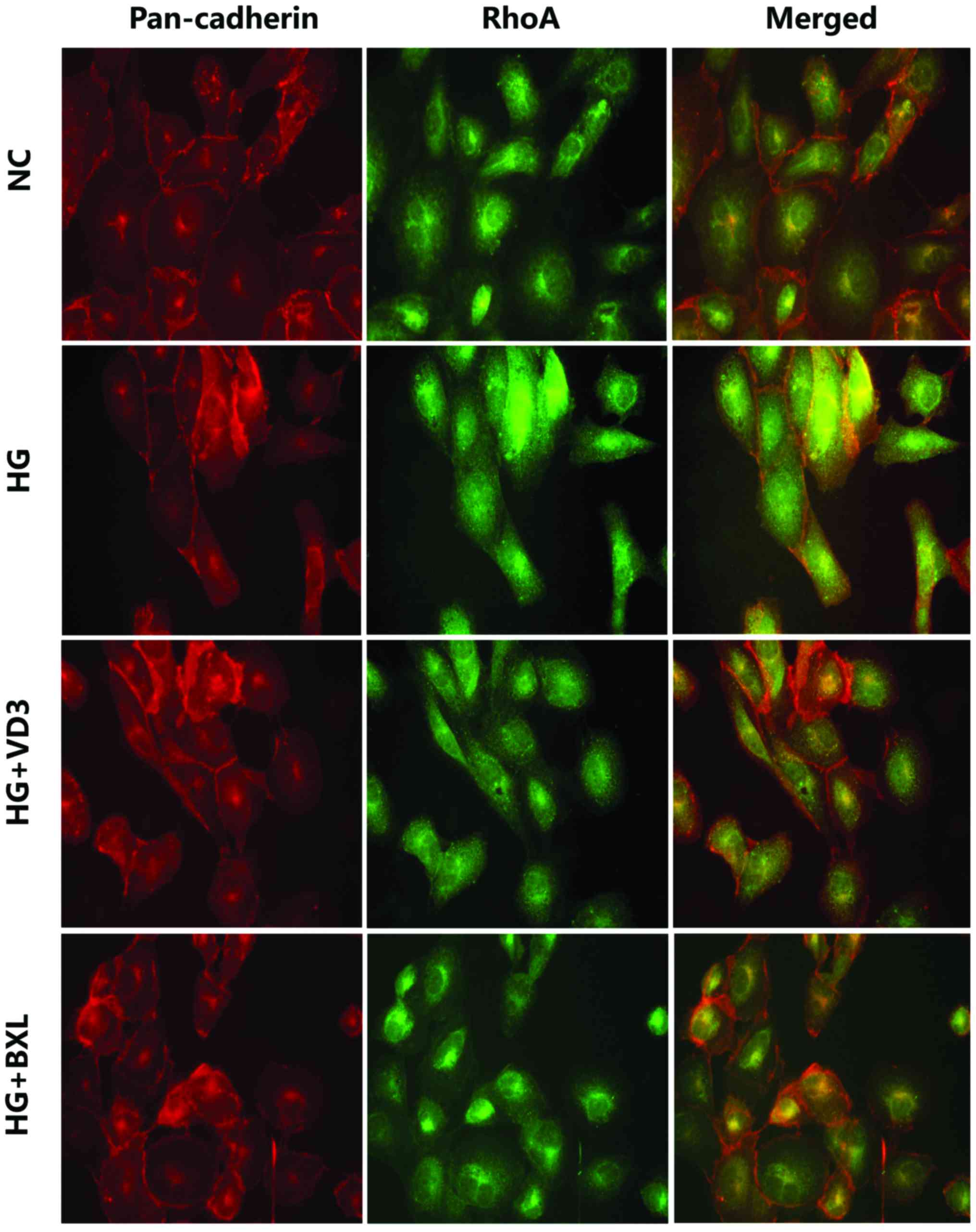 | Figure 2.1,25-(OH)2D3
and BXL-628 inhibit RhoA translocation from the cytosol to the
plasma membrane. Confocal immune localization by dual labelling of
pan-cadherin (red, tetramethylrhodamine) and RhoA (green,
fluorescein isothiocyanate) in HK-2 cells from NC, HG, VD3 and BXL
groups following 2 h treatment. Yellow indicates co-localization of
RhoA and pan-cadherin at plasma membrane level. Cells were analyzed
by confocal microscopy and individual and merged stains are shown.
