Introduction
Chronic rhinosinusitis (CRS) is a group of disorders
defined as persistent inflammation involving the nose and paranasal
mucosa, and is characterized by nasal
blockage/obstruction/congestion, nasal discharge, facial
pain/pressure, and/or reduction in or loss of smell (1). The overall prevalence of CRS ranged
from 6.9 to 27.1% (mean, 10.9%) due to obvious geographical
variation (2), which causes a
significant public health problem as well as considerable
socioeconomic costs (1,3). Currently, not all CRS cases can be
completely controlled due to the complex and multifactorial
etiology of this condition, including the involvement of exogenous
pathogens, microbial biofilms and individual host factors (4,5).
Glucocorticoids are effectively used as
anti-inflammatory drugs to improve airway inflammatory diseases,
and are considered as the first-line treatment for CRS (1,6,7). In addition, glucocorticoids have been
reported to regulate the water balance in various tissues and
organs, including the lungs, peritoneum and middle ear (8).
Aquaporin 5 (AQP5) is a crucial protein formed by
four subunits that passively transports water in and out of cells
in accordance with the osmotic gradient across the membrane
(9). AQP5 has been described to
serve a role in several diseases associated with dysfunction of
water regulation, and is key in this process (10–12).
Furthermore, differences in the cellular location and mRNA
expression pattern of AQP5 were observed in the nasal tissues from
CRS patients compared with those of healthy controls (13,14).
Based on their hypothesized functions,
glucocorticoids may also alleviate edema and improve the symptoms
of CRS by means of their regulatory role on AQP5. No previous
studies have examined the effects of glucocorticoids on AQP5 in
CRS, to the best of our knowledge. In the present study, the
expression pattern of AQP5 and the effect of glucocorticoids on
AQP5 expression were studied using a rat model of CRS.
Materials and methods
Animal model and treatment
A total of 30 male Sprague-Dawley rats (age, 6
months; Animal Laboratory Center, Nanjing Drum Tower Hospital,
Nanjing, China) weighing between 220 and 250 g were used in this
study. All animals were handled according to the guidelines of the
Animal Care and Use Committee of Nanjing Drum Tower Hospital,
Nanjing University School of Medicine.
The rats were randomly divided into three equal
groups, as follows: i) CRS; ii) dexamethasone (dexa) treatment; and
iii) control groups. Animals in the first two groups were
anesthetized by intraperitoneal injection of a mixture of ketamine
(50 mg/kg; Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu,
China) and diazepam (5 mg/kg; Tianjin Jinyao Pharmaceutical Co.,
Ltd., Tianjin, China), and a polyvinyl acetal material (3×5 mm)
with Staphylococcus aureus (ATCC 25923; American Type
Culture Collection, Manassas, VA, USA), produced in the Laboratory
of Clinical Microbiology, Nanjing Drum Tower Hospital) was inserted
into the left nasal cavity of each rat from the CRS and dexa
groups. The control group did not receive any treatment. On the
90th postoperative day, the dexa group received dexa (2 mg/kg/day)
via intraperitoneal injection for 7 days. All rats were sacrificed
under anesthesia via an intraperitoneal injection of a mixture of
ketamine (50 mg/kg) and diazepam (5 mg/kg) on the 97th
postoperative day, and the left sinonasal mucosa were removed for
subsequent experiments (Fig. 1).
Some samples were used immediately for histology and
immunohistochemical staining, others were stored in eppendorf tubes
at −70°C.
