Introduction
Atrial arrhythmias are the most common complications
following cardiac surgery and contribute to hemodynamic
instability, cognitive impairment, thromboembolic events, and
congestive heart failure and prevention of atrial fibrillation
following cardiac surgery reduces morbidity and mortality (1,2). During
surgery and anesthesia, many patients undergo hemodynamic
depression and this is more often seen in patients with
hypertension or myocardial insufficiency (3). Many of the available clinical
approaches for addressing this are not completely safe and
dexmedetomidine has been shown to have many positive effects on
cardiovascular stability in patients undergoing cardiac surgery
(4,5). Dexmedetomidine, a highly selective and
potent α2-adrenoreceptor agonist, with analgesic as well as
anaesthetic effects also has other beneficial effects including
reduction in the release of catecholamine (6), incidence of postoperative delirium
(7,8), and also need for anesthetics (9,10).
Because of these multiple beneficial effects dexmedetomidine is
widely used in various surgeries and intensive care units. Many
studies have shown that dexmedetomidine lowers the myocardial
complications following cardiovascular surgery in adults (11,12). It
has been reported that postoperative myocardial injury and
arrhythmic events were decreased in patients administered
dexmedetomidine (13).
Besides its use in adult patients, dexmedetomidine
has also been found to be effective in children undergoing surgery
for congestive heart failure in reducing post-operative cardiac
complications (14). It has been
observed that dexmedetomidine usage in children led to more stable
intraoperative hemodynamics, reduced mechanical ventilation times
and analgesia requirements along with lower incidence of agitation
and delirium, similar to the effects in adults. However,
perioperative bradycardia and hypotension are common in patients
receiving dexmedetomidine and care must be taken to control the
extent of bradycardia and hypotension. Considering that hypotension
and bradycardia are often seen in patients undergoing cardiac
surgery, the possibility that dexmedetomidine may further aggravate
their incidence exists (3). Even
though many studies with a smaller number of patients and also some
meta-analysis studies indicated the beneficial effects of
dexmedetomidine, the power of the analysis and conclusion of the
studies is rather weak due to relatively smaller number of patients
and included studies.
Materials and methods
Objectives
In the present meta-analysis, we included studies
from 2003 to 2016 and a large number of patients, both children and
adults, undergoing cardiac surgery, to address the efficacy of
dexmedetomidine to control adverse effects of surgery and the
results are strongly suggest that dexmedetomidine has many
beneficial effects both in children as well as adults, following
cardiac surgery.
Methods
Criteria for considering studies for this
review
In the present meta-analysis, both randomized and
non-randomized studies that examined and compared the efficacy of
dexmedetomidine in patients of all ages, undergoing different types
of cardiac surgery are included. All the studies included a control
group of patients, receiving either placebo or other
analgesic/anaesthetic, for comparison with dexmedetomidine group.
Studies that addressed the effectiveness of dexmedetomidine during
non-cardiac surgeries were not included. In order to increase the
strength of the analysis, we included studies on children as well
as adults as the type of surgeries and the outcomes measured were
the same and thus relate to the effectiveness of
dexmedetomidine.
Search methods
Publications describing the relevant information
were searched in PubMed, Google Scholar, Scopus and Web of Science
databases. Search MeSH terms included dexmedetomidine, cardiac
surgery, atrial fibrillation, arrhythmias, myocardial infarction,
cardiac protection, tachycardia, bradycardia, hypotension and
systolic blood pressure. Papers published in English language in
the last 20 years were searched. All the authors of these
meta-analysis citations initially screened the articles for
relevance at the title and abstract level and full reports and
supplemental information files were retrieved as per the relevance
of the selected study.
Data collection and analysis and quality
assessment
The data extracted from the included studies are as
follows: Institutional details where the study was conducted and
the authors, publication details, the total number of patients
studied and the number of patients in dexmedetomidine treated and
control groups, age, and gender of the studied patients. Also, the
type of cardiac surgery and the dosage of dexmedetomidine employed
were collected. Besides, results on heart rate and systolic blood
pressure before and after dexmedetomidine treatment, and
tachycardia, bradycardia and atrial fibrillation events in control
and dexmedetomidine treated groups were collected. Co-authors of
this meta-analysis study independently screened all the data items
and the full texts of all selected studies. The collected
information was then combined and reviewed collectively and final
data collected were decided by discussion and consensus.
Statistical analysis
The statistical analyses were performed by Review
Manager (RevMan) version 5.3 (The Cochrane Collaboration, London,
UK). The comparative effect of dexmedetomidine administration
versus control (placebo or other anaesthetic/analgesic) was
analyzed by Mantel-Haenszel statistics in the random-effect model.
For heart rate and systolic blood pressure effects, mean difference
was calculated and for tachycardia, bradycardia and atrial
fibrillation analyses, odds ratios (OR) were derived at 95%
confidence intervals (CI). P<0.05 was considered to indicate a
statistically significant difference.