Magnification, ×400. ROCK, Rho associated protein kinase; NC,
normal control group; HG, high glucose group; VD3,
1,25-(OH)2D3 group; BXL, BXL-628 group. |
Effects of
1,25-(OH)2D3 and BXL-628 on the expression of
E-cadherin and α-SMA
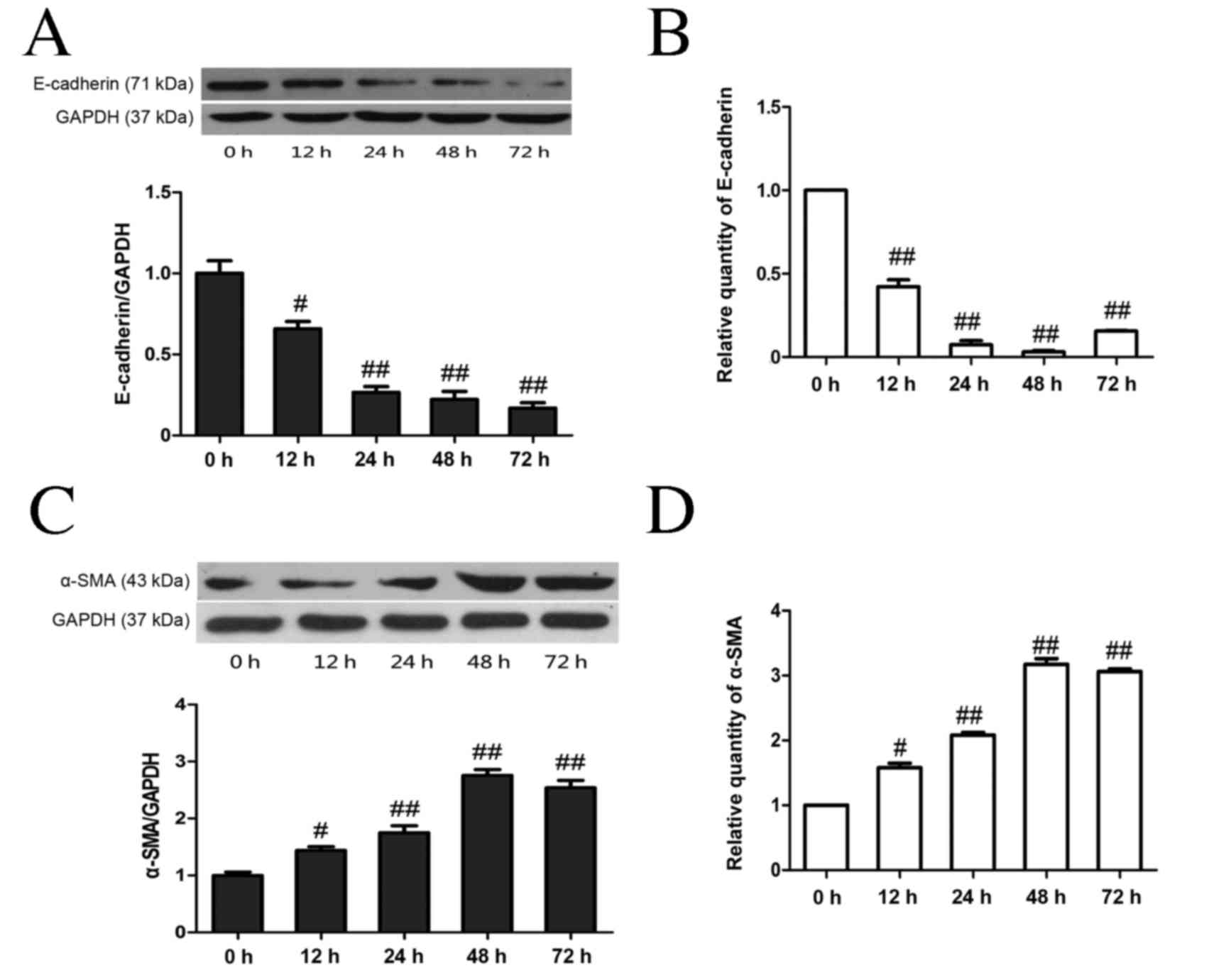
As shown in Fig. 3,
high glucose stimulation resulted in a significant time-dependent
decrease in E-cadherin protein levels (P<0.05 at 12 h, P<0.01
from 24–72 h; Fig. 3A) and mRNA
(P<0.01; Fig. 3B) compared with
the NC group, whereas high glucose stimulation resulted in a
significant time-dependent increase in α-SMA at both the protein
and mRNA levels (both P<0.05 at 12 h, P<0.01 from 24–72 h;
Fig. 3C-D). Treatment with either
1,25-(OH)2D3 or BXL-628 attenuated the
downregulation in E-cadherin expression at the protein (P<0.05;
Fig. 4A) and mRNA levels (P<0.01;
Fig. 4B) compared with the HG group.
Both 1,25-(OH)2D3 and BXL-628 treatments
