Introduction
Temporomandibular joint osteoarthritis (TMJOA) is
the most serious category in temporomandibular joint disorder
disease in the oral and maxillofacial region, which can lead to
severe joint pain and joint movement disorder and seriously damage
the life quality of the patient. The pathological characteristics
of TMJOA mainly include condylar cartilage degeneration and
abnormal subchondral bone sclerosis (1). The subchondral bone remodeling cannot
only change the stress characteristics of subchondral bone, but the
new blood vessels at the boundary of the cartilage and subchondral
bone can also carry factors to influence chondrocyte metabolism
through ‘dialogue’ (2). The
preliminary studies found that under normal physiological
conditions, the expression of hypertrophic chondrocytes of rat
condylar cartilage can promote angiogenic factor vascular
endothelial growth factor (VEGF) and inhibit anti-angiogenic factor
CHM-1, and its expression quantity decreased with age (3). The overexpression of VEGF can promote
proliferation of chondrocytes and the deposition of new bones under
the cartilage, and if the matrix synthesis is greater than the
degradation, the growth of the mandible will be promoted. However,
under the action of abnormal bite force, the condylar cartilage of
TMJOA rats will be degraded, the ubchondral bone will be lost and a
large amount of the inflammatory factor and matrix
metalloproteinase (MMP) will be secreted. At the osteochondral
boundary, VEGF and CTGF can promote high expressions of angiogenic
factor and the number of new blood vessels will be increased
significantly, breaking through the tidemark and reaching calcified
and non-calcified cartilage.
There are still some issues to be investigated e.g.,
type of pro-angiogenic factors to induce the formation of new blood
vessels under the action of the abnormal force and whether there is
a synergistic effect between it and VEGF, role of pro-angiogenic
factors in the chondrocyte differentiation and apoptosis under the
action of the abnormal force, role of antergic pro-angiogenic
factors in inhibiting the cartilage degradation and osteophyte
formation. The answer to these issues can further clarify the
pathogenesis of TMJOA lesions and be more conducive when looking
for effective drug targets for the treatment of TMJOA. To this end,
the study will explore the significance of new blood vessels in the
pathogenesis of TMJOA by constructing animal models.
Materials and methods
Animals model
Fifteen 8-week-old female Sprague-Dawley (SD) rats
with the average weight of ~200 g were kept under conventional
breeding; 3M orthodontic apron (no. 1/8; G&H Orthodontics,
Franklin, IN, USA). The TMJOA models were established in gradually
induced occlusal disorder. The animals were anesthetized and the
orthodontic apron was placed between the first and the second
molars in the lower right and upper left positions. The mesial
movement of the first molar were made by nearly 1 mm after 1 week,
and then the apron was removed and placed in self-curing plastics
in the gap so as to make it below the occlusal plane. Subsequently,
the same operations was repeated between the second and third
molars at the lower right and upper left position at 4 weeks after
the first separation so as to make the distal movement of the third
molar. Next, 5 rats were sacrificed at 4, 8 and 16 weeks after
successful modeling, and TMJ was removed at the right side to fix
it with 4% paraformaldehyde for 48 h. It was followed by
decalcification with sodium formate for 1 week, then the
decalcification solution was cleaned-up, graded dehydration with
ethanol was performed, then embedded with paraffin, the sections
were cut longitudinally in proximal, distal and immediate
directions to make condylar specimens of 5 µm. The approval for
animal experiments was received from the Animal Ethics Committee of
Guangzhou University Animal Center.
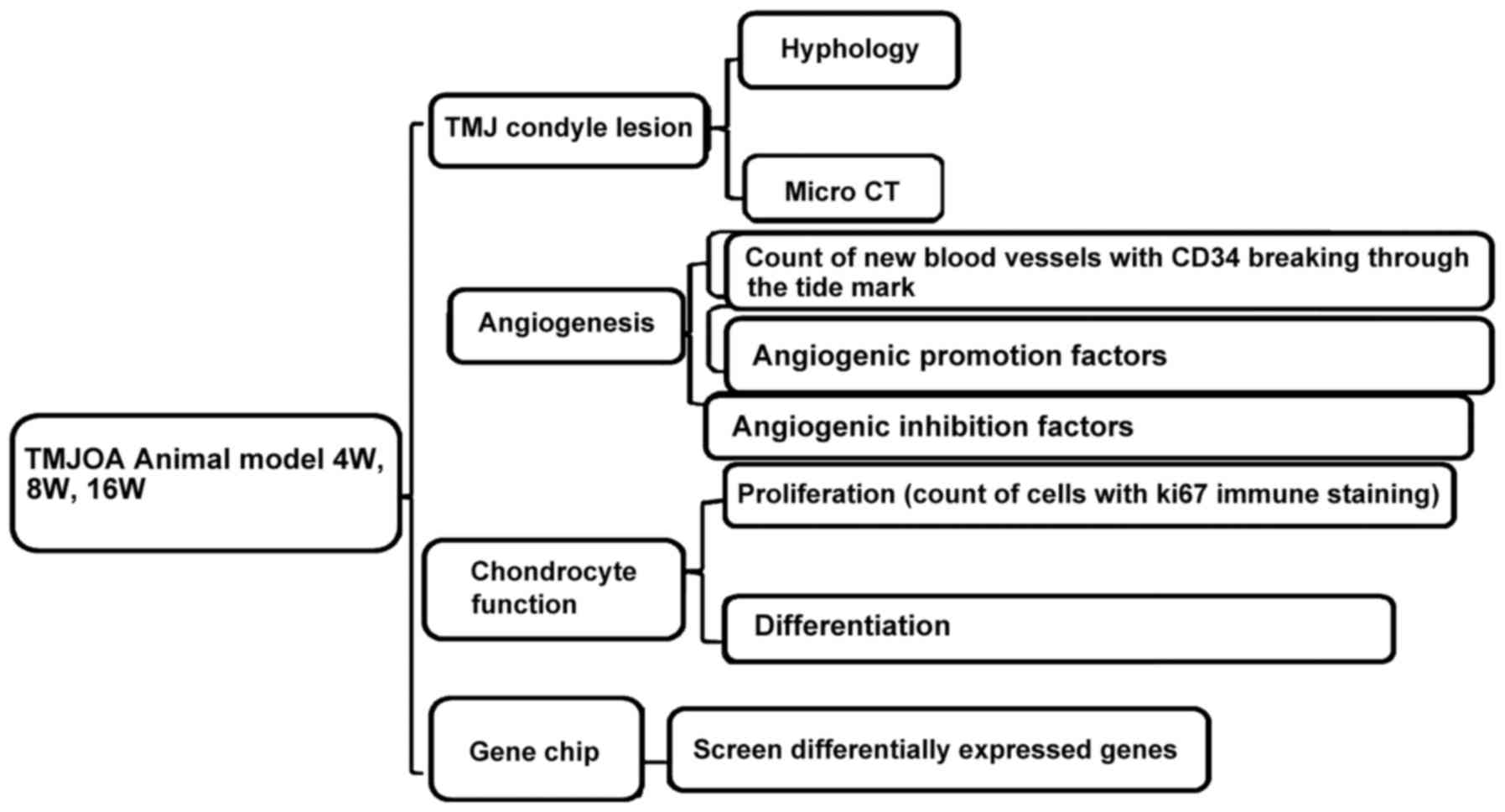
Study method
Histological and micro-computed tomography
(Micro-CT) observations were performed on the condyle specimens.