Results
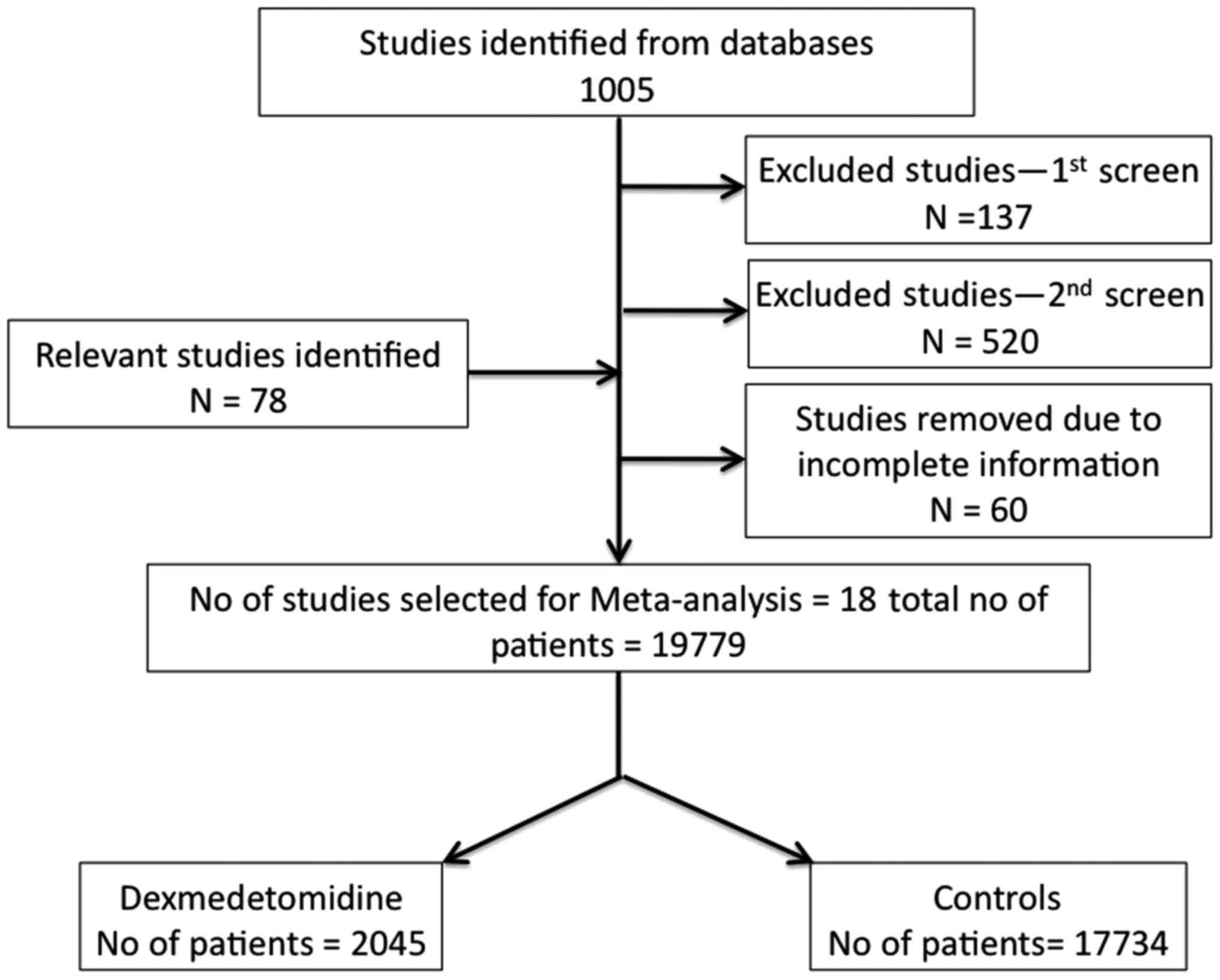
A total of 1,005 studies were identified in database
search, that contained the search terms, in title, abstract or main
text. All the authors of this meta-analysis participated in
deciding on the studies to be included the present meta-analysis.
In accordance with the selection criteria, a total of 18 studies
(13,15–31)
published between 2003 and 2015 were selected. A total of 19,225
patients were included in these studies and formed part of the
present meta-analysis. Flow chart of study selection (Fig. 1) gives the details of selected
studies based on inclusion and exclusion criteria. The total number
of patients treated with dexmedetomidine was 2,045 and the number
of control patients was 17,734.
The demographic characteristics of patients included
in this study are given in Table I.
In most of the included studies, there was a higher proportion of
males and the age of patients ranged from less than a year to 71
years, as the age was not an exclusion criteria. All the patients
were suffering from cardiac abnormalities and underwent surgery for
ventricle or septal repair, or aortic defects, cardiopulmonary
bypass, coronary artery bypass grafting or off-pump coronary artery
bypass grafting surgery. Dosage of dexmedetomidine was in the range
of 0.5–1 µg/kg body weight loading followed by continuous infusion
at a rate of 0.2–0.7 µg/kg/h. The body weights of patients ranged
from 3 to 89 kg (Table I).
 | Table I.Demographic characteristics of
patients in the included studies. |
Table I.
Demographic characteristics of
patients in the included studies.