Histology and immunohistochemical
staining
The samples were prepared by cardiac perfusion with
physiological saline, and were then fixed with 4% paraformaldehyde
for 24 h at 4°C. The specimens were dehydrated by a graded ethanol
series and embedded in paraffin. Tissues were cut into 4-µm
sections, deparaffinized, and hydrated with phosphate-buffered
saline (PBS; pH 7.4). A number of sections were stained with
hematoxylin and eosin (HE) for morphological examination, as
described previously (15), whereas
other samples from the same rats were treated with 3%
H2O2 for 30 min at room temperature to block
the endogenous peroxide, and then incubated with rabbit polyclonal
antibody against AQP5 (ab92320; Abcam, Cambridge, UK; 1:800
dilution) for 18 h at 4°C. The sections were then washed with PBS
and incubated with a free biotin-conjugated anti-rabbit IgG
secondary antibody (PV8000; PowerVision Two-Step Histostaining
Reagent; Zhongshan Golden Bridge, Beijing, China; 1:100 dilution)
for 30 min at room temperature. The sections were incubated with
0.05% 3,3-diaminobenzidine and counterstained with Mayer's
hematoxylin. The negative control was incubated with 0.01 M PBS
instead of the primary antibody.
RNA extraction, reverse transcription,
and quantitative PCR (qPCR)
Total RNA of samples from the left sinonasal muscosa
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific Inc., Waltham, MA, USA), and 1 µg of total RNA was
reverse-transcribed into cDNA using the Transcriptor First Strand
cDNA Synthesis kit (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturer's instructions. qPCR was conducted
using specific primers (10 µmol/l; Invitrogen; Thermo Fisher
Scientific, Inc.) and SYBR Premix Ex Taq kit (ABI, USA) with an ABI
7900HT Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The concentration of mRNA used in each reaction
was 1 µg/µl. The sequences of the primers were as follows: AQP5
forward, 5′-AGGAGAGGAAGAAGACCATCGA-3′, and reverse,
5′-TGCTTCAAACTCTTCGTCTTCCTT-3′; β-actin forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. The amplification reaction consisted
of 40 cycles of denaturation (95°; 20 sec), annealing (60°; 60 sec)
and extension (72°; 60 sec). The level of mRNA was assessed using
the comparative cycle threshold (Cq) method (16), relative to β-actin.
Statistical analysis
Statistical analyses were performed using SAS
software 9.1.3 (SAS Institute Inc., Cary, NC, USA). Comparisons of
relative mRNA expression between groups were analyzed using the
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histology
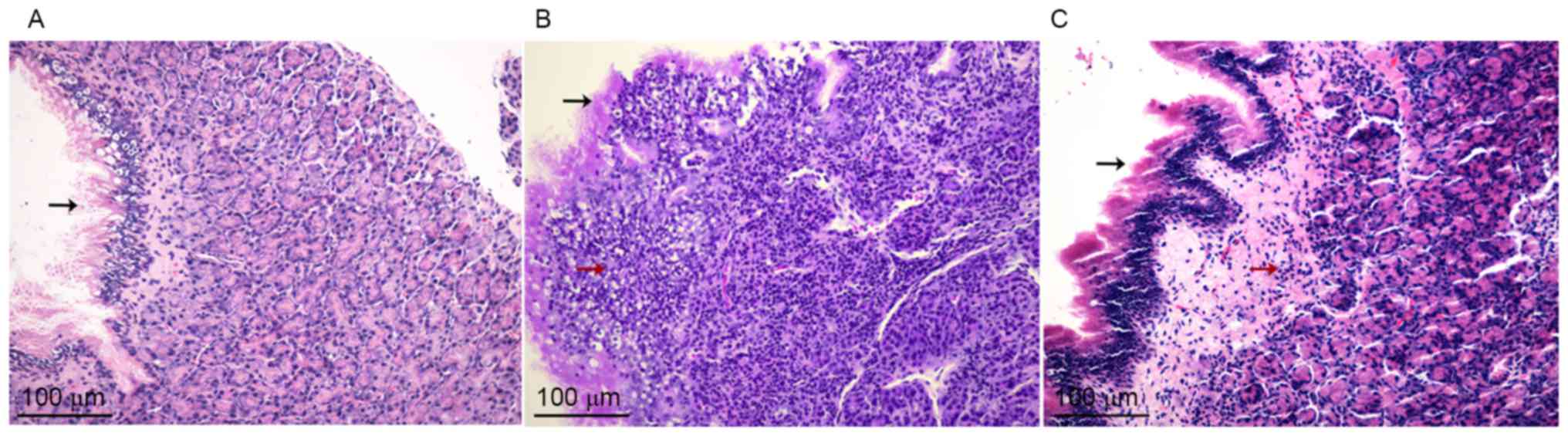
HE staining revealed no evidence of inflammatory
clusters in the mucosa of the maxillary sinus obtained from control
rats (Fig. 2A). However, erosions of
epithelial cilia, gland damage, vessel dilation, edema and
dispersed lymphocytes in the connective tissue were observed in the
sinonasal mucosa in the CRS and dexa groups (Fig. 2B and C), which confirmed chronic
sinonasal inflammation.