The distribution and number of new blood vessels breaking were
observed through the tidemark through CD34 immunofluorescence
staining. The proliferation condition of chondrocytes were observed
through Ki67 immunohistochemical staining, the differentiation
functions of chondrocytes were conducted through PTHrP and IHH
immunohistochemical staining. The degradation functions of
cartilage matrix were measured through MMP-9 immunohistochemical
staining. The expression of vascular growth promotion and
inhibition factors with VEGF, CTGF and CHM-1 immunohistochemical
staining and then screened differentially expressed genes through
gene chip analysis method (Fig.
1).
Histological observation
For conventional hematoxylin and eosin (H&E)
staining, Leica DFC490 system (Leica Microsystems, Wetzlar,
Germany) was used for collection of microscopic images. The condyle
surface was divided into the front, middle and rear parts with
articular disc front and rear joint as the boundary, quarter each
part. The Leica QWin software (Leica Microsystems Imaging Solution,
Ltd., Cambridge, UK) was applied to measure the fiber layer
thickness, calcified cartilage layer thickness and full-layer
cartilage thickness at a total of 9 positions in each quartering
point and then taking the mean value for analysis. The Micro-CT (GE
Healthcare, London, ON, Canada) was used to scan the specimen with
the resolution of 8 µm and the peak voltage of 80 kV. Microview
software (version 2.5.0; GE Healthcare) was used to re-construct
the condylar specimen axial, coronal and sagittal images and to
calculate the bone mineral density (BMD) and bone microstructure
parameters [bone surface area-to-volume ratio (BS/BV), trabecular
number (Tb.N), trabecular thickness (Tb.Th) and trabecular bone gap
(Tb.Sp)] of each specimen. The 0.5×0.5×0.5 mm region of interest
(ROI) at the midpoint of the condyle postmedian was selected and
just below the boundary of the cartilage and subchondral bone
software was used to automatically calculate subchondral
bone-related parameters in the region of interest.
CD34 immunofluorescence staining
CD34 polyclonal antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), immunofluorescence kit (Zhongshan
Biologics Co., Zhongshan, China) was used. The tissue slides were
placed for antigen digestion at 37°C for 10 min after xylene
dewaxing and graded alcohol dehydration. The slides were washed
with phosphate-buffered saline (PBS) for 5 min, 3 times and placed
in the antigen retrieval solution at 37°C for 15 min. Subsequently,
the sides were washed with PBS for 5 min, 3 times, and incubated in
serum at 37°C for 20 min. After discarding serum, the sections were
covered with CD34 primary antibody (1:50) at 4°C overnight. It was
followed by washing the slide with PBS for 5 min, 3 times, and
addition of fluorescently labeled secondary antibody Cy3 to
incubate at 37°C for 60 min. It was again followed by washing with
PBS for 5 min, 3 times, and slides were fixed with glycerol to take
an image by confocal microscopy.
Ki67 immunohistochemical staining
Ki67 monoclonal antibody (1:200 dilution, Lab Vision
Corp., Fremont, CA, USA) was used. The staining was performed as
previously described (4). Likewise,
immunohistochemical staining was performed for PTHrP, IHH, MMP-9,
VEGF, CTGF and CHM-1 with monoclonal antibodies (1:200 dilution;
Sigma-Aldrich, St. Louis, MO, USA).