| Study Author
(Refs.) | Year | Patients on Dex (%
males) | Age | BW (kg) | Dex
dose/administration | Cardiac
diagnosis |
|---|
| Herr et al
(15) | 2003 | 148 |
|
| 1.0 µg/kg loading;
0.2–0.2 µg/kg/h infusion | Cardiac
surgeries |
| Corbett et al
(16) | 2005 | 43 | 63 years | 89 | 1 µg/kg loading; 0.4
µg/kg continuous infusion | Coronary artery
bypass grafting |
| Chrysostomou et
al (17) | 2008 | 14 (79) | 2 months | 4 | 1.1±0.5 µg/kg
loading; 0.9 µg/kg continuous infusion | Arrhythmias |
| Shehabi et al
(20) | 2009 | 152 | 60 years |
| 0.1–0.1 µg/kg/h | Cardiac
surgeries |
| Chrysostomou et
al (18) | 2010 | 51 (61) | 0.5 year | 3.4 | 1 µg/kg loading; 0.9
µg/kg continuous infusion | Ventricle and septal
defects repair |
| Anger et al
(21) | 2010 | 28 |
|
| 0.6±0.1 µg/kg/h | Cardiac
surgeries |
| Hosokawa et al
(22) | 2010 | 56 | 1 year |
| 0.4–0.4 µg/kg/h | Cardiac
surgeries |
| Chrysostomou et
al (19) | 2011 | 32 (66) | 4.8 months | 5.3 | 1 µg/kg loading;
0.5 µg/kg continuous infusion | Ventricle, aortic,
septal defects repair |
| Ji et al
(23) | 2013 | 568 (72) | 63 years |
| 0.24–0.24
µg/kg/h | Cardiopulmonary
bypass |
| Ren et al
(13) | 2013 | 81 (31) | 60 years |
| 0.2–0.2
µg/kg/h | Coronary artery
bypass grafting |
| Tosun et al
(24) | 2013 | 18 (72) | 60.4 years | 77.5 | 0.5 µg/kg loading;
0.5 µg/kg/min continuous infusion | Coronary artery
bypass grafting |
| Gu et al
(25) | 2014 | 14 (86) | 5.2 years | 22.5 | 1 µg/kg loading;
0.01 µg/kg/min continuous infusion | Laparoscopic
surgery |
| Rajput et al
(26) | 2014 | 110 (86) | 2.8 years | 10 | 0.5 µg/kg loading;
0.5 µg/kg/min | Tetralogy of Fallot
continuous infusion |
| Turan et al
(27) | 2014 | 765 (70) | 58 years |
|
| Cardiac
surgeries |
| Narisawa et
al (28) | 2015 | 16 (75) | 71.3 years |
| 0.3±0.2 µg/kg/h
during night time | Cardiac
surgeries |
| Jiang et al
(29) | 2015 | 77 | 17.7 years |
| 0.25–0.25
µg/kg/h | Congenital heart
disease |
| Cheng et al
(30) | 2015 | 29 | 6.6 months | 5.5 | 0.5–0.5
µg/kg/h | Congenital heart
disease |
| Chi et al
(31) | 2016 | 34 (65) | 56 years | 69 | 1 µg/kg loading;
0.6 µg/kg continuous infusion | Off-pump coronary
artery bypass grafting surgery |
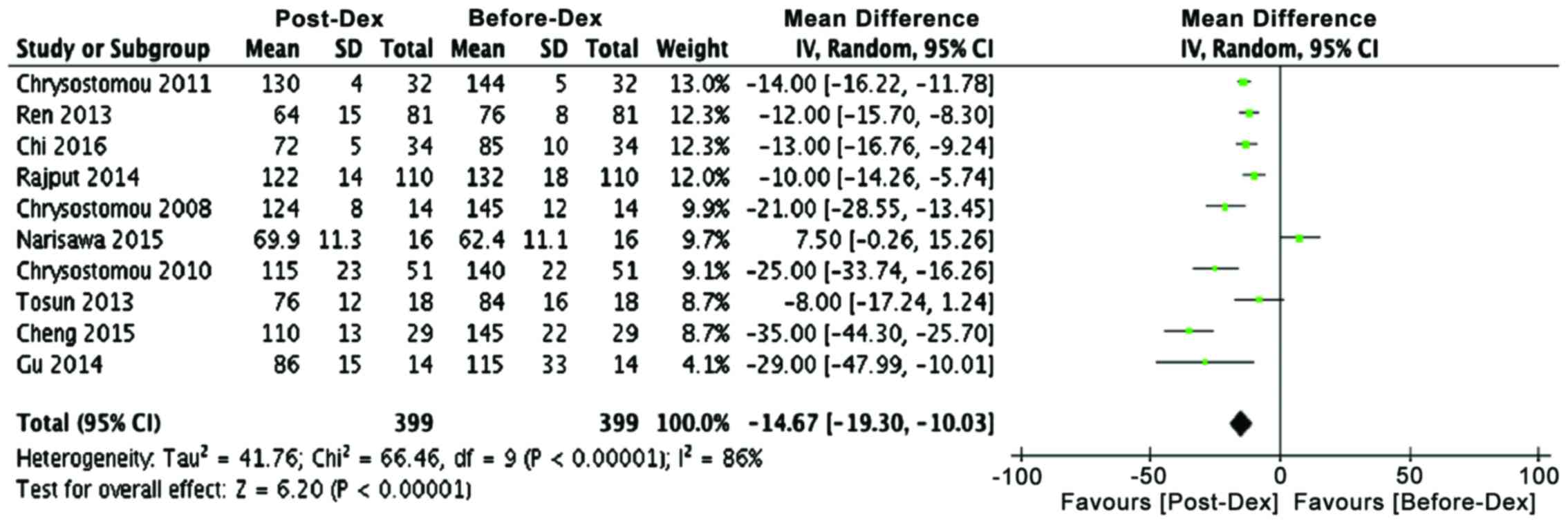
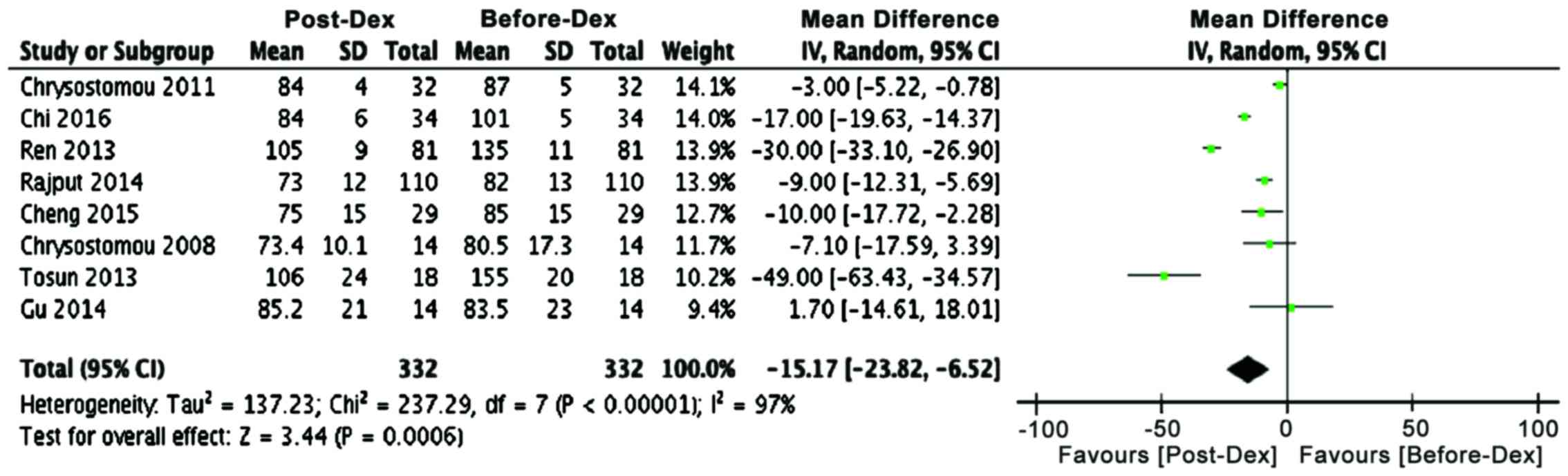
Comparative analysis of cardiac parameters before