Immunohistochemical staining
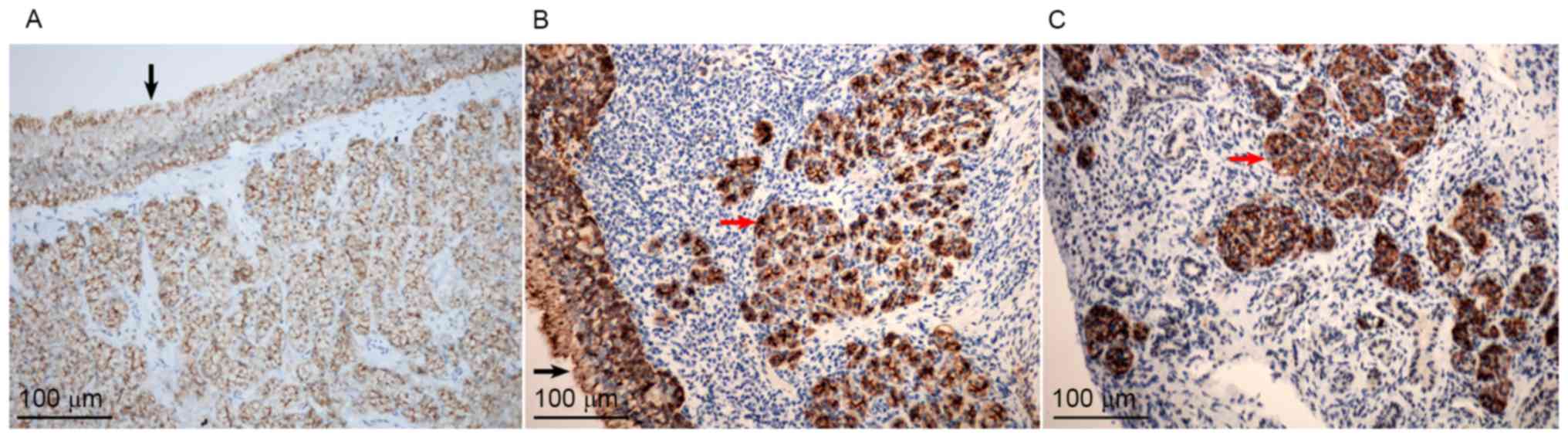
The sinonasal mucosal specimens from all three
groups demonstrated positive AQP5 staining, as indicated by the
brownish color (Fig. 3). The
immunoreactivity of AQP5 was primarily noted in the epithelial
lining, glandular cells, vascular endothelium and goblet cells in
the sinonasal mucosa (Fig. 3A). No
AQP5 expression was observed in the damaged gland areas
demonstrating lymphocyte infiltration, whereas a marked reaction
was observed in the residual glands (red arrows; Fig. 3B and C).
