Introduction
Rheumatoid arthritis (RA) is a chronic disease
involving various immune cells, particularly T lymphocytes, such as
CD4+ T-helper (Th) cells (1,2). RA is
characterized by chronic inflammation of the joints, synovial
hyperplasia and abnormal systemic immune responses. The joint
damage that underlies the pathogenesis of RA resembles the process
of immune-mediated tumor destruction (3). The formation of blood vessels and
degradation of the extracellular matrix are crucial steps in the
process of erosion, and T lymphocytes have an important role in
these coronary events (4).
CD4+ T cells may differentiate into
various lineages that are characterized by their profiles of
secreted cytokines. CD4+ regulatory T (Treg) cells and
Th17 cells have been described as two original subsets that are
distinct from Th1 and Th2 cells. Treg cells, which are
characterized by the expression of forkhead/winged helix
transcription factor (Foxp3), are essential for regulating
self-tolerance and have exhibited anti-inflammatory functions via
contact-dependent suppression or by the release of
anti-inflammatory cytokines (5). A
lack of Treg cells has previously been associated with autoimmune
diseases (6). Th17 cells express
retinoic acid-related orphan receptor γt and have critical roles in
the development of autoimmune diseases and human inflammatory
conditions by producing a novel cytokine, interleukin (IL)-17
(7). It has been demonstrated that
an imbalance in Th17/Treg cells had an important role in joint
inflammation and destruction in an animal model (8). A previous study demonstrated that the
imbalance between Th17 and Treg cells is an important mechanism
which may lead to RA (2).
Artesunate is a low toxicity and highly efficient
immune inhibitor (9). It has
previously been demonstrated that artesunate is able to suppress
lipopolysaccharide-induced secretion of tumor necrosis factor
(TNF)-α by synovial cells by regulating nuclear factor-κB signaling
pathways (10,11), thereby inhibiting the synovial
inflammation associated with RA. In addition, artesunate inhibited
the secretion of IL-17, TNF-α and other inflammatory factors by
synovial cells in previous studies (12,13);
thus reducing the formation of pannus and erosion of cartilage and
bone. However, the effect of artesunate on the balance of Th17/Treg
cells in patients with RA remains unknown.
The present study established a rat model of type II
collagen-induced arthritis (CIA) in order to investigate the effect
of artesunate on the Th17/Treg cell imbalance in vivo and to
elucidate the underlying mechanisms.
Materials and methods
Experimental animals
A total of 70 male Sprague Dawley rats (weight, ~100
g; age, 4 weeks) were obtained from the Guilin Medical College
Experimental Animal Center (Guilin, China). The rats were
maintained at 22–26°C and 55±10% humidity, under a 12-h light/dark
cycle with ad libitum access to food and water. The present
study was approved by the Ethics Committee of Guilin Medical
University (Guilin, China).
Reagents
Collagen type II (5 ml/bottle) was purchased from
Chondrex, Inc., (Redmond, WA, USA); Complete Freund's adjuvant
(CFA; 10 ml/bottle) was obtained from Sigma-Aldrich (St. Louis, MO,
USA); artesunate was purchased from Guilin Pharmaceutical Co.,
Ltd., (Guilin, China); Leukocyte Activation Cocktail with BD
GolgiPlug™ and Lysing Buffer were purchased from BD Pharmingen (San
Diego, CA, USA); phycoerythrin (PE)-conjugated anti-rat IL-17A,
PE-conjugated anti-rat Foxp3, allophycocyanin (APC)-conjugated
anti-rat CD4, PE-conjugated rat IgG2a Isotype Control and the
Foxp3/Transcription Factor Staining Buffer Set were obtained from
eBioscience, Inc., (San Diego, CA, USA); anti-IL-17 and anti-Foxp3
antibodies were purchased from Abcam (Cambridge, UK).
Establishment of a CIA murine
model
Collagen type II (with acetic acid, 2 mg/ml) was
slowly added to an equal volume of CFA to a final concentration of
1 mg/ml and maintained on ice until further use. Subsequently, rats
were anesthetized with pentobarbital sodium (0.1 ml/100 g;
Sigma-Aldrich) and divided into the normal control (n=9) and the
CIA model (n=8) groups. The CIA model group was administered a
mixed emulsion (0.2 ml) of collagen and adjuvant (1 mg/ml acetic
acid) by intradermal injection in the rat tail, and the normal
control group was administered an equal volume of saline. Primary
immunization was performed on day 0 with 200 mg (0.2 ml), followed
by a second immunization on day 7 with 100 mg (0.1 ml). Rats that
scored >6 points in the arthritis index (AI) scoring system
(14) were used for subsequent
analyses.
Treatment with artesunate
CIA rats were divided into four sub-groups, as
follows: i) CIA model plus 2 ml saline; ii) CIA model plus 5
mg/kg/day artesunate; iii) CIA model plus 10 mg/kg/day artesunate;
and iv) CIA model plus 20 mg/kg/day artesunate. Daily artesunate
gavage was initiated on day 14 following primary immunization. All
rats were sacrificed in week 20 by cervical dislocation following
intraperitoneal injection with 10% chloral hydrate (OriGene
Technologies, Inc., Beijing, China). The normal control group was
administered an equal volume of physiological saline.