significantly inhibited the expression of α-SMA at the protein
(P<0.05; Fig. 4C) and mRNA
(P<0.01; Fig. 4D) levels compared
with the HG group (Fig. 4B and
D).
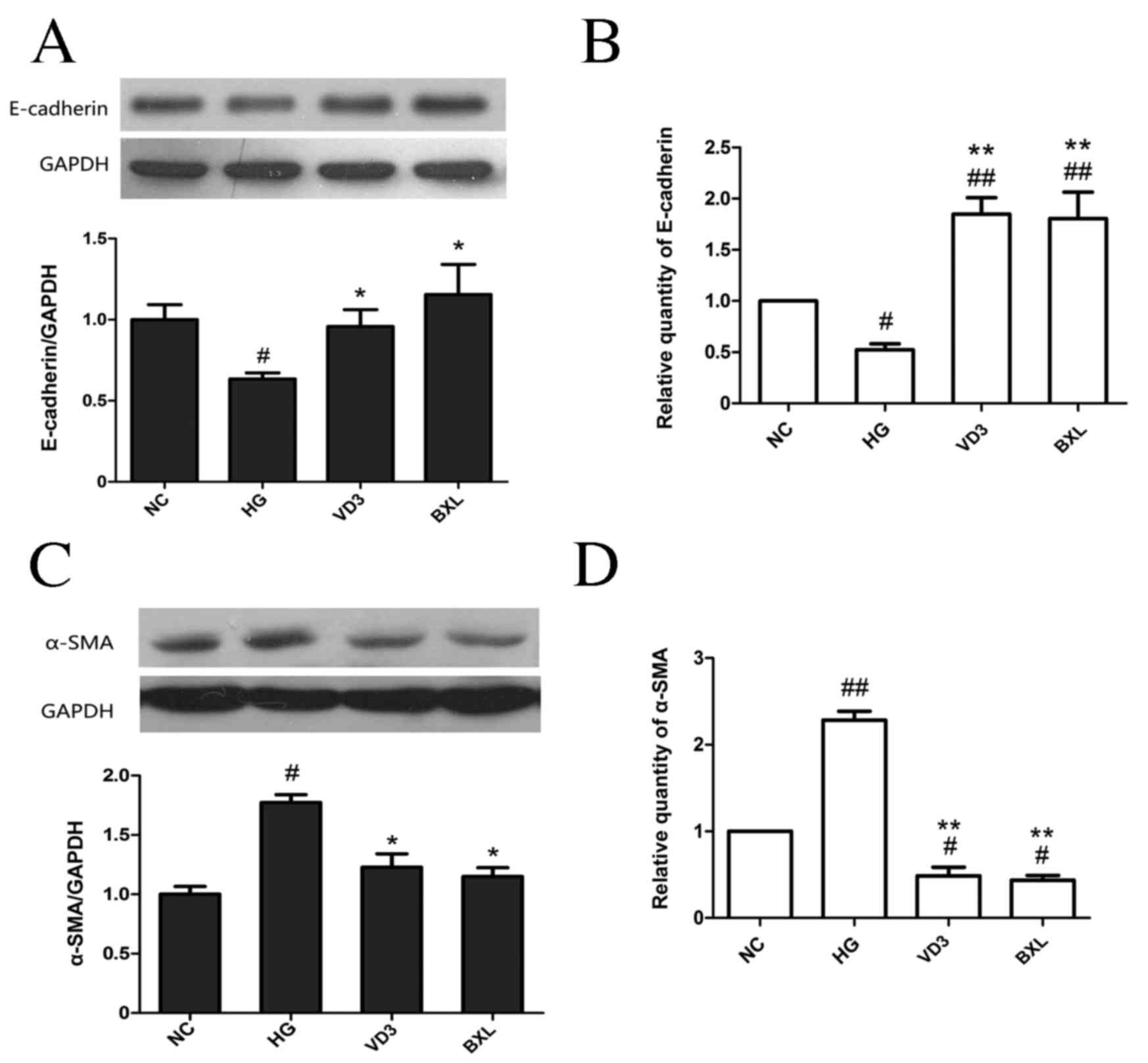 | Figure 4.The effect of
1,25-(OH)2D3 and BXL-628 on E-cadherin and
α-SMA expression. (A) Expression of E-cadherin protein in HK-2
cells from NC, HG, VD3 and BXL groups following 48 h treatment. (B)
Relative expression of E-cadherin mRNA. (C) Expression of α-SMA
protein in HK-2 cells from NC, HG, VD3 and BXL groups following 48
h treatment. (D) Relative expression of α-SMA mRNA. Data are
expressed as the mean ± standard error of the mean (n=3).
#P<0.05, ##P<0.01 vs. NC group;
*P<0.05, **P<0.01 vs. HG group. E, epithelial; SMA, smooth
muscle actin; NC, normal control group; HG, high glucose group;
VD3, 1,25-(OH)2D3 group; BXL, BXL-628
group. |
Effects of
1,25-(OH)2D3 and BXL-628 on the expression of
ECM in HK-2 cells
To further analyze the effects of
1,25-(OH)2D3 and its analog BXL-628 on ECM
accumulation induced by high glucose, the expressions of collagen I
and fibronectin in the culture supernatant were detected using
ELISA. Cellular mRNA expression was evaluated using qPCR. The
results indicated that high glucose stimulation significantly
increased the expression of collagen I protein (P<0.05; Fig. 5A) and mRNA (P<0.01; Fig. 5B) compared with the NC group. High
glucose stimulation also induced a significant increase in
fibronectin protein (P<0.05; Fig.
5C) and mRNA (P<0.01; Fig.
5D) compared with the NC groups. Notably, both
1,25-(OH)2D3 and BXL-628 significantly
decreased the expression of collagen I and fibronectin compared
with the HG group (P<0.01 for collagen protein, collagen mRNA
and fibronectin mRNA; P<0.05 for fibronectin protein; Fig. 5A-D).