qPCR
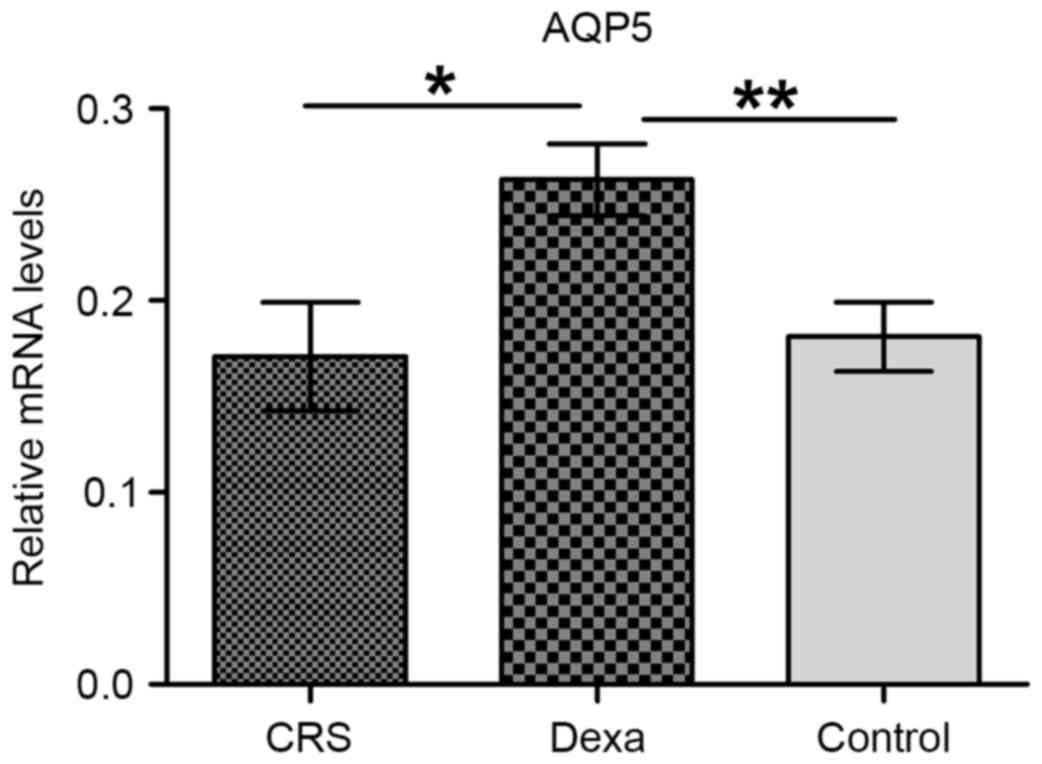
AQP5 mRNA expression was confirmed by qPCR, and was
detected in the sinonasal mucosa from all three groups. As reported
in Fig. 4, the AQP5 mRNA level was
significantly higher in the dexa group than in the CRS and control
groups (P=0.014 and P=0.006, respectively). However, no significant
difference was found between the CRS and control groups
(P=0.760).
Discussion
Among the multiple etiological hypotheses of
inflammation involving exogenous and host factors, bacterial
colonization is the most cited factor (1,17,18). As
the most common colonizer of the nasal passages and sinuses, S.
aureus is also regarded as a primary causal pathogen of CRS,
owing to its high prevalence in CRS patients (17,19),
ability to secrete superantigens that alleviate airway inflammation
(20) and tendency to form a
biofilm, which negatively affects treatment outcomes in CRS
patients (21).
In the present study, with the aim of stimulating
and maintaining chronic sinonasal inflammation, a model of CRS was
established in rats using intranasally administered S.
aureus. The experimental CRS that developed in the current
model is similar to that reported in previous studies (22,23).
AQP5 was confirmed to be primarily expressed in the
epithelial lining, subepithelial glandular cells, vascular
endothelium and goblet cells of the sinonasal mucosa of the rats.
This distribution is consistent with previous findings in humans
(14,24). The subepithelial glandular cells are
established to participate in mucus secretion and consistency,
which maintain the function of the mucociliary system. Furthermore,
the epithelial cilia are crucial in osmotic transport to facilitate
cilia-dependent movement of mucus. Thus, the present distribution
pattern suggests that AQP5 serves notable and coordinated roles in
osmotic homeostasis in the sinonasal mucosa.
AQP5 mRNA was also detected in the sinonasal mucosa
of the rat model. Specifically, the AQP5 mRNA level in the dexa
group was significantly higher than that of the other two groups,
with no significant difference between the CRS and control groups.