administration of dexmedetomidine, preoperatively and following
treatment with dexmedetomidine post-operatively revealed that heart
rate in most studies decreased significantly in dexmedetomidine
receiving patients, both young and old (Table II and Fig. 2). Mean difference analysis showed
that dexmedetomidine treatment favors lower heart rate
(P<0.00001). Similarly, another cardiac parameter, systolic
blood pressure, showed a decline in many studies and mean
difference analysis that dexmedetomidine has a strong tendency for
lowering systolic blood pressure in both adult and pediatric
patients (Table II; Fig. 3, P<0.0006). These analyses
confirmed the earlier meta-analysis results in a small group of
studies with much lower number of pediatric patients (14).
 | Table II.Effect of dexmedetomidine treatment
on heart rate and systolic blood pressure of patients undergoing
cardiac surgery. |
Table II.
Effect of dexmedetomidine treatment
on heart rate and systolic blood pressure of patients undergoing
cardiac surgery.
|
|
| Heart rate | Systolic blood
pressure |
|---|
|
|
|
|
|
|---|
| Study Author
(Refs.) | Year | Before Dex | Post-Dex | Before Dex | Post-Dex |
|---|
| Chrysostomou et
al (17) | 2008 | 145±12 | 124±8 | 80.5±17.3 | 73.4±10.1 |
| Chrysostomou et
al (18) | 2010 | 140±22 | 115±23 | – | – |
| Chrysostomou et
al (19) | 2011 | 144±5 | 130±4 | 87±5 | 84±4 |
| Ren et al
(13) | 2013 | 76±8 | 64±15 | 135±11 | 105±9 |
| Tosun et al
(24) | 2013 | 84±16 | 76±12 | 155±20 | 106±24 |
| Gu et al
(25) | 2014 | 115±33 | 86±15 | 83.5±23 | 85.2±21 |
| Rajput et al
(26) | 2014 | 132±18 | 122±14 | 82±13 | 73±12 |
| Narisawa et
al (28) | 2015 | 62.4±11.1 | 69.9±11.3 | – | – |
| Cheng et al
(30) | 2015 | 145±22 | 110±13 | 85±15 | 75±15 |
| Chi et al
(31) | 2016 | 85±10 | 72±5 | 101±5 | 84±6 |
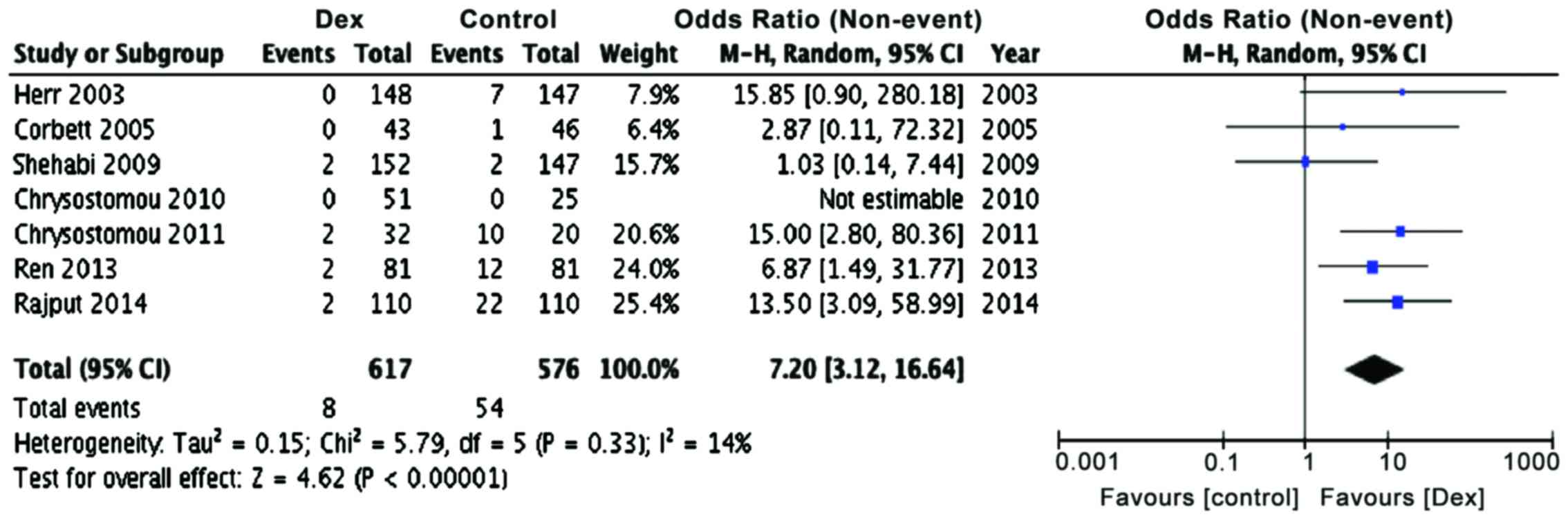
Incidence of tachycardia and arrhythmias is common
during surgery due to the procedures involved such as tracheal
intubation, which elevates sympathetic and sympathoadrenal
activity. The increased sympathoadrenal activity not only leads to
tachycardia and arrhythmias, but also to increased myocardial
oxygen consumption and ischemia (32). In the present meta-analysis, we found
that the number of tachycardia events (Table III; Fig.