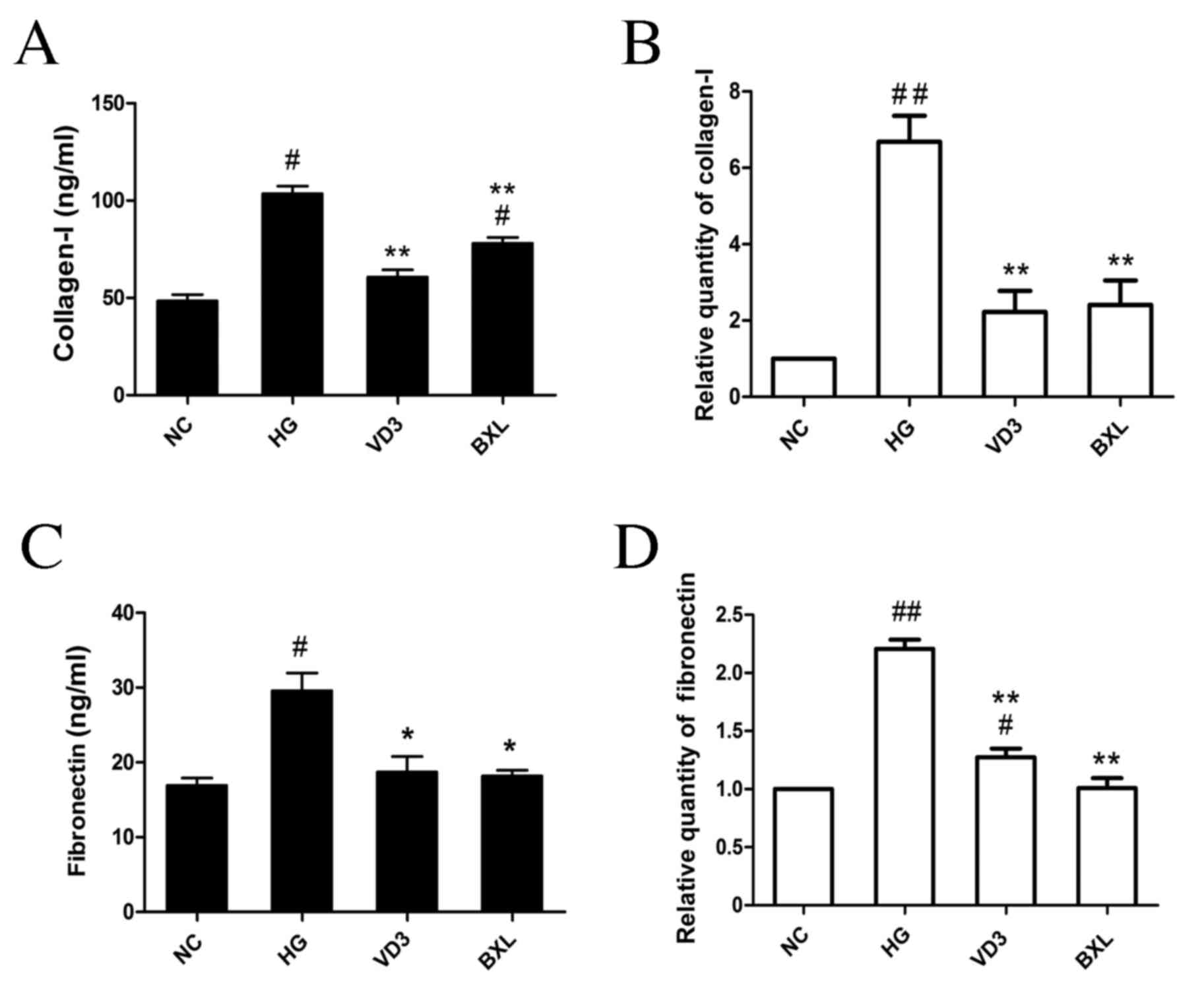 | Figure 5.The effect of
1,25-(OH)2D3 and BXL-628 on collagen I and
fibronectin expression. (A) Protein level of collagen I in the
culture supernatant of HK-2 cells from NC, HG, VD3 and BXL groups
following 48 h treatment. (B) Relative expression of collagen I
mRNA. (C) Protein level of fibronectin in the culture supernatant
of HK-2 cells from NC, HG, VD3 and BXL groups following 48 h
treatment. (D) Relative expression of fibronectin mRNA. Data are
expressed as the mean ± standard error of the mean (n=4).