Gene chip analysis method
HumanHT-12 v4 Expression BeadChip (Illumina, Inc.,
San Diego, CA, USA) was used. Total RNA was extracted and purified,
micro-sample in vitro amplification, probe labeling and
hybridization and chip scanning were performed to obtain the
original signal value of each gene point on the chip. The Database
for Annotation, Visualization and lntegrated Discovery (DAVID)
analysis tool was used to screen the difference in expression
genes. The genes with more than 1.5 times expression change (i.e.,
the genes with the fold value >1.5 or <0.67) was considered
as differentially expressed genes according to the signal intensity
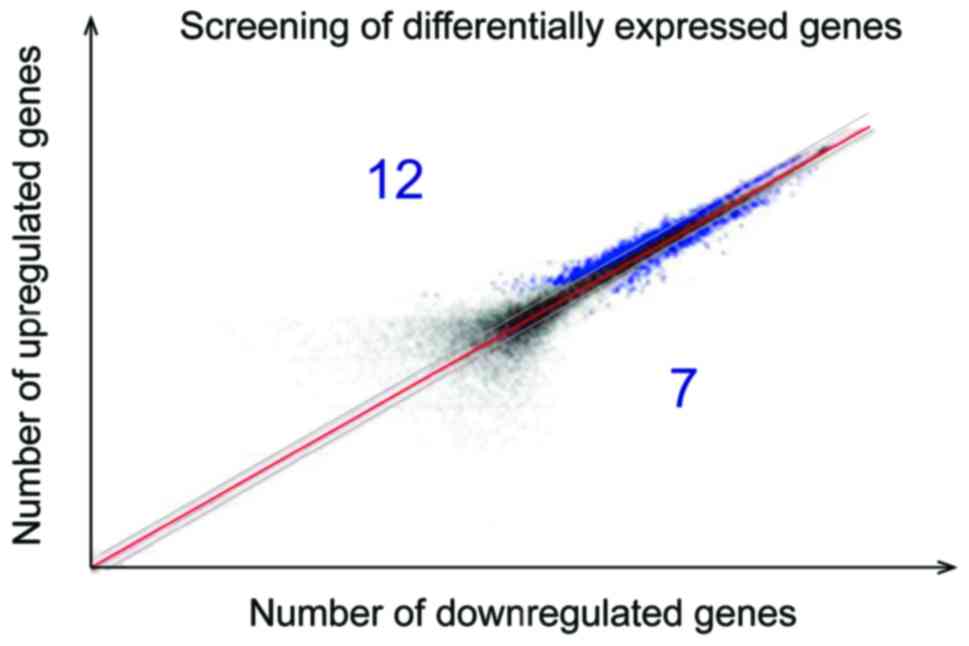
ratio. The screened different mRNA were presented with logarithmic
scatter diagram and was divided along the red diagonal line. The
upper was a point with the fold >1.5, the lower was the point
with fold <0.67 and the blue represented differentially
expressed genes.
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for entry and data analysis. The quantitative
data were expressed as mean ± standard deviation and single factor
analysis of variance (ANOVA) was used for the data analysis in
terms of the comparison between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Model of histological observation and
Micro-CT quantitative detection
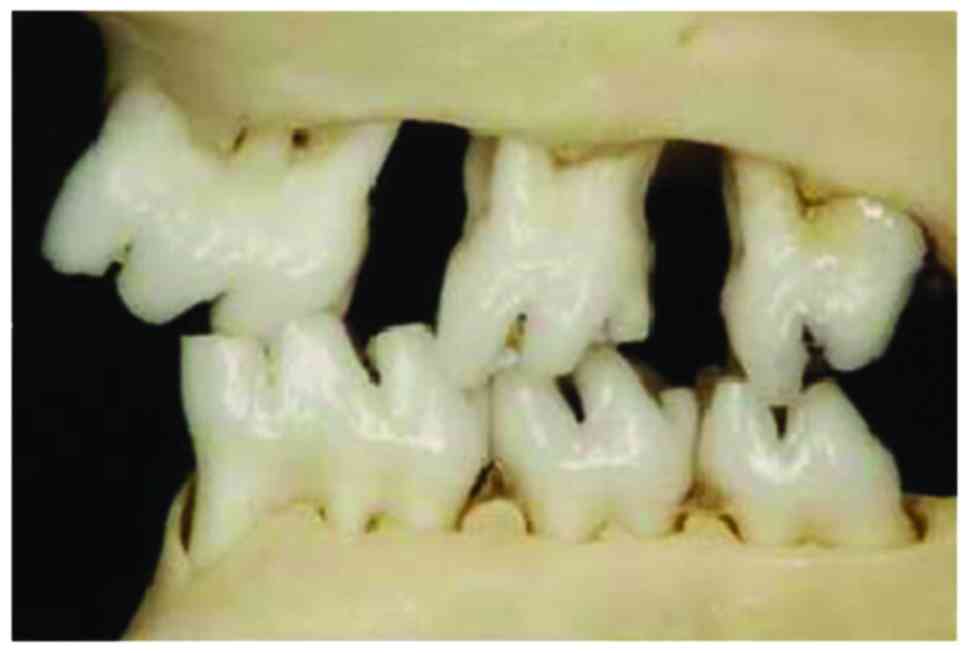
After respective mesial and distal movement of the
first and third molars of rats in the lower right and upper left
positions, the original relationship between the sharpness and
concaveness was destroyed and an experimental occlusal disorder was
formed (Fig. 2). The fibrous layer
structure of the condyle surface was incomplete, the chondrocytes
were in disorder, and it could be seen that the mast cell clusters
were integrated in an island shape, the chondrocyte nail-like
hyperplasia was protruded into the subchondral bone, there was an
acellular region, tangent crack was formed and similar
osteophyte-like hyperplasia occurred. The lesions were mainly
concentrated in the middle and rear parts of the condylar
cartilage, and under the observation by microscope, the tide line
showed a basophilic wavy line, which clearly separated the
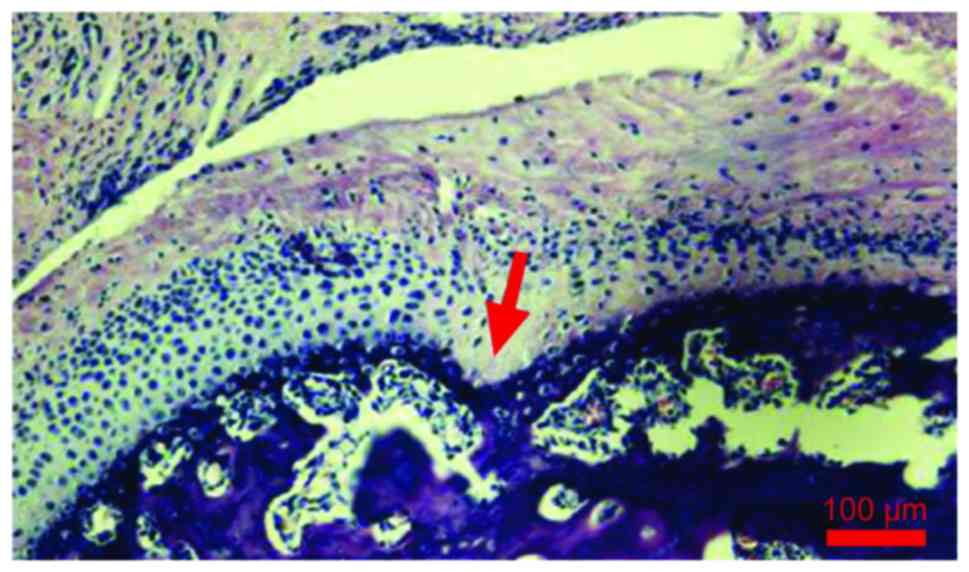
calcified cartilage from the non-calcified cartilage. The condyle
tissue full thickness, fiber layer thickness and calcified
cartilage layer thickness were increased with the time and the
difference was statistically significant (P<0.05) (Fig. 3 and Table
I). BMD, Tb.Th and Tb.Sp increased with time, BS/BV and Tb.N
were decreased with time and the difference was statistically
significant (P<0.05) (Table
II).