4, P<0.00001) were significantly lower in patients receiving
dexmedetomidine, as compared to control patients (OR, 7.2; 95% CI
limits 3.12, 16.64). The number needed to treat (NNT) is 431,
indicating a significant benefit by dexmedetomidine treatment.
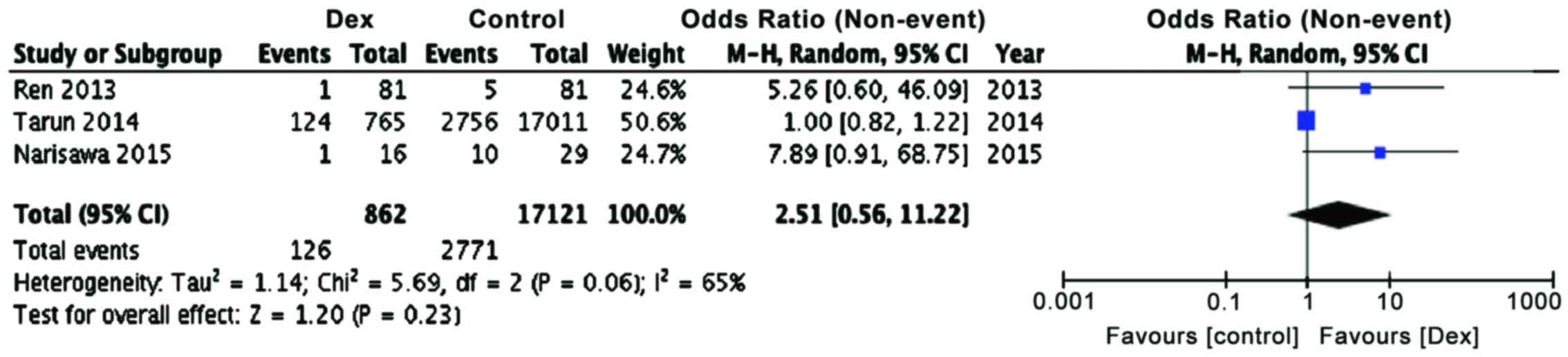
However, the number of atrial fibrillation events (Table III; Fig.
5, P=0.23) in dexmedetomidine treated group is not
significantly different from control patients, even though there
was a tendency towards a lower incidence (OR, 2.51; 95% CI limits
0.56, 11.22). This is presumably because only three studies were
included in this meta-analysis as other studies did not report the
incidence of atrial fibrillation events in their patients and
because of this, the power of this analysis for atrial fibrillation
events was less.
 | Table III.Effect of dexmedetomidine treatment
on post-operative cardiac parameters in patients undergoing cardiac
surgery. |
Table III.
Effect of dexmedetomidine treatment
on post-operative cardiac parameters in patients undergoing cardiac
surgery.