Evaluation of clinical features
Clinical features of the rats were analyzed once a
week following primary immunization. This included an assessment of
the general health of the rats, including their weight, fur color,
feeding behaviour and AI. In addition, the drainage method was used
to assess the degree of swelling of the hind legs, as described
previously (15).
Degree of joint swelling
A vernier caliper was used to measure the radius of
the left leg ankle (from the medial malleolus to the external
ankle) and the diameter from the foot heel to the middle point of
joint clearance, in order to assess the general incidence degree of
joint swelling and the effect of treatment by analyzing the
anteroposterior and transverse diameters.
AI
The AI of each rat was calculated prior to primary
immunization, following primary immunization, once every 3 days in
the acute phase during the initial 6 weeks and once every week in
the chronic phase, according to a previous study (16). Briefly, each claw was assigned 4
points, thus the maximum possible score for each rat was 16 points.
However, as pathological changes predominantly occurred in the hind
legs of rats in the present study, a score of 6–8 points was deemed
to indicate severe arthritis.
Molybdenum target X-ray
Joint damage in the left posterior ankle was
observed by molybdenum target X-ray mammography at 10 and 21 weeks
following primary immunization of the normal control, CIA model and
CIA model plus artesunate (20 mg/kg/day) groups. The stage of
arthritis was subsequently determined according to the American
Rheumatism Association criteria for the classification of
rheumatoid arthritis (17), as
follows: Normal (0), osteoporosis period (+), bone destruction
period (++), bone serious destruction period (+++) and ankylosis
period (++++).
Histopathological analysis
Rats were sacrificed at week 20 and the left
anklebone was harvested, fixed with 10% neutral formalin for 24 h,
decalcified using 10% EDTA, cut vertically, embedded in paraffin
and cut into 2-µm slices. Subsequently, the slices were stained
with hematoxylin and eosin in order to observe inflammation in the
joint under a light microscope, according to a previous study
(18). The slices were observed for
infiltration of inflammatory cells, including neutrophils,
lymphocytes and plasma cells, synovial cell hyperplasia,
proliferation of fibrous tissue and erosion of bone and
cartilage.
Immunohistochemical analysis
Sections were dewaxed and incubated with citric acid
in a pressure cooker for antigen retrieval. After this, the
sections were incubated with 0.3% H2O2 for 3
min, washed three times for 3 min each with phosphate-buffered
saline (PBS) and incubated with PE-conjugated anti-IL-17A and
anti-Foxp3 antibodies for 1 h at 37°C. Subsequently, the sections
were washed three times for 3 min each with PBS, followed by
incubation with the secondary antibody for 1 h at room temperature.
Antibody complexes were visualized with 3,3′-diaminobenzidine,
after which the sections were stained with hematoxylin, dehydrated
and mounted with gum, prior to visualization under a fluorescent
microscope.
Extraction of spleen lymphocytes
