Introduction
Dilated cardiomyopathy (DCM) is a common
cardiomyopathy leading to heart failure (HF), and is characterized
by the enlargement of one or both of the ventricles with associated
systolic dysfunction (1). In adults,
the prevalence is ~1 in 2,500 individuals, with an incidence of
7/100,000 per year; however, it maybe underdiagnosed. The
prevalence of DCM in the United States (adjusted for age) is
36/100,000 of the population (2).
DCM is an important cause of sudden cardiac death (SCD) and HF and
is the leading indication for cardiac transplantation in children
and adults worldwide (3).
Ventricular remodeling is the key process of DCM and
HF pathogenesis, and was originally referred to an alteration in
the ventricular architecture (4). It
is characterized by a structural rearrangement of the cardiac
chamber wall that involves not only cardiomyocyte hypertrophy, but
also the fibroblast proliferation, and an increased deposition of
extracellular matrix (ECM) proteins (5). It refers to the aberrant deposition of
ECM in the heart, and is characterized by an increase of collagen
in the interstitium. As shown experimentally, an increase in
collagen content increases myocardial stiffness and promotes
abnormalities of cardiac function, whereas its regression
normalizes stiffness and function (6). Myocardial fibrosis is an important
pathophysiological process in which the accumulation of collagen
contributes to DCM and HF (7). To
understand the components of myocardial fibrosis and remodeling,
current medication has been used to counteract the compensatory
mechanism of ventricular remodeling and, consequently, to reduce
morbidity and mortality (8).
Traditional Chinese medicine (TCM) has been applied
to treat HF for thousands of years, and some herbal formulas have
been proven to be effective (9).
Baoxin decoction (BXD), is a compound that has been prepared and
formulated on the basic theory of TCM, and consists of a complex
combination of natural herbs where every herb contains various
chemical compounds (10). These
compounds can work together in mutual coordination and auxiliary,
which has been used to treat DCM and leads to HF. Clinical studies
have demonstrated that BXD can improve myocardial dysfunction in
DCM patients. In the present study, we aimed to examine the
hypothesis that BXD exerts its cardioprotective effect by
preventing myocardial fibrosis in a rat model of
doxorubicin-induced DCM.
Materials and methods
Drug preparation
Traditional Chinese herb granules manufactured by
Guangdong Yifang Pharmaceutical Co., Ltd. (Guangdong, Guangzhou,
China) were purchased from the pharmacy of Traditional Chinese
Medicine Hospital. The granules were prepared from
Astragalus (30 g), Salvia miltiorrhiza (10 g),
Cassia twig (6 g), Wolfiporia extensa (10 g),
Forsythia suspense (10 g), Turtle shell (10 g),
Angelica sinensis (10 g), Codonopsis pilosula (10 g),
Ophiopogon japonicus (10 g) and Schisandra chinensis
(6 g). After preparation, the herbal mixtures were freeze-dried.
Prior to use, each 2 g of dry herbal mixture was resuspended in 1
ml of distilled water.
Experimental animals
Male Sprague-Dawley rats (n=70) weighing 220±10 g,
were purchased from the Laboratory Animal Center of Xuzhou Medical
College [Xuzhou, Jiangsu, China, license no. SCXK (Su) 2010-0003].
The rats were housed in a normal 12-h light/dark rhythm under
standard conditions of temperature (21±2°C) and humidity (55±5%)
and fed with a standard diet and tap water. Animals were allowed a
1-week acclimatization period prior to the experimental protocol.
The animal care, use and experimental protocols were approved by
the Animal Experimental Ethics Committee of Xuzhou Medical
University.
Animal models
A total of 62 animals were subjected to
intraperitoneal injections of doxorubicin hydrochloride (Zhejiang
Haizheng Pharmaceutical Industry, Zhejiang, China) 2.5 mg/kg weekly
for 6 consecutive weeks (11). Eight
rats received the same volume of physiological saline as control.
Two weeks after cessation of the doxorubicin injection, the two
groups of rats that survived were examined by transthoracic
echocardiography to determine whether the model was successful.
Grouping and treatment
With the exception of the control animals, the
surviving animals developed ventricular dysfunction with a left
ventricular ejection fraction (LVEF) of <60% (n=42). The rats
were randomly divided into five groups: the DCM group (DCM, n=9,
saline), the low-dose BXD group (BXD-L, n=9, 7.5/kg), the
middle-dose BXD group (BXD-M, n=8, 15 g/kg), the high-dose BXD
group (TXL-H, n=8, 30 g/kg) and the positive group (n=8, captopril,
8.75 g/kg). Doses were selected on the basis of human clinical
dosage and the control group and DCM group rats were administered
saline.
Echocardiography measurements
Before and after treatment, transthoracic
echocardiography was performed in all the groups. The rats were
anesthetized using chloral hydrate. Images were captured using a 12
MHz linear transducer connected to a Vivid 7 echocardiography
machine (GE Healthcare, Aurora, OH, USA). A two-dimensional
short-axis view of the left ventricle was obtained at the level of
the papillary muscle and two-dimensional targeted M-mode tracings
were recorded. The detection index was as follows: left ventricular
ejection fraction (LVEF), left ventricular end-diastolic diameter
(LVEDD), left ventricular end-systolic diameter (LVESD) and left
ventricular fractional shortening (LVFS). All the parameters were
measured over three consecutive cardiac cycles.
Measurement of serum indicators by
ELISA
Blood samples were collected from the abdominal
artery and the serum was separated. Procollagen type I
carboxy-terminal peptide (PICP) and procollagen type III
aminoterminal peptide (PIIINP) in serum were determined by
enzyme-linked immunosorbent assay (ELISA) kits (hCG ELISA kit,
Ontario, Canada) following the manufacturers instructions.