|
| Control
patients | Dex-treated
patients |
|---|
|
|
|
|
|---|
| Study Author
(Refs.) | No. of
patients | Tachycardia
events | Bradycardia
events | Atrial fibrillation
events | No. of
patients | Tachycardia
events | Bradycardia
events | Atrial fibrillation
events |
|---|
| Herr et al
(15) | 147 | 7 | 2 | – | 148 | 0 | 2 | – |
| Corbett et
al (16) | 46 | 1 | – | – | 43 | 0 | – | – |
| Shehabi et
al (20) | 147 | 2 | 9 | – | 152 | 2 | 25 | – |
| Chrysostomou et
al (18) | 25 | 0 | 0 | – | 51 | 0 | 1 | – |
| Anger et al
(21) | 28 | – | 4 | – | 28 | – | 5 | – |
| Hosokawa et
al (22) | 85 | – | 7 | – | 56 | – | 12 | – |
| Chrysostomou et
al (19) | 20 | 10 | 2 | – | 32 | 2 | 2 | – |
| Ren et al
(13) | 81 | 12 | – | 5 | 81 | 2 | – | 1 |
| Rajput et al
(26) | 110 | 22 | – |
| 110 | 2 | – |
|
| Turan et al
(27) | 17,011 | – | – | 2,756 | 765 | – | – | 124 |
| Jiang et al
(29) | 5 | – | 0 | – | 9 | – | 6 | – |
| Narisawa et
al (28) | 29 | – | – | 10 | 16 | – | – | 1 |
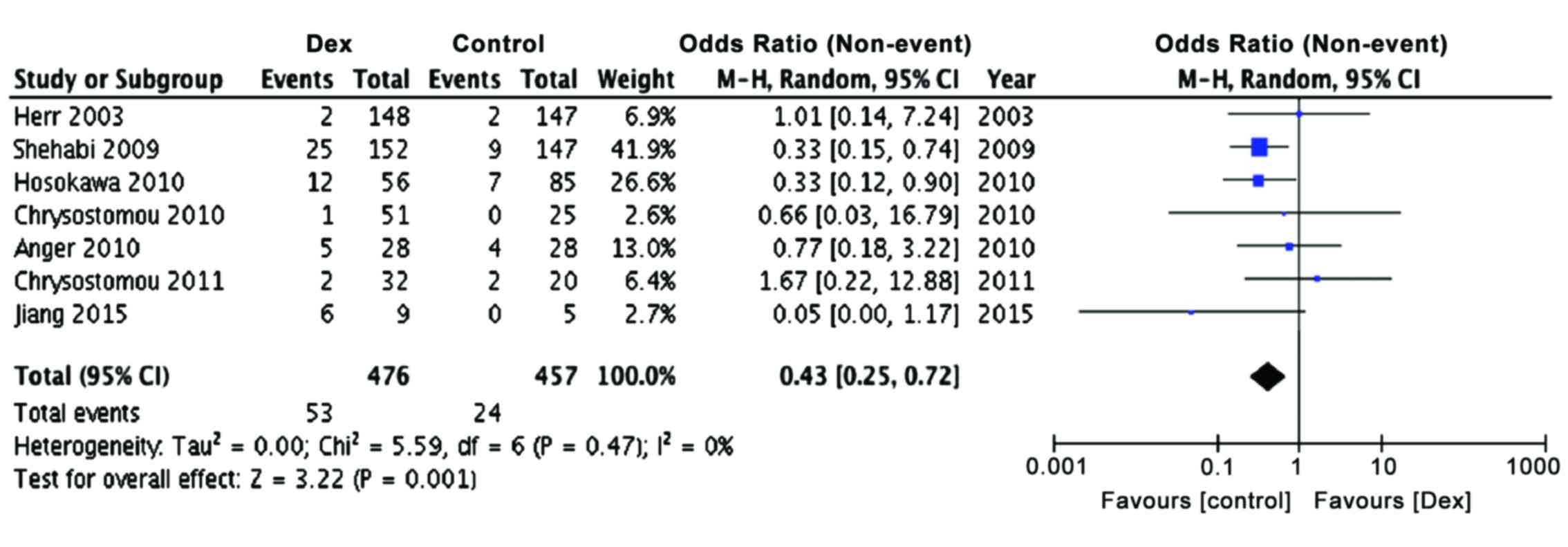
Since it has been reported earlier that
perioperative bradycardia and hypotension are common in patients
receiving dexmedetomidine (3) and
considering that hypotension and bradycardia are often seen in
patients undergoing cardiac surgery, we examined the effect of
dexmedetomidine treatment on the incidence of bradycardia, both in
children as well as adults. The present meta-analysis also showed a
significant preponderance of patients with bradycardia in
dexmedetomidine group, as compared to controls (Table III; Fig.
6, P<0.001), confirming earlier observations, but adding
more power to the conclusions reached (OR, 0.43; 95% CI limits
0.25, 0.72). The Relative Risk of developing bradycardia was found
to be 26.3 (95% CI limits, 16.3, 42.4; P<0.0001) and NNT was
29.3, indicating strong possibility for the development for
bradycardia in dexmedetomidine treated patients.
Discussion
There have been few meta-analysis studies addressing
the effects of dexmedetomidine specifically in cardiac surgery
patients, even though there are many clinical studies indicating
the beneficial effects of this drug in cardiac protection following
surgery. Also, there were few studies indicating a lack of cardiac
protection effects by dexmedetomidine in adult patients undergoing
coronary artery bypass grafting with cardiopulmonary bypass
(24). This raised questions
regarding the wider usefulness of dexmedetomidine, as a
cardioprotective drug during cardiac surgery. We addressed this
issue with respect to few important parameters, known to be
influenced by dexmedetomidine, such as heart rate, systolic blood
pressure, tachycardia, arrhthmias and bradycardia. In a
meta-analysis of patients undergoing non-cardiac surgeries, it was
noted that dexmedetomidine improved cardiac outcomes including
myocardial ischemia and non-fatal myocardial infarction while
increasing peri-operative hypotension and bradycardia (33). In another meta-analysis of studies
including randomized clinical trials of non-cardiac surgery
patients, it was observed that dexmedetomidine significantly
lowered the length of ICU stay, shortened the duration of
mechanical ventilation, even though this drug increased the
incidence of bradycardia (34). In
patients with cardiac surgery, a meta-analysis showed that
dexmedetomidine greatly reduces the length of mechanical
ventilation and also lowers risk of delirium and ventricular
tachycardia, following surgery (7).
However, heart rate and systolic blood pressure were not analyzed
in this study. A more recent meta-analysis on pediatric clinical
studies with patients undergoing surgery for congenital heart
disease, concluded that dexmedetomidine likely improves cardiac
function in children; but the included studies were few and are
mostly observational studies and thus the conclusions of this
analysis were based on less number of patients and studies.