Following sacrifice, the rats were soaked in 75%
ethanol for 10 min and the spleen was harvested under sterile
conditions. Subsequently, the spleen was cut into small pieces
using ophthalmic scissors, homogenized and filtered using a
200-mesh steel sieve with the core of a 5 ml syringe to remove
large pieces of tissue. The homogenate was centrifuged at 400 × g
for 5 min at 4°C, the supernatant was discarded and 5 ml Lysing
Buffer was added to the pellet. Spleen cells were subsequently
washed 1–2 times with PBS, after which 106 cells/ml
underwent flow cytometry in four tubes.
Flow cytometry
Leukocyte Activation Cocktail, with BD GolgiPlug™
(0.5 µl) was added to each tube and incubated at 37°C in 5%
CO2 for 6 h. Subsequently, 1 ml fluorescence-activated
cell sorting (FACS) buffer (50 ml 10X PBS, 20 g bovine serum
albumin, 0.02% NaN3, distilled water) was added to each
tube, followed by centrifugation at 400 × g for 5 min at 4°C. The
supernatant was discarded and the pellet was resuspended in 100 µl
FACS buffer, followed by the addition of 5 µl APC-conjugated CD4
and centrifugation at 350 × g for 5 min at 4°C. In order to rupture
the cell membranes, 1 ml Foxp3/permeabilization buffer (BD
Pharmingen) was added to each tube and the tubes were incubated at
4°C for 40 min in the dark. Subsequently, 2 ml permeabilization
buffer (1X) was added to each tube and the tubes were centrifuged
at 350 × g for 5 min at 4°C. The resulting pellet was resuspended
in 100 µl permeabilization buffer (1X), followed by the addition of
5 or 10 µl PE-conjugated rat IgG2a isotype control, 5 µl
PE-conjugated anti-Foxp3 or 10 µl PE-conjugated anti-IL-17 at 4°C
for 30 min in the dark. The tubes were analyzed on a flow cytometer
using BD FACSDiva™ Software 6.0 (BD Pharmingen).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to conduct statistical analyses. Data were presented as
the mean ± standard deviation. One-way analysis of variance was
performed to analyze differences among groups. Dunnett's T3 test
was used to compare non-parametric data. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical features of CIA rats
Of the 61 rats in the CIA model group, 56 exhibited
arthritic symptoms in 9–21 days following primary immunization
(success rate, ~93.3%). Arthritis appeared at 9 days following the
primary immunization as redness and swelling in the small toe, foot
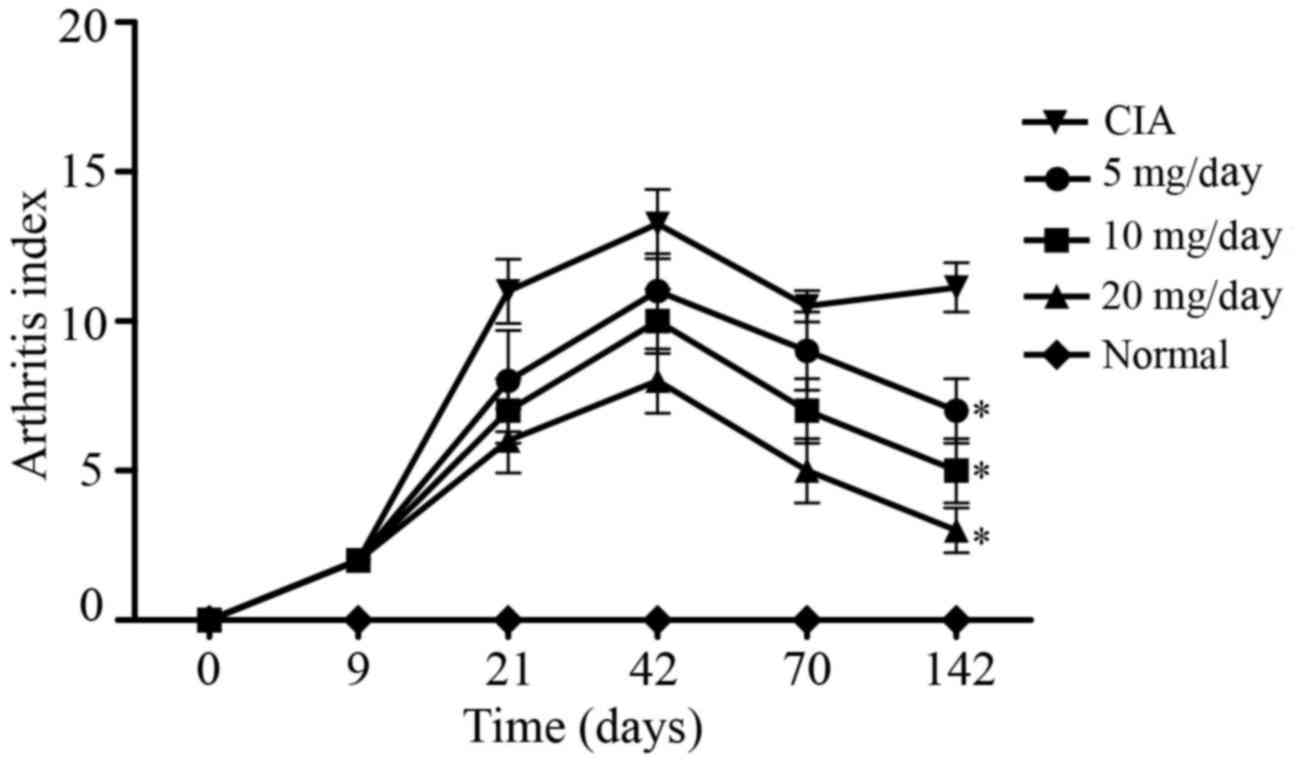
or ankle. As demonstrated in Fig. 1,
AI peaked at ~42 days following immunization in the CIA model
group, which was followed by a chronic arthritis phase. At 70 days
post-primary immunization, the mean AI was 9 points for the 5
mg/kg/day artesunate group, 7 points for the 10 mg/kg/day
artesunate group, 5 points for the 20 mg/kg/day artesunate group,
and 10.5 points for the CIA group. At 142 days post-primary
immunization, the mean AI was 7 points for the 5 mg/kg/day
artesunate group, 5 points for the 10 mg/kg/day artesunate group, 3
points for the 20 mg/kg/day artesunate group and 11.1 points for
the CIA group. These results suggest that 20 mg/kg/day artesunate
is able to improve joint symptoms in a rat model of RA.