It is therefore hypothesized that the underlying reason for this
result is as follows: The persistent inflammation induced by S.
aureus damaged the natural structure of the nasal mucosa,
including the ciliated epithelium and glandular tissue, and AQP5
was predominantly localized to these two cell types. It may be
speculated that there may, in fact, be increased expression of AQP5
mRNA in the sinonasal mucosa of CRS rats; however, as the remaining
cell counts were reduced in this model, there was an overall
similar value of the mRNA quantity compared with the control
group.
The valuable role of glucocorticoids in the
conservative treatment of CRS is undeniable; in addition to their
anti-inflammatory effects, glucocorticoids can regulate the water
balance in multiple tissues (8,25,26).
Water transport of the sinonasal mucosa is also noteworthy in the
pathogenesis of CRS. Abnormal water homeostasis leads to nasal
obstruction, purulent discharge and polyp formation. Previous
studies revealed that glucocorticoids increased the expression of
AQP5 in human airway epithelial cells (25), and may alleviate pulmonary edema in
asthmatic rats (26).
In the present study, elevated expression of AQP5
mRNA was observed following glucocorticoid stimulation by
intraperitoneal administration for 7 days. It was therefore
hypothesized that the regulatory effect of glucocorticoids on AQP5
results in osmotic homeostasis of the nasal mucosa via promotion of
glandular secretion and alleviating edema, which consequently
improves local inflammation. However, additional studies are
required to confirm the protein expression pattern of AQP5, and to
elucidate whether glucocorticoids modulate the expression of AQP5
in a dose- and/or time-dependent manner.
In summary, the current study investigated the
expression of AQP5 and the effect of glucocorticoids on AQP5
expression in a rat model of CRS. The results demonstrated that
glucocorticoids enhance the functional expression of AQP5. These
findings may provide evidence for a novel target in CRS
treatment.
Acknowledgements
The current work was supported by the Medical
Science Program of Nanjing Municipality (grant no. YKK13081) and
the Medical Youth Talent Cultivation Project of Nanjing
Municipality (grant no. QRX11193).
References
|
1
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: EPOS 2012: European position paper on rhinosinusitis and nasal
polyps 2012. A summary for otorhinolaryngologists. Rhinology.
50:1–12. 2012.PubMed/NCBI
|
|
2
|
Hastan D, Fokkens WJ, Bachert C, Newson
RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A,
Dahlén SE, et al: Chronic rhinosinusitis in Europe-an
underestimated disease. A GA2LEN study. Allergy.
66:1216–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subspecialty Group of Rhinology, Editorial
Board of Chinese Journal of Otorhinolaryngology Head and Neck
Surgery; Subspecialty Group of Rhinology, Society of
Otorhinolaryngology Head and Neck Surgery, Chinese Medical
Association: Guidelines for diagnosis and treatment of chronic
rhinosinusitis (2012, Kunming). Zhonghua Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 48:92–94. 2013.PubMed/NCBI
|
|
4
|
Mahoney EJ and Metson R: Palliative care
for the patient with refractory chronic rhinosinusitis. Otolaryngol
Clin North Am. 42:39–47, vii-viii. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan BK, Schleimer RP and Kern RC:
Perspectives on the etiology of chronic rhinosinusitis. Curr Opin
Otolaryngol Head Neck Surg. 18:21–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T,
Schünemann HJ, et al: Allergic rhinitis and its impact on asthma
(ARIA) guidelines: 2010 revision. J Allergy Clin Immunol.
126:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okubo K, Kurono Y, Fujieda S, Ogino S,
Uchio E, Odajima H and Takenaka H: Japanese Society of Allergology:
Japanese guideline for allergic rhinitis 2014. Allergol Int.