Pathology and histology
Left ventricular tissues of the same region were
fixed in 4% paraformaldehyde. Paraffin-embedded tissues were
sectioned into slices (4 µm) and stained with hematoxylin and eosin
staining (H&E). Masson's trichrome staining was performed to
assess the degree of myocardial fibrosis according to protocol
(12). In the Massons-stained
sections, myocardial cells were stained red while the collagen was
stained blue. Six randomly selected microscopic fields of each
section were analyzed for collagen deposition using Image-Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA), which was
expressed as collagen volume fraction (CVF), the percentage of the
area stained blue for collagen to the total area of each
microscopic field. The CVF of each animal represents the mean of 6
randomly selected microscopic fields.
RNA extraction and RT-qPCR
Quantitative analysis of the mRNA levels of target
genes was performed with RT-qPCR by the relative standard curve
method using the SYBR-Green PCR Master mix (Promega, Madison, WI,
USA). Total RNA was extracted from frozen ventricular tissue
samples using a TRIzol reagent (Takara Bio, Inc., Otsu, Japan).
Reverse transcription of RNA was carried out according to the
instructions of RT-PCR kit (Promega). The primer sequences used
were as follows: galectin-3 (Gal-3) forward, 5-CCCAACGCAAACA
GTATA-3 and reverse, 5-TGTCTTTCTTCCCTTCCC-3′; collagen I (Col I)
forward, 5-GCCAAGAAGACATCCCT GAA-3′ and reverse,
5-CTTCTGGGCAGAAAGGA CAG-3; collagen III (Col III) forward,
5-GGTGGCTTTCAGTTCAG CTATG-3 and reverse, 5-GTCTTGCTCCATTCACCAG
TGT-3; GAPDH forward, 5-CTGCACCACCAACTGCT TAG-3 and reverse,
5-GGATG CAGGGATGATGTTCT-3.
Western blot analysis
Total protein was extracted from frozen heart tissue
by RIPA buffer containing 1 mM PMSF, followed by determination of