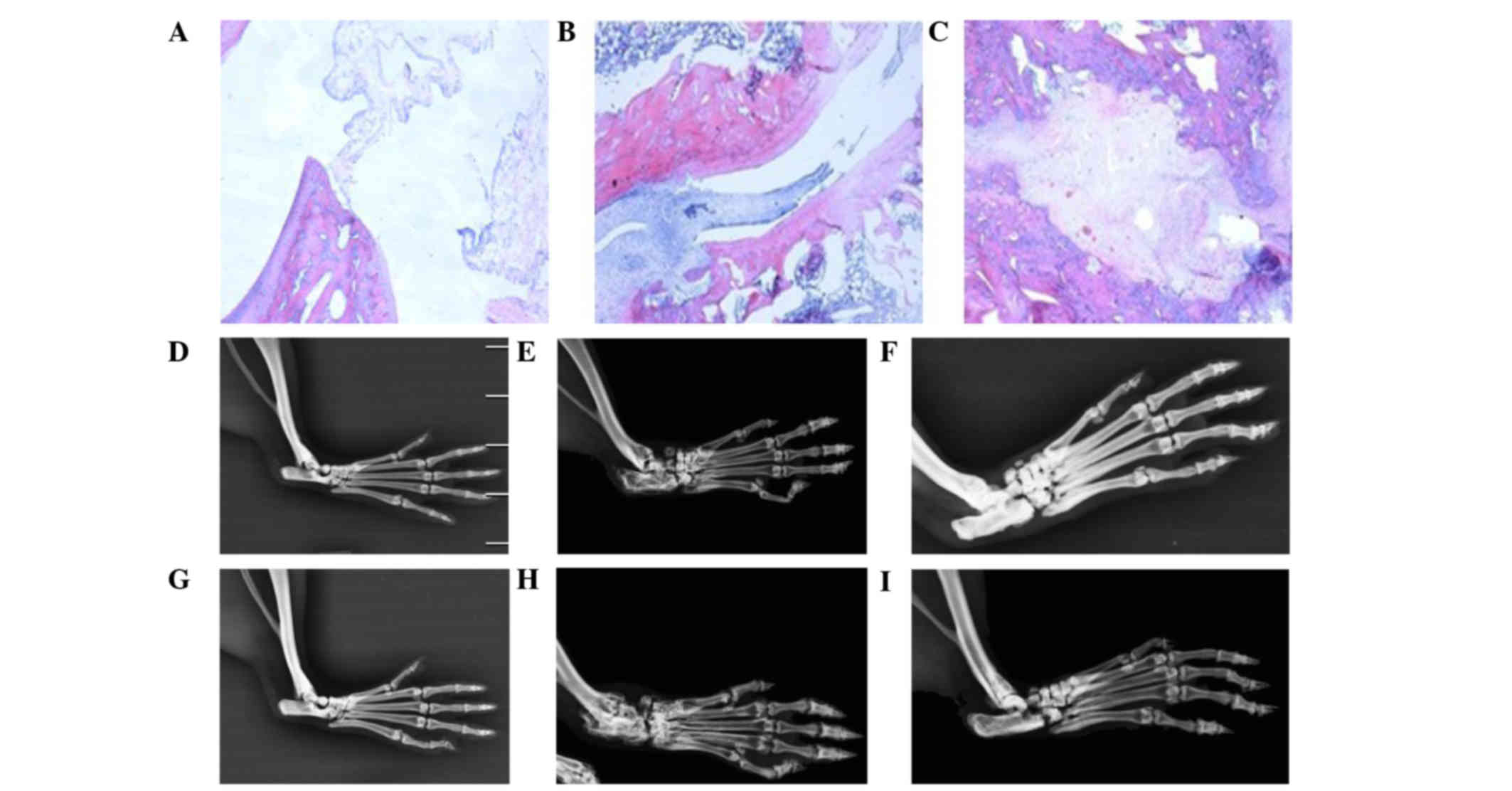
Histopathological analysis of rat
ankle joints
As compared with the normal control rats, the levels
of synovial fluid in the CIA rats were increased, the joint cavity
was disorganized, and infiltration of inflammatory cells, in
particular lymphocytes and monocytes, and synovial pannus invasion
of joint cartilage and bone, were observed at 10 weeks following
primary immunization (Figs. 2A and
B). At 21 weeks following the primary immunization, the levels
of synovial fluid were increased, the joint cavity was disordered,
and infiltration of inflammatory cells and cartilage and bone
damage were observed in the CIA rats (Fig. 2C). These results suggested that the
rat model of CIA had been established successfully.
Molybdenum target X-rays of rat limb
joints
The structure of the joint cavity was integrated in
normal rats. Conversely, CIA rats exhibited signs of osteoporosis,
bone and articular cartilage destruction and narrowed joint spaces
at weeks 10 and 21 following primary immunization. Rats in the 20
mg/kg/day artesunate group exhibited signs of osteoporosis;
however, bone destruction was mild (Fig.
2). These results suggest that artesunate is able to attenuate
the bone destruction associated with RA.
Expression of Foxp3 and Il-17 in the
synovium
Immunohistochemical analysis demonstrated that Foxp3
was dispersed in the synovial lining cell layer, surrounding blood
vessels and cartilage surface, and IL-17 was predominantly located
in the synovial lining cell layer and surrounding blood vessels.
Expression levels of Foxp3 were markedly increased in the 20
mg/kg/day artesunate group, as compared with the other drug
intervention groups and CIA group; however, no marked difference
was observed between the 20 mg/kg/day artesunate group and the
normal control group. The expression levels of IL-17 were markedly
reduced in the 20 mg/kg/day artesunate group, as compared with the
CIA group and the other drug intervention groups (Fig. 3).