 | Table I.Histological condyle tissue
observation (µm). |
Table I.
Histological condyle tissue
observation (µm).
| Items | 4 weeks | 8 weeks | 16 weeks | F-value | P-value |
|---|
| Full-layer
thickness | 166.4±23.5 | 183.2±26.8 | 221.7±30.3 | 6.569 | 0.012 |
| Fiber layer
thickness | 45.5±7.2 | 51.2±7.6 | 56.8±7.7 | 6.238 | 0.015 |
| Calcified cartilage
layer thickness | 71.4±8.3 | 78.2±8.5 | 84.4±8.6 | 6.425 | 0.013 |
 | Table II.Micro-CT scanning of condyle
tissue. |
Table II.
Micro-CT scanning of condyle
tissue.
| Items | 4 weeks | 8 weeks | 16 weeks | F-value | P-value |
|---|
| BMD
(mg/cm3) | 412.3±56.5 | 526.4±62.3 | 729.2±75.8 | 12.532 | <0.001 |
| BS/BV(mm) | 42.5±4.7 | 33.6±4.2 | 25.2±3.3 | 10.527 | <0.001 |
| Tb.N (mm) | 11.5±2.3 | 8.6±1.7 | 6.2±1.4 |
8.624 | <0.001 |
| Tb.Th (mm) | 0.04±0.01 | 0.06±0.02 | 0.10±0.03 |
9.466 | <0.001 |
| Tb.Sp (mm) | 0.05±0.01 | 0.06±0.01 | 0.07±0.01 |
7.452 | <0.001 |
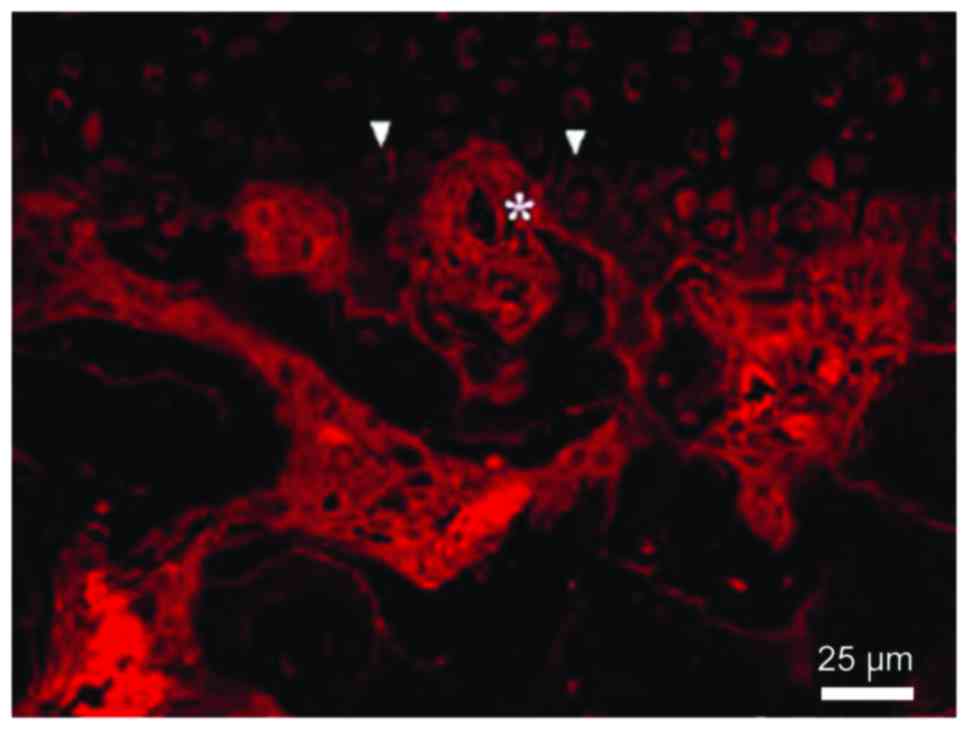
Distribution and number of new blood
vessels
The vessel could reach the deep layer of the
calcified cartilage until the breakthrough of the tide line and the
invasion of non-calcified cartilage. The number of vessels was
increased significantly with time (P<0.05) (Fig. 4 and Table III).
 | Table III.Comparison of the number of new blood
vessels (vessel/HP). |
Table III.