the protein concentrations with a BCA protein assay kit. The
protein was separated using a 12% SDS polyacrylamide gel in
electrophoresis sample buffer and then transferred onto a PVDF
membrane. Following washing and blocking, the membrane was
incubated overnight at 4°C with mouse monoclonal Gal-3 antibody
(dilution, 1:500; cat. no. sc-25279), rabbit polyclonal TGF-β1
antibody (dilution, 1:500; cat. no. sc-7892), mouse monoclonal
Smad3 antibody (dilution, 1:500; cat. no. sc-101154) (all from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After
washing, the membrane was incubated with bovine anti-mouse
secondary antibody (dilution, 1:2,000; cat. no. sc-2371; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. After washing, the membrane
was incubated with a secondary antibody at 37°C for 1 h. A
chemiluminescence detection system was used to detect the western
blot analysis. Target proteins levels were normalized by GAPDH.
Statistical analysis
Data are presented as mean ± SD. Statistical
analysis was performed using a one-way analysis of variance
(ANOVA). P<0.05 was considered to be statistically
significant.
Results
BXD improves cardiac function in rats
with DCM
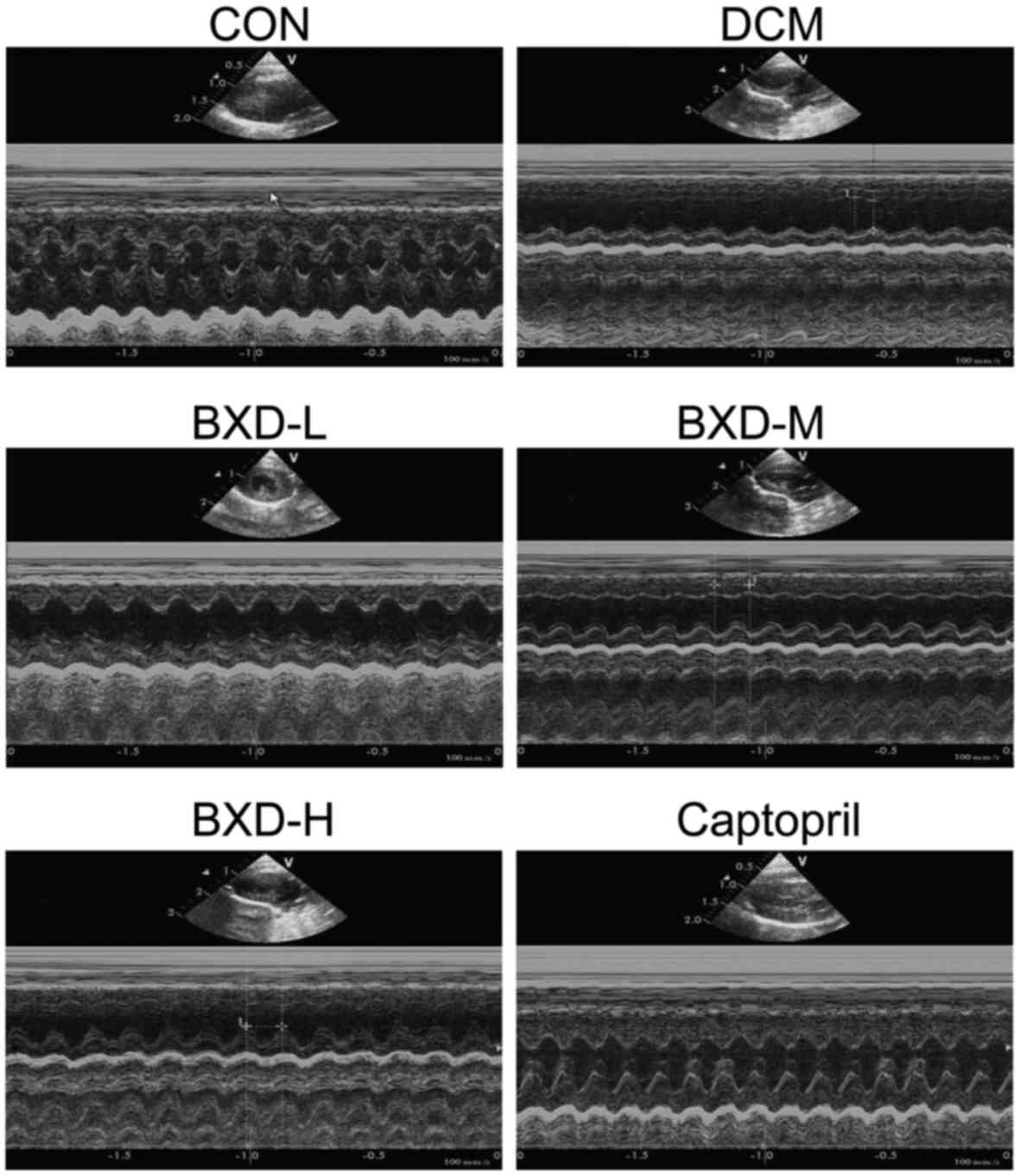
In order to determine whether BXD improved cardiac
function, we performed echocardiography 4 weeks post-treatment.
Echocardiographic parameters of the respective groups of rats are
shown in Table I. It shows a
representative echocardiography view of hearts from each group
after treatment. As shown in Table
I, echocardiography analyses after treatment indicated that
compared to those in the CON group, the rats in the DCM, BXD and
captopril groups exhibited significant left ventricular dilation
and systolic dysfunction. The LVEDD and LVESD were significantly