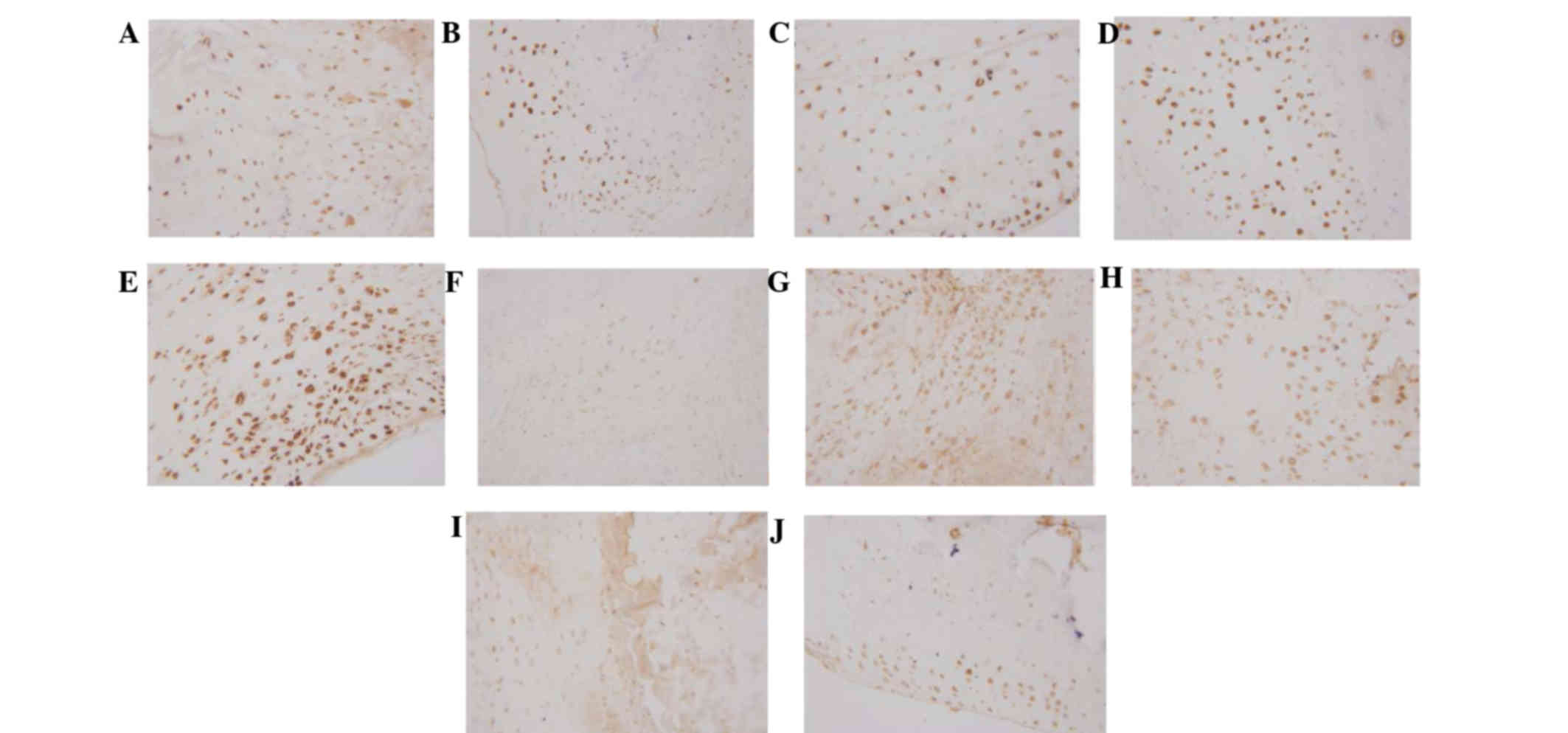 | Figure 3.Expression of Foxp3/IL-17 in the
synovium, as determined by immunohistochemial staining
(magnification, ×400). Foxp3 expression in the (A) normal control,
(B) collagen-induced arthritis model, (C) 5 mg/kg/day artesunate,
(D) 10 mg/kg/day artesunate and (E) 20 mg/kg/day artesunate groups.
IL-17 expression in the (F) normal control, (G) CIA model, (H) 5
mg/kg/day artesunate, (I) 10 mg/kg/day artesunate and (J) 20
mg/kg/day artesunate groups. Foxp3, forkhead/winged helix
transcription factor; IL-17, interleukin-17. |
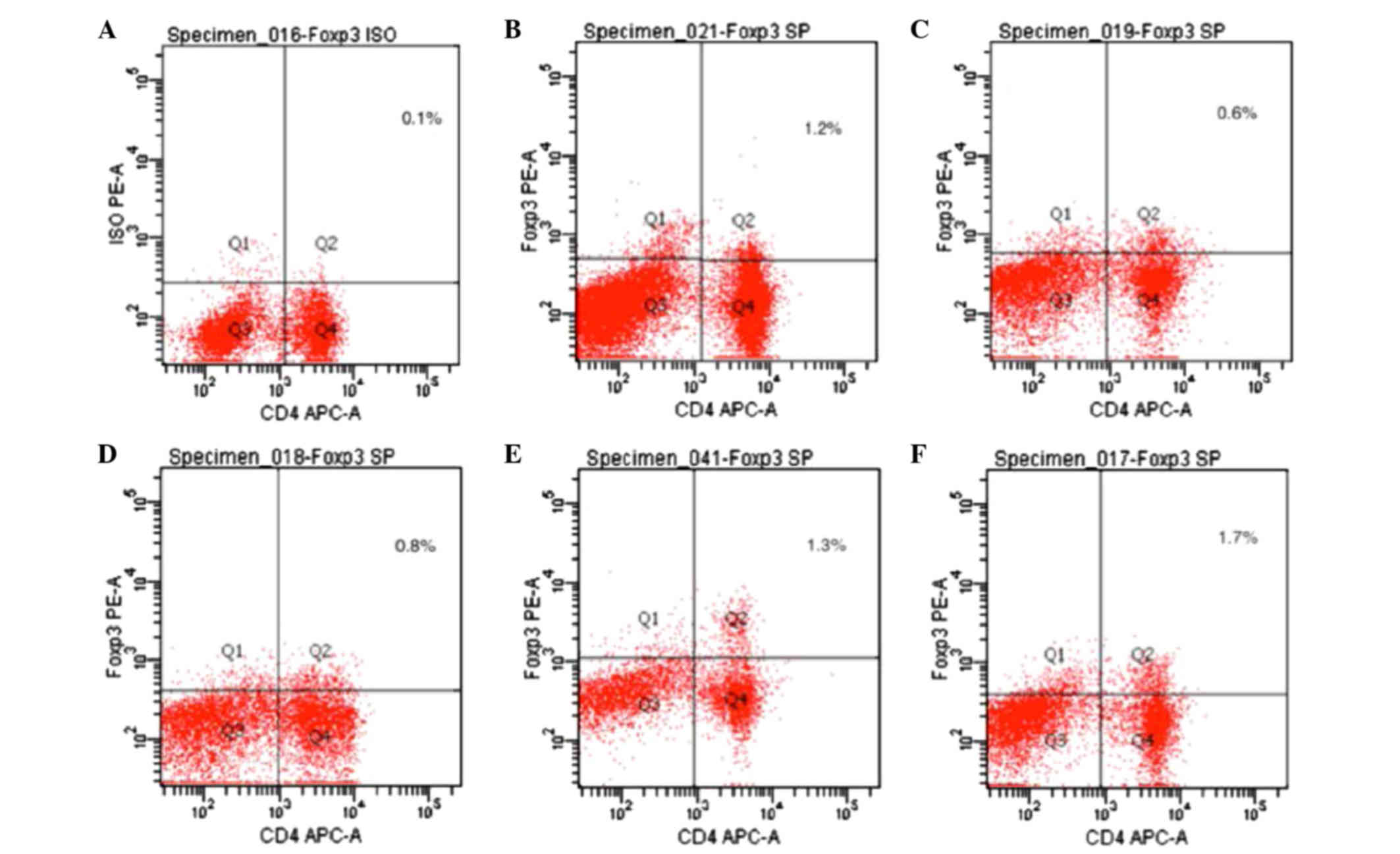
Expression of Foxp3 in spleen T
lymphocytes, as detected by flow cytometry
Foxp3 expression is an indicator of Treg cells. As
compared with the normal control group, the expression levels of
Foxp3 were significantly decreased in the CIA model group, as
compared with the normal control group (P<0.05). The expression
levels of Foxp3 were significantly increased in the 20 mg/kg/day
artesunate group, as compared with the 10 mg/kg/day artesunate and
5 mg/kg/day artesunate groups (P<0.05; Table I and Fig.
4).
 | Table I.Expression of Foxp3/IL-17 in
CD4+ T lymphocytes. |
Table I.
Expression of Foxp3/IL-17 in
CD4+ T lymphocytes.
| Group | Foxp3 (%) | IL-17 (%) |
|---|
| Normal |
1.171±0.191a | 0.514±0.352 |
| CIA model |
0.529±0.138a,b |
1.471±0.180a,b |
| Artesunate
(mg/kg/day) |
|
|
| 5 |
0.729±0.095a |
1.257±0.134a,b |
| 10 |
1.342±0.217a,b |
0.829±0.040a,b |
| 20 |
1.671±0.123b | 0.600±0.038 |
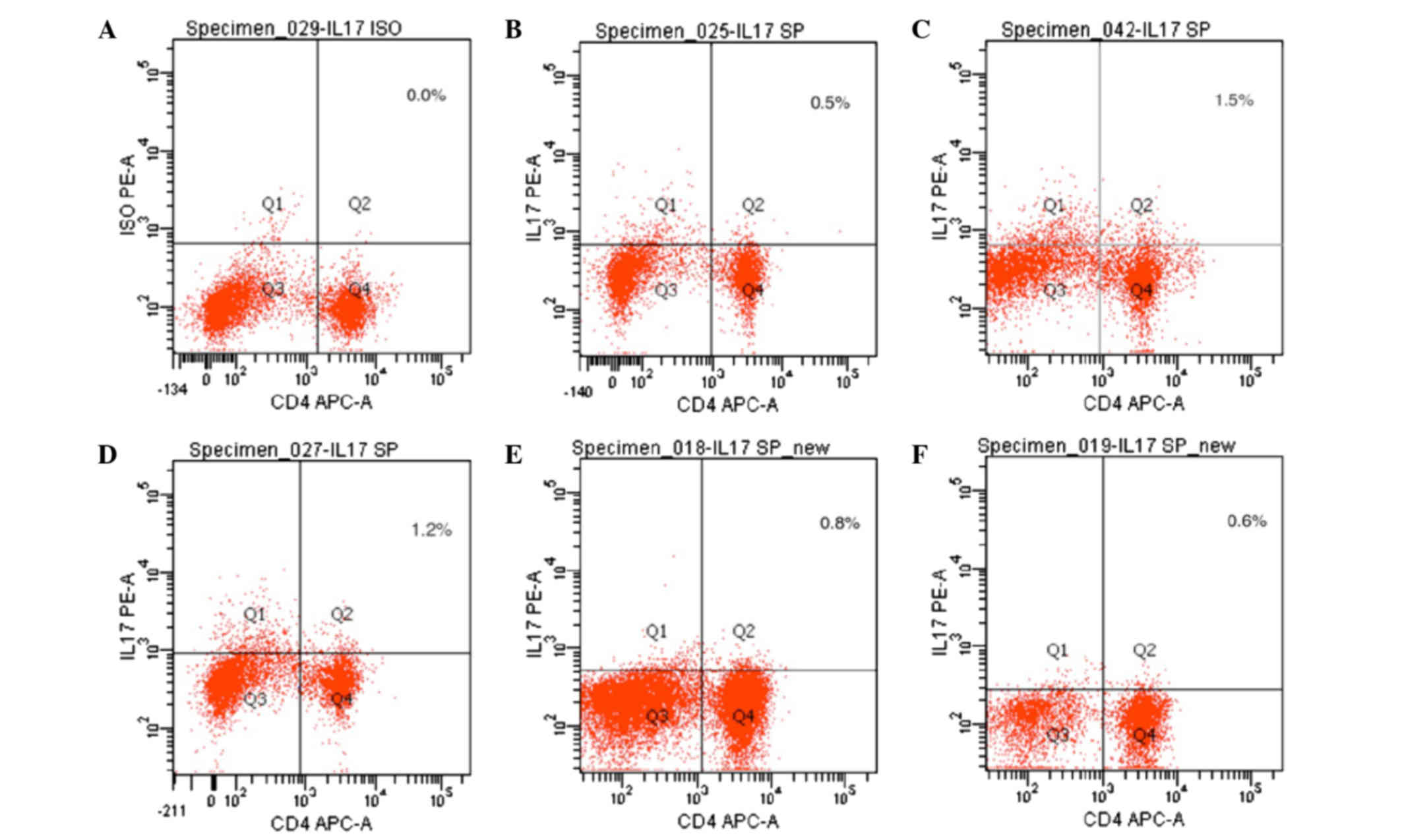
Expression of IL-17 in spleen T
lymphocytes, as detected by flow cytometry
IL-17 expression is an indicator of Th17 cells. As
compared with the normal control group, the expression levels of