Comparison of the number of new blood
vessels (vessel/HP).
| Items | 4 weeks | 8 weeks | 16 weeks | F-value | P-value |
|---|
| Number of blood
vessels ending in the calcified cartilage layer | 15.3±4.2 | 17.2±4.6 | 19.6±4.8 | 7.629 | <0.001 |
| Number of blood
vessels ending in the non-calcified cartilage layer |
1.5±0.3 |
2.2±0.5 |
3.7±0.9 | 9.432 | <0.001 |
The proliferation and differentiation
functions of chondrocytes
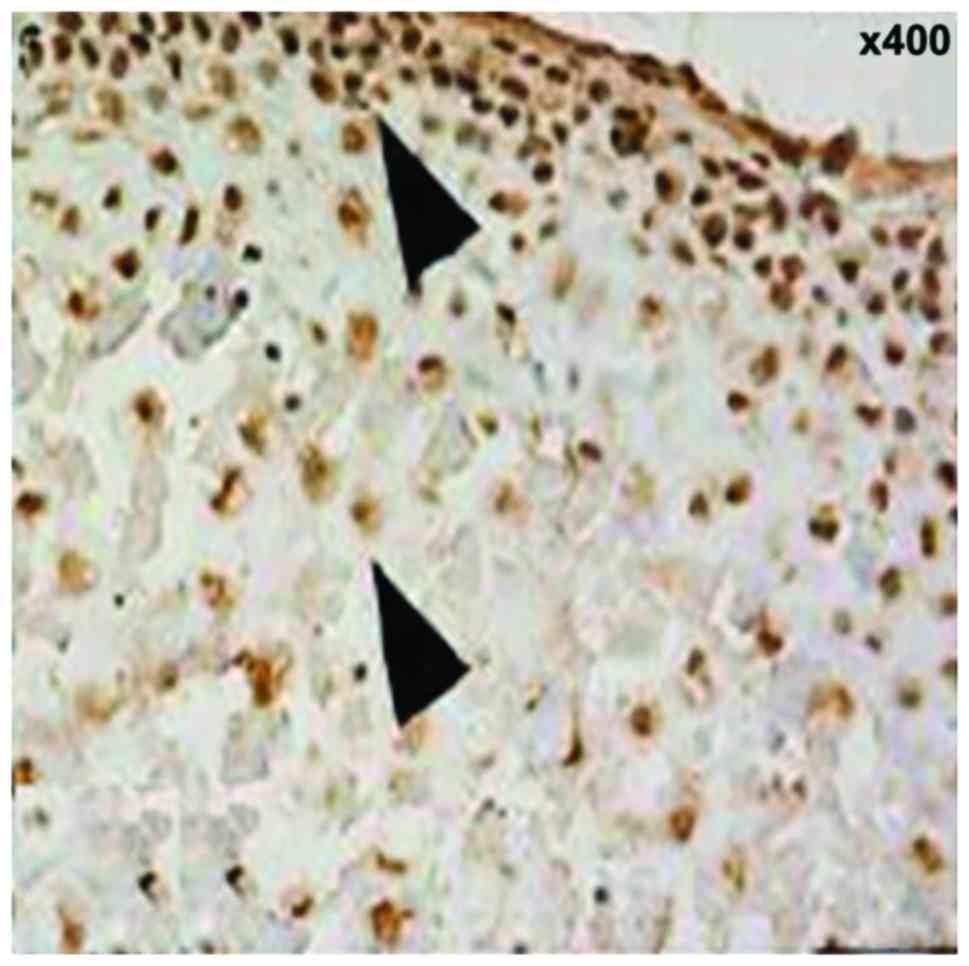
Ki67 showed brown, or brown nuclei, which were
gradually extended from the fiber layer and the proliferation layer
to the maturity layer and hypertrophic layer. The proliferation
rate was increased with time and the difference was statistically
significant (12.5±3.3, 15.7±3.6 and 18.4±3.7%, F=7.648, P<0.001)
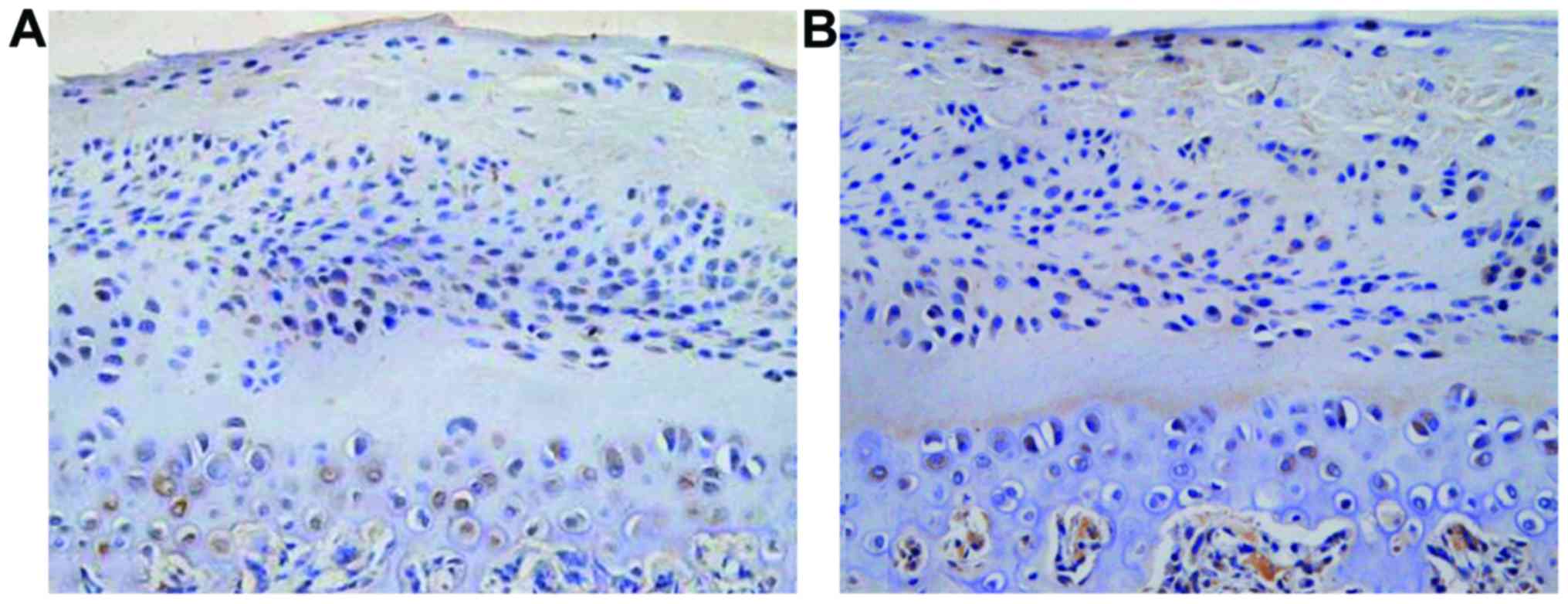
(Fig. 5). PTHrP and IHH were
distributed mainly in shallow layer of condylar cartilage
hypertrophy, the positive rate was increased with time and the
difference was statistically significant (22.4±3.6, 35.6±5.2 and
56.4±7.3%, F=11.427, P<0.001); (16.7±3.2, 22.5±4.4 and
43.6±6.6%), F=10.326, P<0.001) (Fig.