increased while the LVEF and LVFS were significantly decreased
(P<0.01, Fig. 1). The LVESD,
LVEDD, LVEF and LVFS in the BXD and captopril-treated group were
significantly improved after treatment when compared to the DCM
group (P<0.01, Fig. 1),
demonstrating that BXD treatment markedly ameliorated cardiac
dysfunction.
 | Table I.LVEDD, LVESD, LVEF and LVFS in the
different subgroups of patients. |
Table I.
LVEDD, LVESD, LVEF and LVFS in the
different subgroups of patients.
| Group | LVEDD | LVESD | LVEF | LVFS |
|---|
| CON |
6.14±0.19a |
3.69±0.14a |
83.09±5.33a |
45.86±3.80a |
| DCM |
7.80±0.35b |
4.92±0.23b |
53.42±3.83b |
26.09±2.77b |
| BXD-L |
7.43±0.24b,c |
4.58±0.19b,c |
61.09±4.80a,b |
30.18±1.73b,c |
| BXD-M |
7.27±0.24a,b |
4.52±0.26a,b |
64.45±3.75a,b |
31.61±2.09a,b |
| BXD-H |
7.14±0.27a,b |
4.41±0.22a,b |
67.07±3.56a,b |
34.86±1.32a,b |
| CAP |
7.04±0.32a,b |
4.36±0.33a,b |
67.95±2.96a,b |
35.21±1.49a,b |
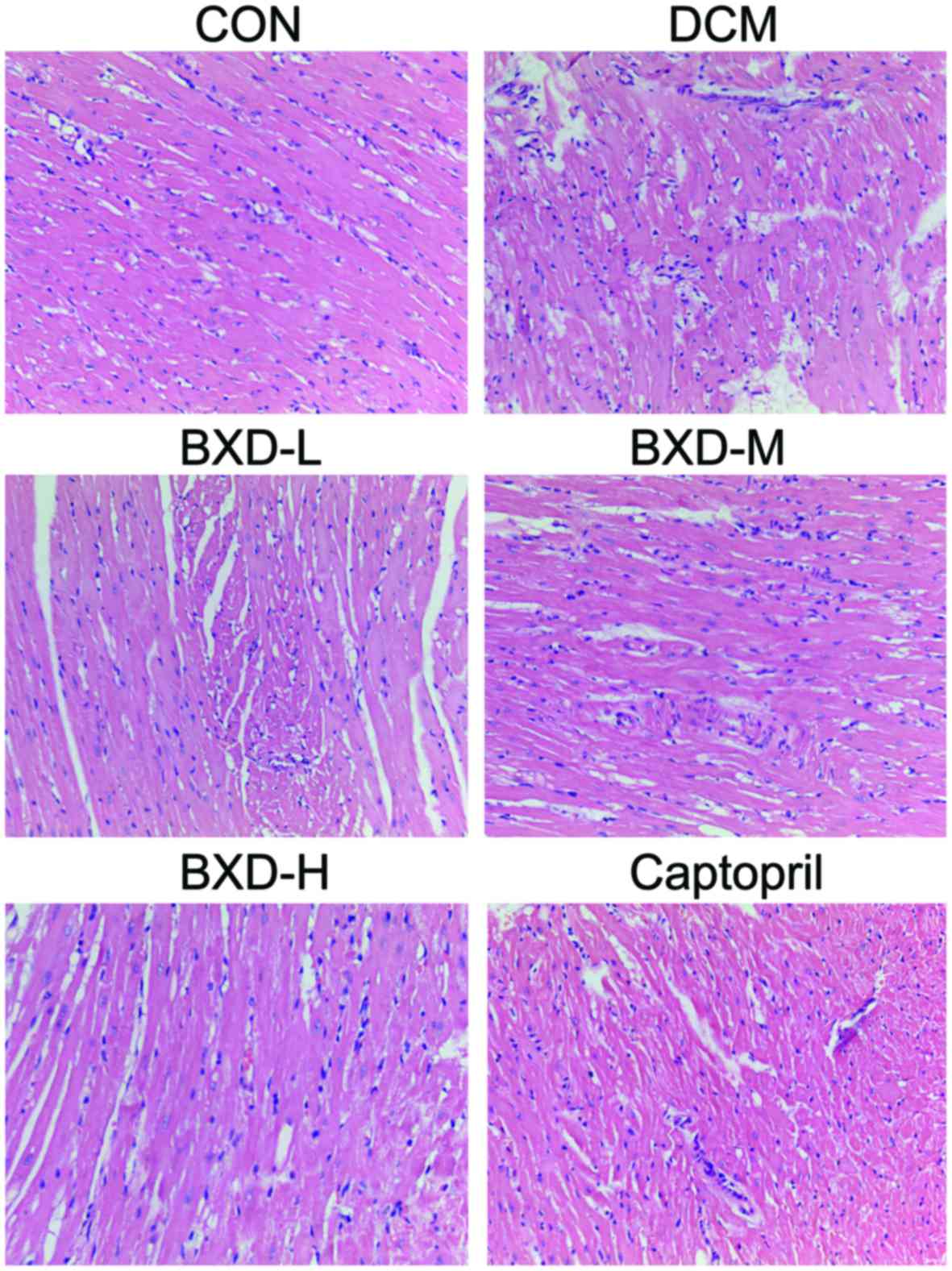
BXD attenuates DCM rat myocardial
pathological change
Fig. 2 shows the
H&E-stained sections of left ventricular myocardial tissue with
the control group showing normal histomorphology. Tissues from the
DCM group showed enhancement and loose arrangement of the
myocardial fibers, hypertrophy, myocardial necrosis, loss of
myocytes and vacuolar degeneration. Administration of BXD and
captopril improved cardiac hypertrophy and alleviated areas of
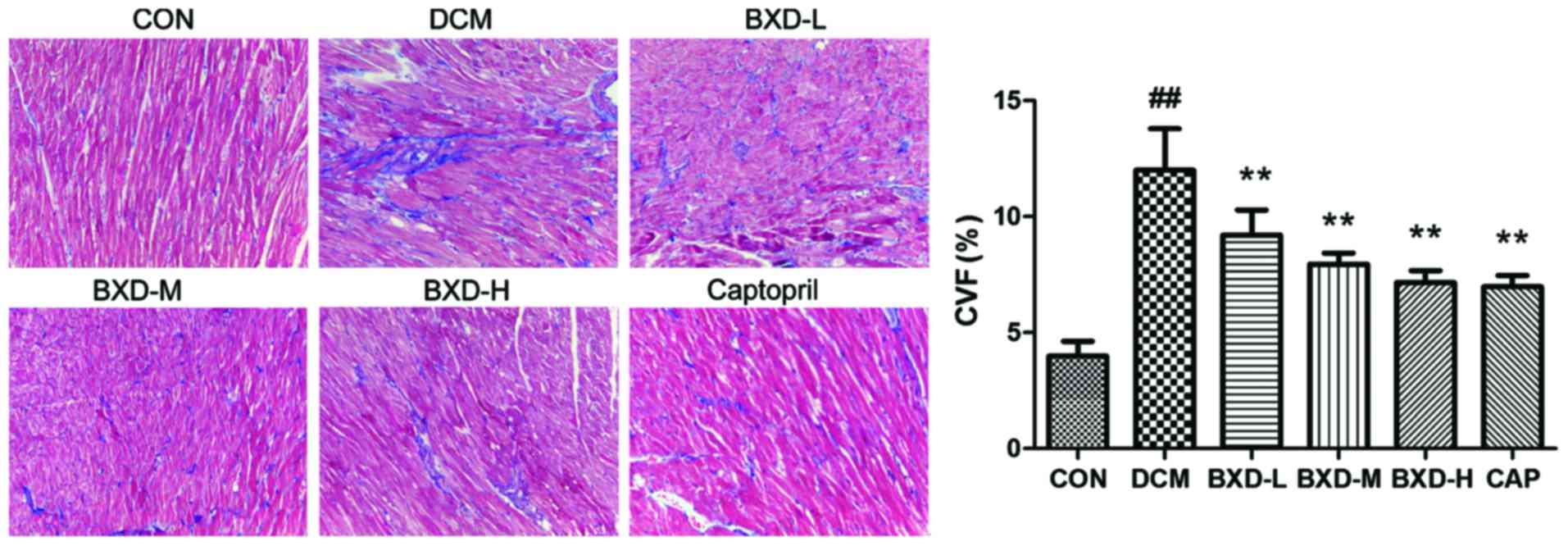
necrosis. As Fig. 3 shows, Massons
trichrome staining for interstitial fibrosis on the left
ventricular myocardial tissue. The areas of fibrosis (blue) show
CVF. CVF of the DCM group were increased (P<0.001) when compared
to the control group. The treatment with either BXD or captopril
was associated with a reduction in CVF (BXD and captopril,
P<0.01).