IL-17 were significantly increased in the CIA model group
(P<0.05). The expression levels of IL-17 were significantly
decreased in the 20 mg/kg/day artesunate group, as compared with
the 10 mg/kg/day artesunate and 5 mg/kg/day artesunate groups
(P<0.05; Table I and Fig. 5).
Discussion
RA is a systemic autoimmune disease characterized by
peripheral arthritis. At present, the CIA model is the most
commonly used animal model for studying RA (19). The underlying etiology and
pathogenesis of RA has yet to be fully elucidated, although the
expression and balance of Th17/Treg lymphocytes has emerged as a
hot research topic in autoimmune diseases. Niu et al
(2) reported that Th17 cells were
significantly increased, whereas Treg cells were significantly
decreased, in the peripheral blood of patients with RA, as compared
with healthy controls; thus suggesting that an imbalance of
Th17/Treg lymphocytes may underlie the pathogenesis of RA. This is
consistent with the present study, which demonstrated that the
expression levels of Foxp3 were decreased, and those of Il-17 were
increased, in spleen lymphocytes derived from CIA rats.
Artemisinin and its derivatives are the most
effective and safe therapeutic agents for the treatment of
malignant and chloroquine-resistant malaria (20). In addition, it has been suggested
that artemisinin and its derivatives may also exert
anti-inflammatory and immunomodulatory effects (21). Artesunate is a semi-synthetic
derivative of artemisinin. In our previous study, it was
demonstrated that artesunate was able to regulate the NF-κB
signaling pathway to inhibit LPS-induced secretion of TNF-α by RA
synovial cells, and it was able to lower the activity of IL-17
(13). One aim of the present study
was to investigate whether artesunate reduces the activity of IL-17