6).
Degradation functions of cartilage
matrix and expression of vascular growth promotion and inhibition
factor
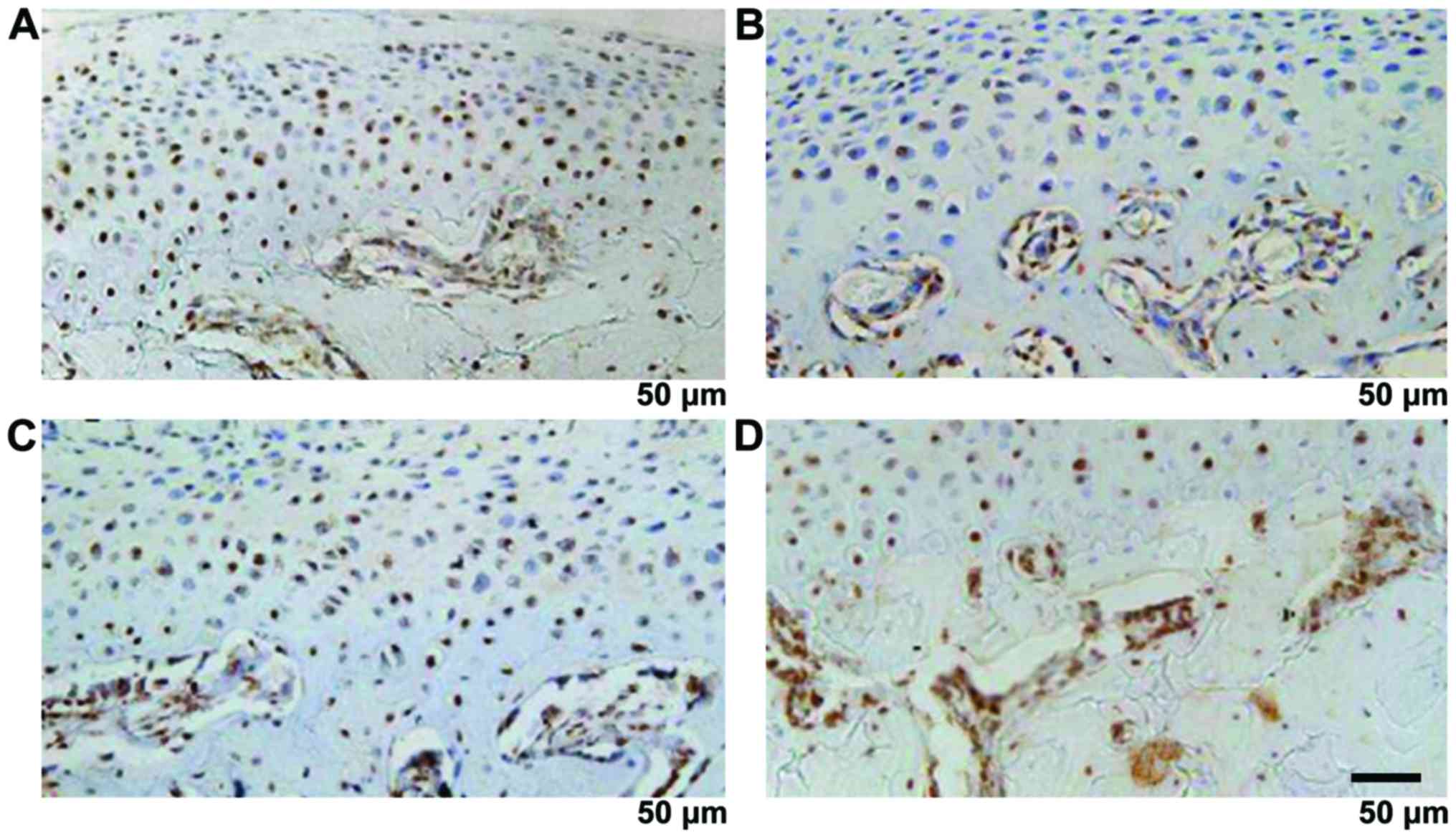
MMP-9, VEGF and CTGF were mainly expressed in the
cartilage proliferative layer and hypertrophy layer, the brown
staining was mainly seen in the nucleus and cytoplasm. The strong
CHM-1 expression was in condylar cartilage full layer and was
clearly expressed in the hypertrophy cartilage deep layer close to
the bone cartilage boundary. MMP-9, VEGF, CTGF and CHM-1 were
increased significantly with time (P<0.05) (Fig. 7 and Table
IV).
 | Table IV.Positive expression rates of MMP-9,
VEGF, CTGF and CHM-1 (%). |
Table IV.