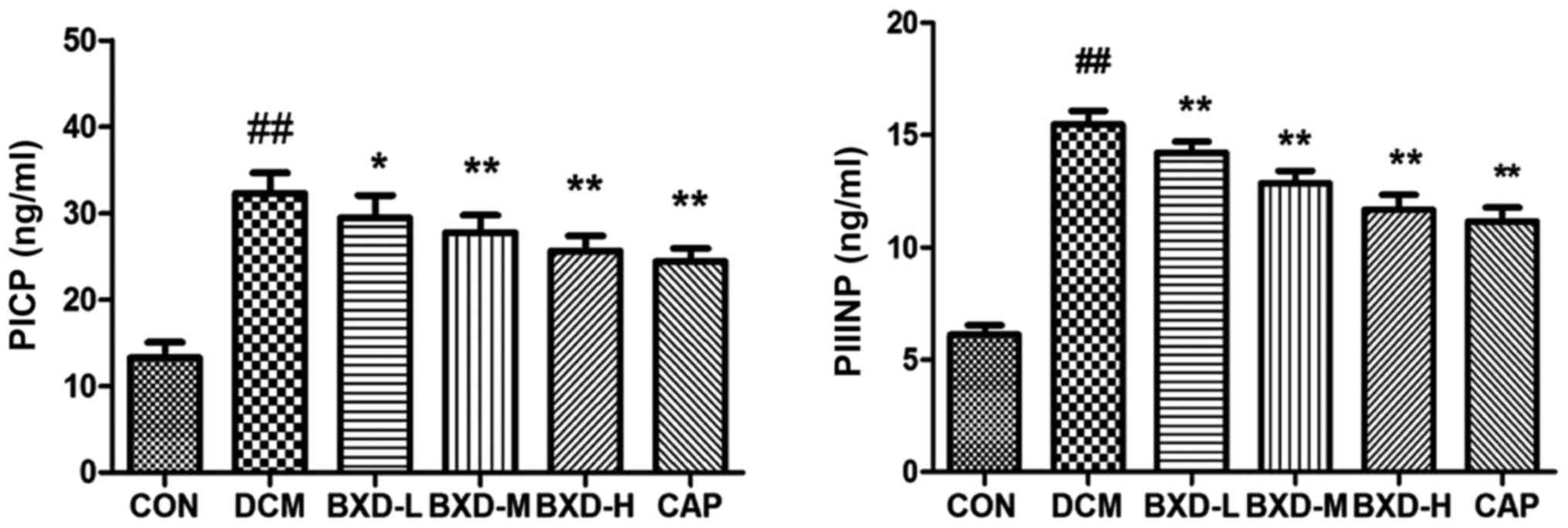
BXD inhibits myocardial collagen
The carboxy-terminal propeptides of collagen I
(PICP) and amino-terminal propeptides of collagen III (PIIINP) are
serum biochemical markers of cardiac ECM. As Fig. 4 shows, when compared to the control
group, the levels of serum PICP and PIIINP were significantly
increased in the DCM group (P<0.01). BXD and captopril-treated
group decreased the concentration of serum PICP and PIIIN
(P<0.05–0.01 vs. the DCM group). These data indicated that BXD
played a protective role in DCM by decreasing the synthesis and
degradation of myocardial collagen.
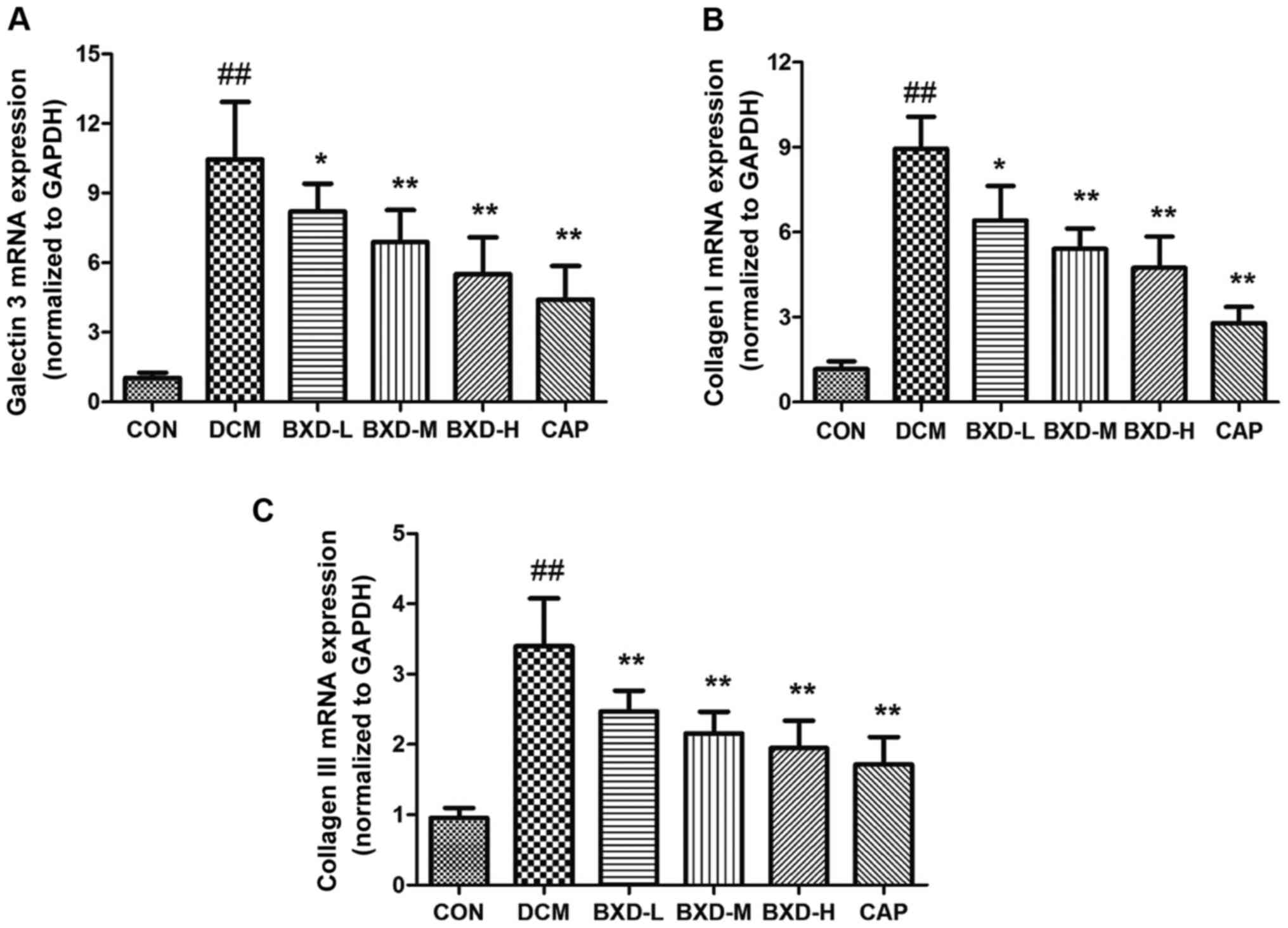
Effects of BXD on heart tissue Gal-3,
Col I and Col III mRNA expression
Gal-3, Col I and Col III mRNA levels in the left