via increasing the expression of Foxp3, thereby exerting
immunomodulatory effects.
The present study demonstrated that intervention
with artesunate resulted in an increase in the number of
Foxp3-positive T-cells and a decrease in the number of
IL-17-positive T cells in a dose-dependent manner. These results
suggested that artesunate was able to correct the imbalance of
Th17/Treg cells in CIA rats. Traditional treatment of RA involves
administration of hydroxychloroquine and methotrexate (22).
The greatest effects were observed with 20 mg/kg/day
artesunate. The results of the present study suggested that
artesunate was able to control and alleviate the symptoms of CIA
rats. A previous study suggested that the effects of artesunate may
involve the inhibition of IL-17, TNF-α, MMP3 and other inflammatory
factors (23), and the present study
demonstrated that these immunomodulatory effects may occur via the
induction of Foxp3 expression.
In conclusion, the present study demonstrated that
artesunate was able to increase the expression of Foxp3 and
decrease the expression of IL-17 in CD4+ T lymphocytes,
thereby reversing the imbalance of Th17/Treg cells associated with
arthritis. Our investigations of artesunate have identified
potential therapeutic targets of artesunate and have highlighted a
potential mechanism underlying the intervention of artesunate in
human synovial cells. However, further studies to elucidate the
potential adverse reactions of artesunate are required prior to the
widespread clinical use of artesunate for the treatment of RA.
Acknowledgements
The present study was supported by grants from the
Chinese National Natural Science Foundation (grant nos. 81160376
and 81360462), the Guangxi Natural Science Foundation (grant nos.