#P<0.05, ##P<0.01 vs. NC group;
*P<0.05, **P<0.01 vs. HG group. NC, normal control group; HG,
high glucose group; VD3, 1,25-(OH)2D3 group;
BXL, BXL-628 group. |
Discussion
The present study demonstrated that
1,25-(OH)2D3 and its analog BXL-628
contribute to renoprotection by preventing high glucose-induced EMT
and ECM accumulation via inactivating the RhoA/ROCK signaling
pathway in vitro. Hyperglycemia is one of the most important
risk factors in diabetes and its complications, including DN
(1,5). Hyperglycemic conditions activate a
number of important intracellular signaling pathways, including the
TGF-β/SMAD, nuclear factor-κB and Rho/ROCK pathways (12,15,27,28),
which serve crucial roles in the diabetic renal inflammation
response, RAS activation, EMT and fibrosis (3,8,19,29).
VitD and VDR have previously been demonstrated to have renal
protective functions via their regulative role in pathways
associated with the prevention of EMT and fibrosis (17,20,30,31).
Peng et al (4) illustrated
that high glucose was able to activate RhoA/Rho-kinase in MCs,
leading to downstream activator protein-1 activation and
fibronectin induction (4). In
accordance with the studies mentioned above, the results of the
present study also demonstrated that high glucose-induced
activation of the RhoA/ROCK pathway subsequently resulted in EMT
and ECM accumulation in HK-2 cells. Furthermore, treatment with
1,25-(OH)2D3 or BXL-628 significantly
inactivated the RhoA/ROCK pathway and attenuated high
glucose-induced EMT and ECM accumulation in HK-2 cells. The present
study found that 1,25-(OH)2D3 and BXL-628
treatment was able to decrease the high glucose-stimulated
upregulation of α-SMA and downregulation of E-cadherin.
Additionally, fibronectin and collagen I accumulation were
significantly suppressed in both the VD3 and BXL groups compared
with the HG group.
VDR acts as a transcriptional factor and a
nongenomic activator of the RhoA/ROCK and p38MAPK/mitogen and
stress activated kinase (MSK)1 pathways, which are required for the
biofunction of 1,25-(OH)2D3 in colon
carcinoma cells (32). By binding to
VDR, 1,25-(OH)2D3 induces an influx of
Ca2+ into cytoplasm and subsequently activates the
RhoA/ROCK and p38MAPK-MSK1 kinase pathways (32). This indicates that the rapid
modulation of ion content and cytosolic GTPases and kinases is
associated with EMT and ECM accumulation (33,34).
However, the present study demonstrated that
1,25-(OH)2D3 acts as an inhibitor of
RhoA/ROCK and its role may depend on cell types. In accordance with
the results of the present study, it has previously been
demonstrated that BXL-628 is able to inhibit RhoA activation in a
dose-dependent manner without altering RhoA expression, and
ameliorates excessive hyperplasia and aberrant differentiation in
bladder smooth muscle cells (24,25). In
the present study, 1,25-(OH)2D3 exerted a
similar effect to BXL-628 at a much higher concentration. This
suggests that BXL-626 may be a more efficient pharmaceutical
choice, although further beneficial value must be investigated
through more clinical studies and fundamental research.
1,25-(OH)2D3 and BXL-628 do not alter the
expression of RhoA and ROCK, however they do increase their
membrane translocation and binding to GTP (24,35).
In conclusion, the results of the present study
demonstrate that 1,25-(OH)2D3 and BXL-628
treatment repress the high glucose-activated RhoA/ROCK pathway in
HK-2 cells. Additionally, activation of the VitD/VDR pathway by
treatment with 1,25-(OH)2D3 or BXL-628
decreased the expression of α-SMA, collagen I and fibronectin, and
increased E-cadherin expression; this is suggestive of an anti-EMT
and anti-fibrosis role of 1,25-(OH)2D3 and
BXL-628. These results indicate that the renal protective effects
of 1,25-(OH)2D3 and its analog are mediated,
at least in part, by the RhoA/ROCK signaling pathway.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470961), the
Natural Science Foundation of Hunan Province (grant no. 14JJ2037)
and the New Xiangya Talent Project of the Third Xiangya Hospital of
Central South University (grant no. 20150313).