The present meta-analysis included much larger
number of studies and patients were undergoing only cardiac related
surgeries, where cardiac protection is the major issue. In this
meta-analysis, we noted that the long-suspected beneficial effects
of dexmedetomidine are true both for adults as well as children,
undergoing cardiac surgery. While dexmedetomidine improves heart
rate, systolic blood pressure and lowers tachycardia and
arrhythmias, this drug also increased the incidence of bradycardia,
indicating that care must be taken while administering
dexmedetomidine, particularly to patients who are in shock and
those with a transduction block. However, earlier studies suggested
that the increased bradycardia did not increase the length of
hospital stay (14).
Overall, this meta-analysis suggests that the use of
dexmedetomidine in cardiac surgery patients has many beneficial
cardioprotective effects, irrespective of the age group of the
patients. The major weakness of this meta-analysis is the inclusion
of both randomized and non-randomized clinical studies, but this
also increased the power of analysis by increasing the number of
included studies and patients to reach valid conclusions with
regard to the beneficiary effects of dexmedetomidine. Another
possible weakness is to combine pediatric and adult patients in the
same analysis and we did this particularly in increase the number
of included and analyzed studies and to enhance the predictive
power of this analysis. This also indicates that there is a need
for larger number of randomized clinical trial studies in either
adult or pediatric patient population, with more number of enrolled
patients. It is interesting to note that only in 2008, the US Food
and Drug Administration (FDA) approved the use of dexmedetomidine
for sedation in adults and this drug represents only 4% of the
currently used drugs outside the operating room (35). Even though dexmedetomidine has not
yet been approved by FDA for use in pediatric patients, clinicians
have found it to be an important drug for cardioprotection and as
an adjunctive anesthetic agent in children undergoing surgery for
repair of congenital heart defects. Despite the setbacks of this
meta-analysis, described above, the results indicate that
dexmedetomidine is an efficacious cardioprotective drug in adults
and children.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81373498 and 81060277).
References
|
1
|
Rostagno C, La Meir M, Gelsomino S, Ghilli
L, Rossi A, Carone E, Braconi L, Rosso G, Puggelli F, Mattesini A,
et al: Atrial fibrillation after cardiac surgery: incidence, risk
factors, and economic burden. J Cardiothorac Vasc Anesth.
24:952–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ad N, Barnett SD, Haan CK, O'Brien SM,
Milford-Beland S and Speir AM: Does preoperative atrial
fibrillation increase the risk for mortality and morbidity after
coronary artery bypass grafting? J Thorac Cardiovasc Surg.
137:901–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Zhao X and Wang Y:
Dexmedetomidine: a review of applications for cardiac surgery
during perioperative period. J Anesth. 29:102–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sulaiman S, Karthekeyan RB, Vakamudi M,
Sundar AS, Ravullapalli H and Gandham R: The effects of
dexmedetomidine on attenuation of stress response to endotracheal
intubation in patients undergoing elective off-pump coronary artery
bypass grafting. Ann Card Anaesth. 15:39–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunisawa T, Nagata O, Nagashima M,
Mitamura S, Ueno M, Suzuki A, Takahata O and Iwasaki H:
Dexmedetomidine suppresses the decrease in blood pressure during
anesthetic induction and blunts the cardiovascular response to
tracheal intubation. J Clin Anesth. 21:194–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keniya VM, Ladi S and Naphade R:
Dexmedetomidine attenuates sympathoadrenal response to tracheal
intubation and reduces perioperative anaesthetic requirement.
Indian J Anaesth. 55:352–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin YY, He B, Chen J and Wang ZN: Can
dexmedetomidine be a safe and efficacious sedative agent in
post-cardiac surgery patients? a meta-analysis. Crit Care.
16:R1692012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pasin L, Febres D, Testa V, Frati E,
Borghi G, Landoni G and Zangrillo A: Dexmedetomidine vs midazolam
as preanesthetic medication in children: a meta-analysis of
randomized controlled trials. Paediatr Anaesth. 25:468–476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariappan R, Ashokkumar H and Kuppuswamy
B: Comparing the effects of oral clonidine premedication with
intraoperative dexmedetomidine infusion on anesthetic requirement
and recovery from anesthesia in patients undergoing major spine
surgery. J Neurosurg Anesthesiol. 26:192–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin E, Ramsay G, Mantz J and Sum-Ping
ST: The role of the alpha2-adrenoceptor agonist dexmedetomidine in
postsurgical sedation in the intensive care unit. J Intensive Care
Med. 18:29–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji F, Li Z, Young N, Moore P and Liu H:
Perioperative dexmedetomidine improves mortality in patients
undergoing coronary artery bypass surgery. J Cardiothorac Vasc
Anesth. 28:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wijeysundera DN, Naik JS and Beattie WS:
Alpha-2 adrenergic agonists to prevent perioperative cardiovascular
complications: a meta-analysis. Am J Med. 114:742–752. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren J, Zhang H, Huang L, Liu Y, Liu F and
Dong Z: Protective effect of dexmedetomidine in coronary artery
bypass grafting surgery. Exp Ther Med. 6:497–502. 2013.PubMed/NCBI
|
|
14
|
Pan W, Wang Y, Lin L, Zhou G, Hua X and Mo
L: Outcomes of dexmedetomidine treatment in pediatric patients
undergoing congenital heart disease surgery: a meta-analysis.