Positive expression rates of MMP-9,
VEGF, CTGF and CHM-1 (%).
| Items | 4 weeks | 8 weeks | 16 weeks | F-value | P-value |
|---|
| MMP-9 | 5.3±1.1 | 6.7±1.2 | 8.2±1.3 | 7.451 | <0.001 |
| VEGF | 3.2±0.5 | 3.6±0.7 | 4.4±0.8 | 6.957 | <0.001 |
| CTGF | 4.3±0.6 | 4.5±0.7 | 4.9±0.8 | 5.865 | <0.001 |
| CHM-1 | 4.5±1.2 | 6.6±1.4 | 8.3±1.6 | 7.702 | <0.001 |
Screening of differentially expressed
genes by gene chip analysis method
TMJOA can upregulate 12 kinds of differentially
expressed genes and downregulate seven kinds of differentially
expressed genes, among which VEGF, CTGF and CHM-1 mRNA can
upregulate the differentially expressed genes (Fig. 8).
Discussion
The new blood vessel of condylar cartilage plays an
important role in the occurrence and development of TMJOA,
elucidating that its mechanism has important theoretical
significance and clinical guidance effect. There are no blood
vessels in the normal condylar cartilage, and its physiological
metabolism relies on the support of the synovial fluid and adjacent
tissues containing rich blood vessels, while the stability of such
a system is essential for the joint tissue integrity and strong
biological force with standing. Under normal physiological
conditions, the articular cartilage has the ability to resist the
invasion of blood vessels, but OA cartilage seems to have lost such
ability (5). The early blood vessels
of OA from subchondral bone can be invaded into the calcified
cartilage and can gradually spread to the cartilage of the
superficial layer with the progression of the disease. The blood
vessel invasion can destroy the integrity of the boundary of
articular cartilage and subchondral bone, the vascular endothelial
cells can secrete a variety of factors simultaneously, thereby
regulating the growth and metabolism of cartilage cells and may
directly involve in the degradation of cartilage matrix (6). It has been believed that the thinning
of OA articular cartilages is not only associated with the
degeneration of the cartilage tissue itself, but also associated
with the bone formation in the cartilage proceeded continuously at
the boundary of bone and cartilage, while the angiogenesis is an
important condition for endochondral bone formation and osteophyte
formation (7). Recent studies also
showed that the level of vascularization of the articular cartilage
may reflect the degeneration of cartilage and severity of OA
clinical symptoms (8). Joint pain is
considered to be an important feature of OA, and there are no
neural tissues in the cartilage under normal conditions. But with
the destruction of bone-cartilage boundary, the sensory nerves may
be invaded into the cartilage tissue with new blood vessels and
simultaneously such nerve tissue that are growing more sensitive to
pain (9). If the angiogenesis of the
articular cartilage is inhibited, the pain symptom of OA will also
be greatly reduced. Therefore, the study on the new blood vessels
between the articular cartilage and subchondral bone under TMJOA
and the finding of the key link and key signal transduction
molecules therein is greatly significant for in-depth understanding
of the occurrence and development TMJOA.
TMJOA condylar cartilage vascular invasion is a
complex process involving multiple-cell proliferation, migration,
degradation, extracellular matrix degradation and remodeling and
may be associated with the disequilibrium of factors regulating
blood vessels, which illustrates the angiogenic factor promotion
and inhibition role and is regarded as an important breakthrough
for studying the TMJOA articular cartilage angiogenesis. The reason
for the start of vascular invasion leading to cartilage
degeneration are still unclear. It is speculated that due to the
effect of the abnormal stress, the blood vessel can break through
the calcified cartilage and tidemark and damage the
microenvironment of articular cartilage independent from the
subchondral bone (10). On the other
hand, the intravascular cells can secrete various factors and cause
damage on the chondrocyte normal physiological functions (11). Further, the vascular invasion is also
a physical disruption for the normal arrangement of the chondrocyte
and collagen fibers (12). The in
vitro experiment also discloses that the vascular endothelial
cells can generate a hypertrophy promotion effect by cascade
reaction similar to the complement system through the secretion of
serine proteases, cysteine proteases and aspartic proteases
(13). It can be seen that the
vascular invasion may promote the cartilage calcification or
ossification and the vascular endothelial cells may regulate the
functions of cartilage cells and promote the degradation of the
cartilage matrix. The condylar cartilage can inhibit the disability
of the vascular invasion mechanism, including promoting the
angiogenic promotion factor expression upregulation and angiogenic
inhibition factor expression downregulation or loss of expression
may be one of the key links. The studies have shown that there is a
high expression of VEGF in OA articular cartilage (14). VEGF is an angiogenic promotion factor
with powerful roles, which may directly or indirectly be involved
in every link of angiogenesis. VEGF is also considered as an
important regulatory factor during the condylar cartilage growth
and development. Under normal physiological conditions, the
hypertrophic chondrocyte can secrete VEGF, promote the invasion of
new blood vessels and raise a lot of broken cartilage chondrocytes
to absorb the cartilage, while it may be also accompanied by a
large number of osteoblasts, promoting new bone deposition.
However, the OA cartilage cells under lesions can secrete VEGF,
promote the adjacent normal cartilage cells to secrete MMP,
interleukin-1β and other inflammatory cytokines (15). The stress change is one of the causes
of OA, in rat models with TMJOA established in abnormal occlusal
disorder, VEGF is mainly expressed in the chondrocytes of the
mature layer and hypertrophic layer of the condylar cartilage and
its expression is enhanced with the extension of the force
application time. The increase in calcified layer osteoclasts may
be associated with VEGF. In addition, the level of expression of
articular cartilage VEGF in patients with OA is positively
correlated with the cartilage vascularization density (16). It can be seen that VEGF may play a
variety of biological effects such as promoting angiogenesis during
the TMJOA development.
However, there is still a certain distance from the
site where VEGF is expressed to the bone, the cartilage boundary,
angiogenesis has synergistic effects on multiple factors and there
may be other more important factors promoting angiogenesis. We also
found that the blood vessel invasion is closely associated with the
area losing collagen, which prompts that under abnormal stress, the
change of cartilage matrix is conductive to the invasion of blood
vessels into the articular cartilage. Therefore, the finding that
there are more effective angiogenic promotion factors and collagen
matrix degradation promotion factors in TMJ joints is helpful in
clarifying the formation mechanism of TMJOA new blood vessels.