ventricles myocardial were significantly increased in the DCM group
compared to the control group (P<0.01, Fig. 5). As expected, treatment with either
BXD or captopril was associated with a significantly lower
expression of Gal-3, Col I and Col III (P<0.01).
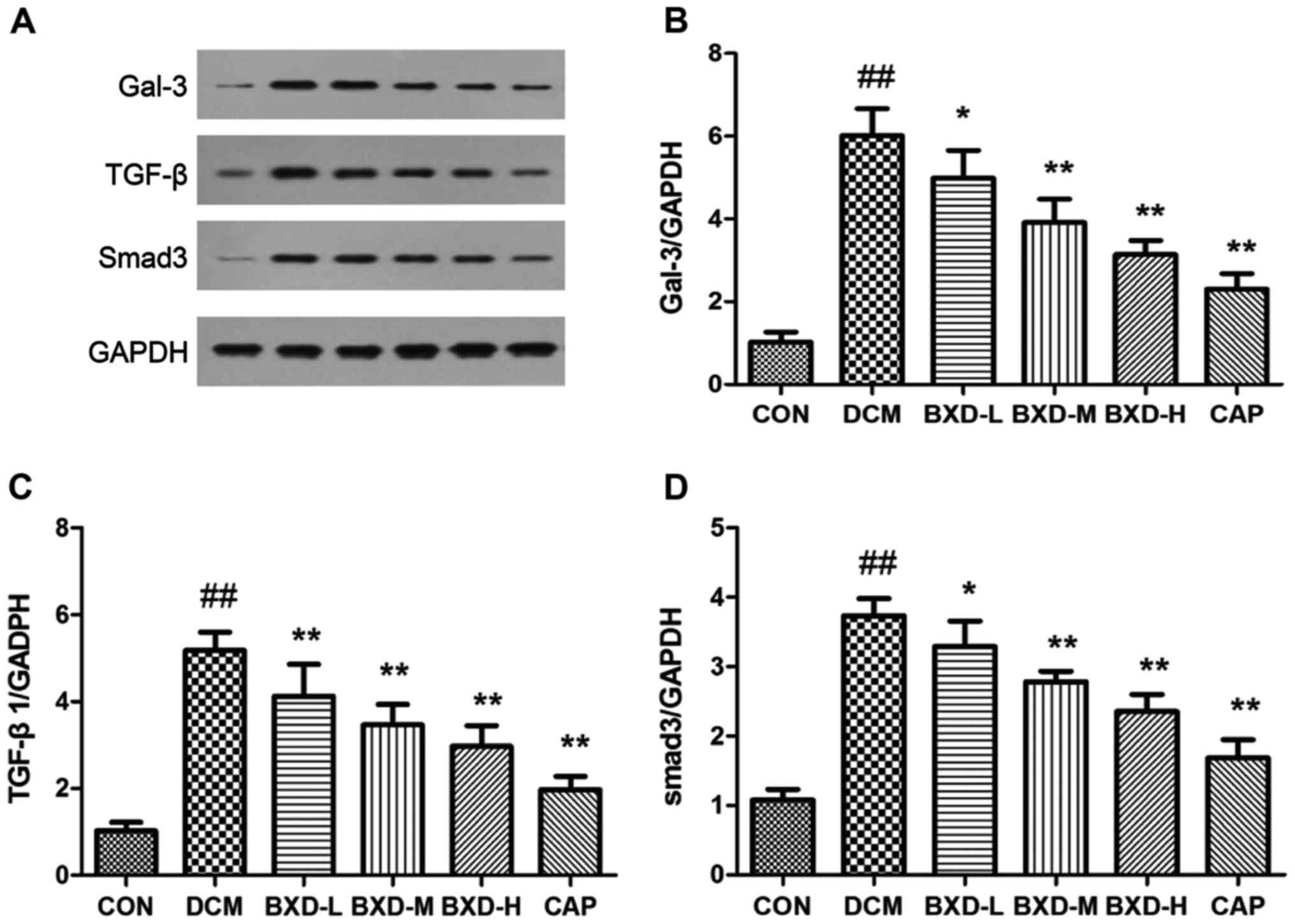
Effects of BXD on heart tissue Gal-3,
TGF-β1 and Smad3 protein expression
Gal-3 protein expression levels and molecules
involved in the Gal-3 signaling pathway (TGF-β and Smad3) were
analyzed by western blot analysis for each experimental group. It
is well known that TGF-β1/Smad3 signaling is a key pathway in the
myocardial fibrosis process. Results indicated that BXD
significantly reduced Gal-3, TGF-β1 and Smad-3 protein expression
(P<0.05, P<0.01, Fig. 6).