Paediatr Anaesth. 26:239–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herr DL, Sum-Ping ST and England M: ICU
sedation after coronary artery bypass graft surgery:
dexmedetomidine-based versus propofol-based sedation regimens. J
Cardiothorac Vasc Anesth. 17:576–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corbett SM, Rebuck JA, Greene CM, Callas
PW, Neale BW, Healey MA and Leavitt BJ: Dexmedetomidine does not
improve patient satisfaction when compared with propofol during
mechanical ventilation. Crit Care Med. 33:940–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chrysostomou C, Beerman L, Shiderly D,
Berry D, Morell VO and Munoz R: Dexmedetomidine: a novel drug for
the treatment of atrial and junctional tachyarrhythmias during the
perioperative period for congenital cardiac surgery: a preliminary
study. Anesth Analg. 107:1514–1522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chrysostomou C, Komarlu R, Lichtenstein S,
Shiderly D, Arora G, Orr R, Wearden PD, Morell VO, Munoz R and
Jooste EH: Electrocardiographic effects of dexmedetomidine in
patients with congenital heart disease. Intensive Care Med.
36:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chrysostomou C, Sanchez-de-Toledo J,
Wearden P, Jooste EH, Lichtenstein SE, Callahan PM, Suresh T,
O'Malley E, Shiderly D, Haney J, et al: Perioperative use of
dexmedetomidine is associated with decreased incidence of
ventricular and supraventricular tachyarrhythmias after congenital
cardiac operations. Ann Thorac Surg. 92:964–972; discussion 972.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shehabi Y, Grant P, Wolfenden H, Hammond
N, Bass F, Campbell M and Chen J: Prevalence of delirium with
dexmedetomidine compared with morphine based therapy after cardiac
surgery: a randomized controlled trial (DEXmedetomidine COmpared to
Morphine-DEXCOM Study). Anesthesiology. 111:1075–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anger KE, Szumita PM, Baroletti SA,
Labreche MJ and Fanikos J: Evaluation of dexmedetomidine versus
propofol-based sedation therapy in mechanically ventilated cardiac
surgery patients at a tertiary academic medical center. Crit Pathw
Cardiol. 9:221–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hosokawa K, Shime N, Kato Y, Taniguchi A,
Maeda Y, Miyazaki T and Hashimoto S: Dexmedetomidine sedation in
children after cardiac surgery. Pediatr Crit Care Med. 11:39–43.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji F, Li Z, Nguyen H, Young N, Shi P,
Fleming N and Liu H: Perioperative dexmedetomidine improves
outcomes of cardiac surgery. Circulation. 127:1576–1584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tosun Z, Baktir M, Kahraman HC, Baskol G,
Guler G and Boyaci A: Does dexmedetomidine provide cardioprotection
in coronary artery bypass grafting with cardiopulmonary bypass? A
pilot study. J Cardiothorac Vasc Anesth. 27:710–715. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu H, Liu J and Wu C: Impact of
dexmedetomidine versus propofol on cardiac function of children
undergoing laparoscopic surgery. Int J Clin Exp Med. 7:5882–5885.
2014.PubMed/NCBI
|
|
26
|
Rajput RS, Das S, Makhija N and Airan B:
Efficacy of dexmedetomidine for the control of junctional ectopic
tachycardia after repair of tetralogy of Fallot. Ann Pediatr
Cardiol. 7:167–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turan A, Bashour CA, You J, Kirkova Y,
Kurz A, Sessler DI and Saager L: Dexmedetomidine sedation after
cardiac surgery decreases atrial arrhythmias. J Clin Anesth.
26:634–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Narisawa A, Nakane M, Kano T, Momose N,
Onodera Y, Akimoto R, Kobayashi T, Iwabuchi M, Okada M, Miura Y, et
al: Dexmedetomidine sedation during the nighttime reduced the
incidence of postoperative atrial fibrillation in cardiovascular
surgery patients after tracheal extubation. J Intensive Care.
3:262015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Ding S, Yan H, Li Y, Zhang L,
Chen X, Yin X, Liu S, Tang X and Zhang J: A retrospective
comparison of dexmedetomidine versus midazolam for pediatric
patients with congenital heart disease requiring postoperative
sedation. Pediatr Cardiol. 36:993–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng X, Zuo Y, Zhao Q, Gu E and Huang Y:
Comparison of the effects of dexmedetomidine and propofol on
hemodynamics and oxygen balance in children with complex congenital
heart disease undergoing cardiac surgery. Congenit Heart Dis.
10:E123–E130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chi X, Liao M, Chen X, Zhao Y, Yang L, Luo
A and Yang H: Dexmedetomidine attenuates myocardial injury in
off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc
Anesth. 30:44–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mikawa K, Nishina K, Maekawa N and Obara
H: Comparison of nicardipine, diltiazem and verapamil for
controlling the cardiovascular responses to tracheal intubation. Br
J Anaesth. 76:221–226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biccard BM, Goga S and de Beurs J:
Dexmedetomidine and cardiac protection for non-cardiac surgery: a
meta-analysis of randomised controlled trials. Anaesthesia.
63:4–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Constantin JM, Momon A, Mantz J, Payen JF,
De Jonghe B, Perbet S, Cayot S, Chanques G and Perreira B: Efficacy
and safety of sedation with dexmedetomidine in critical care
patients: a meta-analysis of randomized controlled trials. Anaesth
Crit Care Pain Med. 35:7–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tobias JD: Dexmedetomidine and ketamine:
an effective alternative for procedural sedation? Pediatr Crit Care
Med. 13:423–427. 2012. View Article : Google Scholar : PubMed/NCBI
|