2013GXNSFAA019111 and 2013GXNSFBA019181), the Guangxi Department of
Education Natural Science Foundation (grant no. 201106LX363) and
the Guilin Natural Science Foundation (grant no. 20120121-1-7)
References
|
1
|
Guggino G, Giardina A, Ferrante A,
Giardina G, Schinocca C, Sireci G, Dieli F, Ciccia F and Triolo G:
The in vitro addition of methotrexate and/or methylprednisolone
determines peripheral reduction in Th17 and expansion of
conventional Treg and of IL-10 producing Th17 lymphocytes in
patients with early rheumatoid arthritis. Rheumatol Int.
35:171–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niu Q, Cai B, Huang ZC, Shi YY and Wang
LL: Disturbed Th17/Treg balance in patient with rheumatoid
arthritis. Rheumatol Int. 32:2731–2736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolb C, Mauch S, Peter HH, Krawinkel U and
Sedlacek R: The matrix metalloproteinase RASI-1 is expressed in
synovial blood vessels of a rheumatoid arthritis patient. Immunol
Lett. 57:83–88. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T: Foxp3+
CD25+ CD4+ natural regulatory T cells in dominant self-tolerance
and autoimmune disease. Immunol Rev. 212:8–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dejaco C, Duftner C, Grubeck-Loebenstein B
and Schirmer M: Imbalance of regulatory T cells in human autoimmune
diseases. Immunology. 117:289–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bettelli E, Oukka M and Kuchroo VK:
T(H)-17 cells in the circle of immunity and autoimmunity. Nat
Immunol. 8:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boissier MC, Assier E, Falgarone G and
Bessis N: Shifting the imbalance from Th1/Th2 to Th17/treg: The
changing rheumatoid arthritis paradigm. Joint Bone Spine.
75:373–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y,
Yang X, Lian F and Sun L: Anti-malarial agent artesunate inhibits
TNF-alpha induced production of proinflammatory cytokines via
inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human
rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology
(Oxford). 46:920–926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang S, Wang Y, Zhou C, Chen G, Shen
W, Li C, Lin W, Lin S, Huang H, et al: Inhibitory effect of the
antimalarial agent artesunate on collagen-induced arthritis in rats
through nuclear factor kappaB and mitogen-activated protein kinase
signaling pathway. Transl Res. 161:89–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Yu M, Pan X, Ren C, Peng W, Li X,
Jiang W, Zheng J and Zhou H: Artesunate reduces serum
lipopolysaccharide in cecal ligation/puncture mice via enhanced LPS
internalization by macrophages through increased mRNA expression of
scavenger receptors. Int J Mol Sci. 15:1143–1161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Ding J, Yang C, Gao Y, Li X, Chen
X, Peng Y, Fang J and Xiao S: Immunomodulatory and
anti-inflammatory properties of artesunate in experimental colitis.
Curr Med Chem. 19:4541–4551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mo HY, Wang LF and Zhang LH: Effects of
artesunate on tumor necrosis factor alpha and chemotactic factors
in the serum and the synoviocyte culture supernate of
collagen-induced arthritis rats. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 32:253–256. 2012.(In Chinese). PubMed/NCBI
|
|
14
|
Cuzzocrea S, Mazzon E, Bevilaqua C,
Costantino G, Britti D, Mazzullo G, De Sarro A and Caputi AP:
Cloricromene, a coumarine derivative, protects against
collagen-induced arthritis in Lewis rats. Br J Pharmacol.
131:1399–1407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brahn E, Peacock DJ and Banquerigo ML:
Suppression of collagen-induced arthritis by combination
cyclosporin A and methotrexate therapy. Arthritis Rheum.
34:1282–1288. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee P, Yang SY, Wu B, Song Z, Myers
LK, Robbins PD and Wooley PH: Tumour necrosis factor receptor gene
therapy affects cellular immune responses in collagen induced
arthritis in mice. Ann Rheum Dis. 64:1550–1556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xinqiang S, Fei L, Nan L, Yuan L, Fang Y,
Hong X, Lixin T, Juan L, Xiao Z, Yuying S and Yongzhi X:
Therapeutic efficacy of experimental rheumatoid arthritis with
low-dose methotrexate by increasing partially CD4+CD25+Treg cells
and inducing Th1 to Th2 shift in both cells and cytokines. Biomed
Pharmacother. 34:463–471. 2010. View Article : Google Scholar
|
|
19
|
Durie FH, Fava RA, Foy TM, Aruffo A,
Ledbetter JA and Noelle RJ: Prevention of collagen-induced
arthritis with an antibody to gp39, the ligand for CD40. Science.
261:1328–1330. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Chen H, Wei N, Mei X, Zhang S, Liu
DL, Gao Y, Bai SF, Liu XG and Zhou YX: Anti-inflammatory and
immunomodulatory mechanisms of artemisinin on contact
hypersensitivity. Int Immunopharmacol. 12:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Dell JR, Leff R, Paulsen G, Haire C,
Mallek J, Eckhoff PJ, Fernandez A, Blakely K, Wees S, Stoner J, et
al: Treatment of rheumatoid arthritis with methotrexate and
hydroxychloroquine, methotrexate and sulfasalazine, or a
combination of the three medications: Results of a two-year,
randomized, double-blind, placebo-controlled trial. Arthritis
Rheum. 46:1164–1170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aldieri E, Atragene D, Bergandi L, Riganti
C, Costamagna C, Bosia A and Ghigo D: Artemisinin inhibits
inducible nitric oxide synthase and nuclear factor NF-κB
activation. FEBS Lett. 552:141–144. 2003. View Article : Google Scholar : PubMed/NCBI
|