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
No authors listed: Retinopathy and
nephropathy in patients with type 1 diabetes four years after a
trial of intensive therapy. The diabetes control and complications
trial/epidemiology of diabetes interventions and complications
research group. N Engl J Med. 342:381–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis EJ, Hunsicker LG, Bain RP and Rohde
RD: The effect of angiotensin-converting-enzyme inhibition on
diabetic nephropathy. The collaborative study group. N Engl J Med.
329:1456–1462. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng F, Wu D, Gao B, Ingram AJ, Zhang B,
Chorneyko K, McKenzie R and Krepinsky JC: RhoA/Rho-kinase
contribute to the pathogenesis of diabetic renal disease. Diabetes.
57:1683–1692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Afkarian M, Sachs MC, Kestenbaum B, Hirsch
IB, Tuttle KR, Himmelfarb J and de Boer IH: Kidney disease and
increased mortality risk in type 2 diabetes. J Am Soc Nephrol.
24:302–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Y, Wan J, Jiang D and Wu X: BMP-7
counteracts TGF-beta1-induced epithelial-to-mesenchymal transition
in human renal proximal tubular epithelial cells. J Nephrol.
22:403–410. 2009.PubMed/NCBI
|
|
7
|
Bhowmick NA, Ghiassi M, Bakin A, Aakre M,
Lundquist CA, Engel ME, Arteaga CL and Moses HL: Transforming
growth factor-beta1 mediates epithelial to mesenchymal
transdifferentiation through a RhoA-dependent mechanism. Mol Biol
Cell. 12:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: Pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolavennu V, Zeng L, Peng H, Wang Y and
Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression
of diabetic nephropathy independent of glucose control. Diabetes.
57:714–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manickam N, Patel M, Griendling KK, Gorin
Y and Barnes JL: RhoA/Rho kinase mediates TGF-β1-induced kidney
myofibroblast activation through Poldip2/Nox4-derived reactive
oxygen species. Am J Physiol Renal Physiol. 307:F159–F171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Liu X, Liu Y, Yi B and Yu X:
Epithelial-mesenchymal transition of rat peritoneal mesothelial
cells via Rhoa/Rock pathway. In Vitro Cell Dev Biol Anim.
47:165–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K, Zhang H, Xiang H, Liu J, Liu Y,
Zhang X, Wang J and Tang Y: TGF-β1 induces the dissolution of tight
junctions in human renal proximal tubular cells: Role of the
RhoA/ROCK signaling pathway. Int J Mol Med. 32:464–468.
2013.PubMed/NCBI
|
|
15
|
Al-Rubeaan K, Youssef AM, Subhani SN,
Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM, David SK and AlNaqeb D:
Diabetic nephropathy and its risk factors in a society with a type
2 diabetes epidemic: A Saudi national diabetes registry-based
study. PLoS One. 9:e889562014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo J, Xia N, Yang L, Zhou S, Zhang Q,
Qiao Y and Liu Z: GSK-3β and vitamin D receptor are involved in
β-catenin and snail signaling in high glucose-induced
epithelial-mesenchymal transition of mouse podocytes. Cell Physiol
Biochem. 33:1087–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meems LM, Cannon MV, Mahmud H, Voors AA,
van Gilst WH, Silljé HH, Ruifrok WP and de Boer RA: The vitamin D
receptor activator paricalcitol prevents fibrosis and diastolic
dysfunction in a murine model of pressure overload. J Steroid
Biochem Mol Biol. 132:282–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Zeeuw D, Agarwal R, Amdahl M, Audhya P,
Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E
and Andress D: Selective vitamin D receptor activation with
paricalcitol for reduction of albuminuria in patients with type 2
diabetes (VITAL study): A randomised controlled trial. Lancet.
376:1543–1551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan X, Li Y and Liu Y: Paricalcitol
attenuates renal interstitial fibrosis in obstructive nephropathy.