Notably, the inhibitory effect of BXD on myocardial fibrosis
signaling was associated with the downregulation of TGF-β1/Smad3
signaling.
Discussion
In this study, we investigated the effects of BXD on
myocardial dysfunction, fibrosis and interrelated signaling
pathways using a rat model of doxorubicin-induced DCM. The main
findings of this study include that BXD reduced left ventricular
dilation and improved left ventricular systolic function in rats of
doxorubicin-induced DCM, BXD effectively attenuated interstitial
fibrosis by inhibiting collagen production and the underlying
mechanism may be associated with the suppression of Gal-3 and
TGF-β1/Smad3 signaling.
Doxorubicin is used as a common chemotherapeutic
agent, which can also lead to cardiotoxicity. Many experimental
animal models of Dox-induced cardiomyopathy have been used to
investigate DCM (13). In this
study, we followed the animal model of DCM. Echocardiography showed
significant dilation of the left ventricle and a significant
reduction of cardiac function. Pathological examination showed a
large accumulation of collagen in the myocardial interstitial
tissues. Our results demonstrated that this model is successful,
which is in agreement with previous findings (14).
A number of previous reports have described herbal
medicines as being effective in preventing or decreasing myocardial
fibrosis in cardiovascular disease. Evidence gathered from a
systematic review shows that herbal medicine, which seems to be
relatively safe and convenient, may offer a much needed alternative
and merit further attention (15).
BXD is a specific TCM that has been developed based on the meridian
theory. Therefore, we evaluated the effects of BXD in an animal
experimental model on myocardial dysfunction, fibrosis and
interrelated signaling pathways.
Gal-3 is a member of the galectin family, and
consists of animal lectins that bind β-galactosides (16). In recent years, Gal-3 has emerged as
a link to the pathophysiology of adverse myocardial remodeling
(17). This has been associated with
activation of fibroblasts and macrophages, which leads to the
development of interstitial and perivascular fibrosis and left
ventricular dysfunction (18). Some
studies have indicated that inhibition of Gal-3 was associated with
a downregulation in collagen production. Furthermore, the
inhibition of Gal-3 has also been shown to attenuate the
progression of cardiac remodeling in a long-term transverse aortic
constriction mouse model (19).
Ac-SDKP has been known to prevent interstitial and perivascular
fibrosis and LV dysfunction was caused by Gal-3. These changes were
shown to be mediated by a transforming growth factor TGF-β/Smad3
pathway (20). The mineralocorticoid
receptor antagonists eplerenone and spironolactone, modulated Gal-3
and TGF-β/Smad3 signaling in an experimental model of left
ventricular systolic dysfunction (21). These findings suggest that
therapeutic agents targeting Gal-3 might result in innovative new
therapies. In our study, BXD treatment was associated with an
inhibition of the observed upregulation of Gal-3.
Myocardial fibrosis is one of the main pathological
changes in DCM. The TGF-β-Smad3 pathway contributes to the long
progress of myocardial fibrosis. TGF-β1 plays a crucial role in
cardiac fibrosis by activating fibroblasts and producing collagen
(22). TGF-β1 inhibition of cardiac
fibroblast proliferation requires Smad3 (23). Therefore, inhibiting Smad3
transduction may prevent cardiac fibrosis. As shown in some
studies, inhibiting the TGF-β1-Smad3 signaling pathway or
modulating the gene expression of Smad3 could effectively interfere
with myocardial fibrosis (24–26).
Many Chinese herbal medicines can inhibit cardiac fibrosis, such as
Gualou Xiebai decoction, by blocking TGF-β1/Smad3 signaling
(27). Shensong Yangxin capsule
prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad3
signaling (28). Captopril, an ACE
inhibitor, prevents the development and progression of subsequent
fibrosis related to the reduction of TGF-β1 levels. The findings of
our study show that BXD may possibly be able to bring about similar
effects in myocardial fibrosis associated with Gal-3 and
TGF-β-Smad3 pathway.
However, the present findings of the study are
limited by the experiment. The relationship between cardiac
fibrosis and Gal-3 and TGF-β/smad3 has not demonstrated causality.
In addition, the mechanism of BXD needs to be further explored.
References
|
1
|
Gopal DM and Sam F: New and emerging
biomarkers in left ventricular systolic dysfunction - Insight into
dilated cardiomyopathy. J Cardiovasc Transl Res. 6:516–527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Towbin JA, Lowe AM, Colan SD, Sleeper LA,
Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, et al:
Incidence, causes, and outcomes of dilated cardiomyopathy in
children. JAMA. 296:1867–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luk A, Ahn E, Soor GS and Butany J:
Dilated cardiomyopathy: A review. J Clin Pathol. 62:219–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carasso S and Amir O: Reverse remodeling
in dilated cardiomyopathy: Dream come true? Isr Med Assoc J.