We showed that the condyle tissue full layer
thickness, fiber layer thickness and calcified cartilage thickness
were increased with time and the difference was statistically
significant. BMD, Tb.Th and Tb.Sp were significantly increased with
time, BS/BV and Tb.N were significantly decreased with time. The
new blood vessels can reach the calcified cartilage deep layer
until the breakthrough of the tide line and invasion into the
non-calcified cartilage. The number of the blood vessels was
increased with time and the difference was statistically
significant. Ki67, PTHrP and IHH-positive rates were increased with
time and the difference was statistically significant. MMP-9, VEGF,
CTGF and CHM-1 were increased with time and the difference was
statistically significant. VEGF, CTGF and CHM-1 mRNA were
upregulated differentially expressed genes. This study did not set
a control group, which has been described in previous studies in
detail. The study mainly analyzed the formation and development
process of the new blood vessels in TMJOA models over time, found
the distribution characteristics and growth changes, proliferation
and differentiation of chondrocytes and matrix degradation and
changes in vascular growth factor promotion and inhibition and
verified them through the differential gene expression. In
comparison with other models of acute injury, TMJOA model was more
consistent with the analysis on whether the better therapeutic
effect was provided against TMJOA through target point intervention
during the course of the body stress chronic disease
progression.
In conclusion, the new blood vessels may be
important in pathogenesis of TMJOA and could be potential targets
for intervention.
References
|
1
|
Bechtold TE, Saunders C, Decker RS, Um HB,
Cottingham N, Salhab I, Kurio N, Billings PC, Pacifici M, Nah HD,
et al: Osteophyte formation and matrix mineralization in a TMJ
osteoarthritis mouse model are associated with ectopic hedgehog
signaling. Matrix Biol. 52(54): 339–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka E, Aoyama J, Miyauchi M, Takata T,
Hanaoka K, Iwabe T and Tanne K: Vascular endothelial growth factor
plays an important autocrine/paracrine role in the progression of
osteoarthritis. Histochem Cell Biol. 123:275–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lingaraj K, Poh CK and Wang W: Vascular
endothelial growth factor (VEGF) is expressed during articular
cartilage growth and re-expressed in osteoarthritis. Ann Acad Med
Singapore. 39:399–403. 2010.PubMed/NCBI
|
|
4
|
Hayashi Y, Takei H and Kurosumi M: Ki67
immunohistochemical staining: the present situation of diagnostic
criteria. Nihon Rinsho. 70 Suppl 7:428–432. 2012.(In Japanese).
PubMed/NCBI
|
|
5
|
Ludin A, Sela JJ, Schroeder A, Samuni Y,
Nitzan DW and Amir G: Injection of vascular endothelial growth
factor into knee joints induces osteoarthritis in mice.
Osteoarthritis Cartilage. 21:491–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XY, Chen Y, Tang XJ, Jiang LH and Ji
P: AMD3100 attenuates matrix metalloprotease-3 and −9 expressions
and prevents cartilage degradation in a monosodium
iodo-acetate-induced rat model of temporomandibular osteoarthritis.
J Oral Maxillofac Surg. 74:927.e1–927.e13. 2016. View Article : Google Scholar
|
|
7
|
Walsh DA, Bonnet CS, Turner EL, Wilson D,
Situ M and McWilliams DF: Angiogenesis in the synovium and at the
osteochondral junction in osteoarthritis. Osteoarthritis Cartilage.
15:743–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tibesku CO, Daniilidis K, Skwara A,
Paletta J, Szuwart T and Fuchs-Winkelmann S: Expression of vascular
endothelial growth factor on chondrocytes increases with
osteoarthritis - an animal experimental investigation. Open Orthop
J. 5:177–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Y, Jin K, Childs JT, Xie L, Mao XO and
Greenberg DA: Vascular endothelial growth factor-B (VEGFB)
stimulates neurogenesis: evidence from knockout mice and growth
factor administration. Dev Biol. 289:329–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walsh DA, McWilliams DF, Turley MJ, Dixon
MR, Fransès RE, Mapp PI and Wilson D: Angiogenesis and nerve growth
factor at the osteochondral junction in rheumatoid arthritis and
osteoarthritis. Rheumatology (Oxford). 49:1852–1861. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jansen H, Meffert RH, Birkenfeld F,
Petersen W and Pufe T: Detection of vascular endothelial growth
factor (VEGF) in moderate osteoarthritis in a rabbit model. Ann
Anat. 194:452–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fazaeli S, Ghazanfari S, Everts V, Smit TH
and Koolstra JH: The contribution of collagen fibers to the
mechanical compressive properties of the temporomandibular joint
disc. Osteoarthritis Cartilage. 24:1292–1301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morjen M, Honoré S, Bazaa A,
Abdelkafi-Koubaa Z, Ellafi A, Mabrouk K, Kovacic H, El Ayeb M,
Marrakchi N and Luis J: PIVL, a snake venom Kunitz-type serine
protease inhibitor, inhibits in vitro and in vivo angiogenesis.
Microvasc Res. 95:149–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Cao W, Wei K, Liu X, Xu Y, Yang
C, Undt G, Haddad MS and Chen W: Expression of VEGF-receptors in
TMJ synovium of rabbits with experimentally induced internal
derangement. Br J Oral Maxillofac Surg. 51:69–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YL, Li XJ, Qin RF, Lei DL, Liu YP, Wu
GY, Zhang YJ, Yan-Jin Wang DZ and Hu KJ: Matrix metalloproteinase
and its inhibitor in temporomandibular joint osteoarthrosis after
indirect trauma in young goats. Br J Oral Maxillofac Surg.
46:192–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fransès RE, McWilliams DF, Mapp PI and
Walsh DA: Osteochondral angiogenesis and increased protease
inhibitor expression in OA. Osteoarthritis Cartilage. 18:563–571.
2010. View Article : Google Scholar : PubMed/NCBI
|