63:357–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu C, Cui X, Chen F, Yang J, Qian X and
Gao X: Effect of glucocorticoids on aquaporin-1 in guinea pigs with
otitis media with effusion. ExpTher Med. 5:1589–1592. 2013.
|
|
9
|
Arbeithuber B, Thuenauer R, Gravogl Y,
Balogi Z, Römer W, Sonnleitner A and Tiemann-Boege I: Aquaporin 5
expression in mouse mammary gland cells is not driven by promoter
methylation. Biomed Res Int. 2015:4605982015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang K, Feng YL, Wen FQ, Chen XR, Ou XM,
Xu D, Yang J and Deng ZP: Decreased expression of human aquaporin-5
correlated with mucus overproduction in airways of chronic
obstructive pulmonary disease. Acta Pharmacol Sin. 28:1166–1174.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Iwata F, Muraguchi M, Ooga K,
Ohmoto Y, Takai M, Mori T and Ishikawa Y: Correlation between
salivary secretion and salivary AQP5 levels in health and disease.
J Med Invest. 56:(Suppl). S350–S353. 2009. View Article : Google Scholar
|
|
12
|
Yoshimura S, Nakamura H, Horai Y, Nakajima
H, Shiraishi H, Hayashi T, Takahashi T and Kawakami A: Abnormal
distribution of AQP5 in labial salivary glands is associated with
poor saliva secretion in patients with Sjögren's syndrome including
neuromyelitis optica complicated patients. Mod Rheumatol.
26:384–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frauenfelder C, Woods C, Hussey D, Ooi E,
Klebe S and Carney AS: Aquaporin expression profiles in normal
sinonasal mucosa and chronic rhinosinusitis. Int Forum Allergy
Rhinol. 4:901–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shikani AH, Sidhaye VK, Basaraba RJ,
Shikani HJ, Alqudah MA, Kirk N, Cope E and Leid JG: Mucosal
expression of aquaporin 5 and epithelial barrier proteins in
chronic rhinosinusitis with and without nasal polyps. Am J
Otolaryngol. 35:377–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kmiec Z: J.A. Kiernan. Histological and
Histochemical Methods: Theory and Practice. 5th edition, Scion
Publishing, 2015, 571 pp. Folia HistochemCytobiol. 54:58–59.
2016.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Mutairi D and Kilty SJ: Bacterial
biofilms and the pathophysiology of chronic rhinosinusitis. Curr
Opin Allergy Clin Immunol. 11:18–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vickery TW and Ramakrishnan VR: Bacterial
pathogens and the microbiome. Otolaryngol Clin North Am. 50:29–47.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sachse F, Becker K and Rudack C: Incidence
of staphylococcal colonization and of the 753Q Toll-like receptor 2
variant in nasal polyposis. Am J Rhinol Allergy. 24:e10–e13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinchuk IV, Beswick EJ and Reyes VE:
Staphylococcal enterotoxins. Toxins (Basel). 2:2177–2197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singhal D, Foreman A, Jervis-Bardy J and
Wormald PJ: Staphylococcus aureus biofilms: Nemesis of
endoscopic sinus surgery. Laryngoscope. 121:1578–1583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn SK, Jeon SY, Khalmuratov R, Kim DJ,
Kim JP, Park JJ and Hur DG: Rat model of staphylococcal enterotoxin
B-induced rhinosinusitis. Clin Exp Otorhinolaryngol. 1:24–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang KL, Jiang RS, Wang J, Shiao JY, Su
MC, Hsin CH and Lin JF: Developing a rabbit model of rhinogenic
chronic rhinosinusitis. Laryngoscope. 118:1076–1081. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seno S, Ogawa T, Shibayama M, Kouzaki H
and Shimizu T: Expression and localization of aquaporin 1, 2, 3, 4
and 5 in human nasal mucosa. Am J Rhinol Allergy. 26:167–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ben Y, Chen J, Zhu R, Gao L and Bai C:
Upregulation of AQP3 and AQP5 induced by dexamethasone and ambroxol
in A549 cells. Respir Physiol Neurobiol. 161:111–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong C, Wang G, Li B, Xiao K, Ma Z, Huang
H, Wang X and Bai C: Anti-asthmatic agents alleviate pulmonary
edema by upregulating AQP1 and AQP5 expression in the lungs of mice
with OVA-induced asthma. Respir Physiol Neurobiol. 181:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|