16:444–445. 2014.PubMed/NCBI
|
|
5
|
Nicolini G, Pitto L, Kusmic C, Balzan S,
Sabatino L, Iervasi G and Forini F: New insights into mechanisms of
cardioprotection mediated by thyroid hormones. J Thyroid Res.
2013:2643872013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jalil JE, Doering CW, Janicki JS, Pick R,
Shroff SG and Weber KT: Fibrillar collagen and myocardial stiffness
in the intact hypertrophied rat left ventricle. Circ Res.
64:1041–1050. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graf K and Schaefer-Graf UM: Is Smad3 the
key to inflammation and fibrosis in hypertensive heart disease?
Hypertension. 55:1088–1089. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreira AS and Lopes AJ: Chinese medicine
pattern differentiation and its implications for clinical practice.
Chin J Integr Med. 17:818–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang P, Deng HY, Li K, Huang GY, Chen Y,
Tu L, Ng PC, Pong NH, Zhao H, Zhang L, et al: Dexrazoxane protects
against doxorubicin-induced cardiomyopathy: Upregulation of Akt and
Erk phosphorylation in a rat model. Cancer Chemother Pharmacol.
63:343–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen FF, Jiang TH, Jiang JQ, Lou Y and Hou
XM: Traditional chinese medicine tongxinluo improves cardiac
function of rats with dilated cardiomyopathy. Evid Based Complement
Alternat Med. 2014:3238702014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin YW, Ye P, He JQ, Sheng L, Wang LY and
Du J: Simvastatin inhibited cardiac hypertrophy and fibrosis in
apolipoprotein E-deficient mice fed a ‘Western-style diet’ by
increasing PPAR α and γ expression and reducing TC, MMP-9, and Cat
S levels. Acta Pharmacol Sin. 31:1350–1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Y, Xu H and Chen K: Natural Polypill
Xuezhikang: Its clinical benefit and potential
multicomponent synergistic mechanisms of action in cardiovascular
disease and other chronic conditions. J Altern Complement Med.
18:318–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayward R and Hydock DS: Doxorubicin
cardiotoxicity in the rat: an in vivo characterization. J Am Assoc
Lab Anim Sci. 46:20–32. 2007.PubMed/NCBI
|
|
15
|
Schwarz ER, Pollick C, Dow J, Patterson M,
Birnbaum Y and Kloner RA: A small animal model of non-ischemic
cardiomyopathy and its evaluation by transthoracic
echocardiography. Cardiovasc Res. 39:216–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barondes SH, Cooper DN, Gitt MA and
Leffler H: Galectins. Structure and function of a large family of
animal lectins. J Biol Chem. 269:20807–20810. 1994.PubMed/NCBI
|
|
17
|
Shah RV and Januzzi JL Jr: Soluble ST2 and
galectin-3 in heart failure. Clin Lab Med. 34:87–97. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu AH, Wians F and Jaffe A: Biological
variation of galectin-3 and soluble ST2 for chronic heart failure:
Implication on interpretation of test results. Am Heart J.
165:995–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Ruifrok WP, Meissner M, Bos EM, van
Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts
JA, et al: Genetic and pharmacological inhibition of galectin-3
prevents cardiac remodeling by interfering with myocardial
fibrogenesis. Circ Heart Fail. 6:107–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma UC, Pokharel S, van Brakel TJ, van
Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius HJ,
Maessen J, et al: Galectin-3 marks activated macrophages in
failure-prone hypertrophied hearts and contributes to cardiac
dysfunction. Circulation. 110:3121–3128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lax A, Sanchez-Mas J, Asensio-Lopez MC,
Fernandez-Del Palacio MJ, Caballero L, Garrido IP, Pastor-Perez FJ,
Januzzi JL and Pascual-Figal DA: Mineralocorticoid receptor
antagonists modulate galectin-3 and interleukin-33/ST2 signaling in
left ventricular systolic dysfunction after acute myocardial
infarction. JACC Heart Fail. 3:50–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du J, Xie J, Zhang Z, Tsujikawa H, Fusco
D, Silverman D, Liang B and Yue L: TRPM7-mediated Ca2+
signals confer fibrogenesis in human atrial fibrillation. Circ Res.
106:992–1003. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobaczewski M, Bujak M, Li N,
Gonzalez-Quesada C, Mendoza LH, Wang XF and Frangogiannis NG: Smad3
signaling critically regulates fibroblast phenotype and function in
healing myocardial infarction. Circ Res. 107:418–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia N, Dong P, Ye Y, Qian C and Dai Q:
Allopurinol attenuates oxidative stress and cardiac fibrosis in
angiotensin II-induced cardiac diastolic dysfunction. Cardiovasc
Ther. 30:117–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M,
Shen Q, Zhu Y and Zhang Y: Metformin attenuates cardiac fibrosis by
inhibiting the TGFβ1-Smad3 signalling pathway. Cardiovasc Res.
87:504–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong
X, Li W and Xuan J: Suppression of TGF-β1/Smad signaling pathway by
sesamin contributes to the attenuation of myocardial fibrosis in
spontaneously hypertensive rats. PLoS One. 10:e01213122015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding YF, Peng YR, Li J, Shen H, Shen MQ
and Fang TH: Gualou Xiebai Decoction prevents myocardial fibrosis
by blocking TGF-β/Smad signalling. J Pharm Pharmacol. 65:1373–1381.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen N, Li X, Zhou T, Bilal MU, Du N, Hu
Y, Qin W, Xie Y, Wang H, Wu J, et al: Shensong Yangxin Capsule
prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad
signaling. J Ethnopharmacol. 157:161–170. 2014. View Article : Google Scholar : PubMed/NCBI
|