J Am Soc Nephrol. 17:3382–3393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong M, Gong J, Liu Y, Xiang R and Tan X:
Loss of vitamin D receptor in chronic kidney disease: A potential
mechanism linking inflammation to epithelial-to-mesenchymal
transition. Am J Physiol Renal Physiol. 303:F1107–F1115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanchez-Niño MD, Bozic M, Córdoba-Lanús E,
Valcheva P, Gracia O, Ibarz M, Fernandez E, Navarro-Gonzalez JF,
Ortiz A and Valdivielso JM: Beyond proteinuria: VDR activation
reduces renal inflammation in experimental diabetic nephropathy. Am
J Physiol Renal Physiol. 302:F647–F657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duffy MM, McNicholas BA, Monaghan DA,
Hanley SA, McMahon JM, Pindjakova J, Alagesan S, Fearnhead HO and
Griffin MD: Mesenchymal stem cells and a vitamin D receptor agonist
additively suppress T helper 17 cells and the related inflammatory
response in the kidney. Am J Physiol Renal Physiol.
307:F1412–F1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding N, Yu RT, Subramaniam N, Sherman MH,
Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, et al: A
vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic
response. Cell. 153:601–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morelli A, Vignozzi L, Filippi S, Vannelli
GB, Ambrosini S, Mancina R, Crescioli C, Donati S, Fibbi B, Colli
E, et al: BXL-628, a vitamin D receptor agonist effective in benign
prostatic hyperplasia treatment, prevents RhoA activation and
inhibits RhoA/Rho kinase signaling in rat and human bladder.
Prostate. 67:234–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manchanda PK, Kibler AJ, Zhang M, Ravi J
and Bid HK: Vitamin D receptor as a therapeutic target for benign
prostatic hyperplasia. Indian J Urol. 28:377–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang SC, Leung JC and Lai KN: Diabetic
tubulopathy: An emerging entity. Contrib Nephrol. 170:124–134.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanlaya R, Sintiprungrat K and
Thongboonkerd V: Secreted products of macrophages exposed to
calcium oxalate crystals induce epithelial mesenchymal transition
of renal tubular cells via RhoA-dependent TGF-β1 pathway. Cell
Biochem Biophys. 67:1207–1215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mezzano S, Droguett A, Burgos ME, Ardiles
LG, Flores CA, Aros CA, Caorsi I, Vío CP, Ruiz-Ortega M and Egido
J: Renin-angiotensin system activation and interstitial
inflammation in human diabetic nephropathy. Kidney Int Suppl.
S64–S70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim CS, Joo SY, Lee KE, Choi JS, Bae EH,
Ma SK, Kim SH, Lee J and Kim SW: Paricalcitol attenuates
4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal
transition in human renal proximal tubular epithelial cells. PLoS
One. 8:e631862013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonçalves JG, de Bragança AC, Canale D,
Shimizu MH, Sanches TR, Moysés RM, Andrade L, Seguro AC and Volpini
RA: Vitamin D deficiency aggravates chronic kidney disease
progression after ischemic acute kidney injury. PLoS One.
9:e1072282014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ordonez-Mórán P, Larriba MJ, Pálmer HG,
Valero RA, Barbáchano A, Duñach M, de Herreros AG, Villalobos C,
Berciano MT, Lafarga M and Muñoz A: RhoA-ROCK and p38MAPK-MSK1
mediate vitamin D effects on gene expression, phenotype, and Wnt
pathway in colon cancer cells. J Cell Biol. 183:697–710. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pertz O, Hodgson L, Klemke RL and Hahn KM:
Spatiotemporal dynamics of RhoA activity in migrating cells.
Nature. 440:1069–1072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wildenberg GA, Dohn MR, Carnahan RH, Davis
MA, Lobdell NA, Settleman J and Reynolds AB: p120-catenin and
p190RhoGAP regulate cell-cell adhesion by coordinating antagonism
between Rac and Rho. Cell. 127:1027–1039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amuchastegui S, Daniel KC and Adorini L:
Inhibition of acute and chronic allograft rejection in mouse models
by BXL-628, a nonhypercalcemic vitamin D receptor agonist.
Transplantation. 80:81–87. 2005. View Article : Google Scholar : PubMed/NCBI
|