Introduction
Carbon tetrachloride (CCl4) is an
experimental hepatotoxicant, able to induce acute liver injury
characterized by necrosis and steatosis (1,2). Once
the metabolism of CCl4 is initiated, its toxicity is
determined by chain chemical reactions that take place in the body.
The initial step reaction is a reduction and heterolytic cleavage
of CCl4 to form a trichloromethyl radical
(•CCl3) (3). This radical
reacts either directly with cellular macromolecules or with oxygen;
reaction with the latter forms the trichloromethylperoxyl radical
(•OOCCl3), which acts more rapidly on lipids than does
•CCl3 (4). Production of
excessive amounts of reactive oxygen species (ROS) generates
oxidative stress which induces major cellular perturbations, such
as alterations in the structures of protein and nucleic acids,
increases in intracellular free calcium levels, perturbation of
membrane permeability, lipid peroxidation and finally cell death
(5). In order to minimize the
effects of ROS, cells have an antioxidant defense system which
includes non-enzymatic antioxidants and enzymes such as superoxide
dismutase, catalase and glutathione peroxidase (6). CCl4 administration
destabilizes this defense system and decreases superoxide
dismutase, catalase and glutathione peroxidase activity (7).
Berberis vulgaris L. (Berberidaceae) (Bv) has
been well known worldwide for its healing properties for >2,500
years (8). The bioactive components
are represented by several alkaloid constituents, such as
berberine, berbamine and palmatine, which confer healing properties
to Berberis extracts (9).
Berberine is the most important isoquinoline alkaloid, obtained
mostly from the roots and bark of Berberis sp. (10). Berberine is known for its multiple
pharmacological properties, such as antimicrobial (11), antitumor (12) and anti-inflammatory effects (13,14).
Berberine is also known for its dose-dependent hepatoprotective
effects on CCl4-induced liver damage, due to its
antioxidant effects (15).
This study was carried out to evaluate for the first
time the increased protective effect of a formulation of Bv bark
extract in β-cyclodextrin (β-CD) against CCl4-induced
cytotoxicity in Huh7 cells. This natural complex was designed for
use in oral formulations, in order to increase the solubility,
dissolution, bioavailability, safety and stability of the extract
via certain properties of β-CD, including its resistance to
hydrolysis by human salivary and pancreatic amylases (16).
Materials and methods
Complex of Bv bark extract and
β-CD
Samples of Bv were collected from the Botanical
Garden of Vasile Goldis Western University of Arad (Arad, Romania)
during October 2008, and certified at the herbarium within the
Faculty of Natural Sciences, where a voucher specimen already
exists. The synthesis and characterization of the Bv bark
extract/β-cyclodextrin complex used in this study have been
previously reported by the authors (2).
Cell culture
The study was carried out using as model a Huh7
human hepatoma cell line (American Type Culture Collection,
Manassas, VA, USA). The Huh7 cell line was chosen due to its
metabolic similarities to normal hepatocytes and in order to avoid
the variability and short life spans of primary human hepatocytes
(17). Cells were grown in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, Irvine,
UK) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich,
Steinheim, Germany), and 1% penicillin-streptomycin (Pen/Strep,
10,000 IU/ml; PromoCell GmbH, Heidelberg, Germany) in a humidified
atmosphere with 5% CO2, at 37°C.
Cell treatment
Cells were plated at a density of 104
cells/cm2 with DMEM medium (high glucose, supplemented
with 10% FCS) and allowed to attach overnight at 37°C. The
CCl4 concentration (0.1 mM) used for cell culture
co-treatment was previously determined and chosen due to its
ability to induce up to 75% cell culture mortality. Three
concentrations (5, 7.5 and 10 µg/ml) of unformulated and
nanoencapsulated β-CD Bv bark extract were tested for protection
against CCl4-induced cytotoxicity in the Huh7 cell line.
Each experiment was performed in triplicate under 48 h of exposure.
Stock solutions were prepared fresh to avoid oxidation. The Bv
extracts were dissolved in dimethylsulfoxide (DMSO) and diluted
with DMEM to the desired concentrations prior to use, while DMSO
alone was used as vehicle control.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cytotoxicity assays
The MTT assay was used to detect the cytotoxicity of
unformulated and formulated Bv extract. Cells were seeded into
96-well plates and allowed to attach overnight. A series of
unformulated and formulated extracts were added (5, 7.5 and 10
µg/ml), alone or together with 0.1 mM CCl4, followed by
48 h incubation. All experiments were conducted in parallel with a
control. The MTT assay was performed using a commercially available
MTT assay (MTT base; Sigma-Aldrich, St. Louis, MO, USA) according
to the manufacturer's protocol. The absorbance (abs) was measured
at 565 nm, using a Tecan Infinite F200 microplate reader (Tecan,
Männedorf, Switzerland). The cell survival rate was calculated as
follows: Survival rate (%) = (Abs treatment - abs blank)/(Abs
control - Abs blank) × 100.
Caspase-3 and −7 activities
Total caspase-3 and −7 activities were measured
using an Apo-ONE Homogeneous Caspase-3/7 assay kit (Promega
Corporation, Madison, WI, USA). Following the various treatments,
100 µl Apo-ONE Caspase-3/7 reagent (substrate and buffer in the
ratio of 1:100) were added to each well of a 96-well plate. After 1
h incubation in the dark at room temperature, the fluorescence of
each well was measured at 485–520 nm (Fluoroskan Ascent FL; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Immunofluorescence
Expression levels of caspase-3 and peroxisome
proliferator-activated receptor-γ (PPARγ) were assessed by
immunofluorescence using a rabbit polyclonal caspase-3 antibody
(orb10273; Biorbyt Ltd., Cambridge, UK) 1:200 dilution, or PPARγ
antibody (sc-7196; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
1:50 dilution and AlexaFluor 488-labeled chicken anti-rabbit IgG
secondary antibody (A-21441; Invitrogen; Thermo Fisher Scientific,
Inc.). Cells were treated with unformulated and nanoencapsulated
β-CD extract for 48 h prior to fixing with cold 100% methanol at
−20°C, for 10 min. The cells treated under experimental conditions
were fixed with cold 100% methanol at −20°C for 10 min. Blocking of
non-specific binding was conducted by incubation with 1% bovine
serum albumin (Antibodies Online Inc., Atlanta, GA, USA) and 0.1%
Triton X (Sigma-Aldrich; Merck Millipore) for 1 h at room
temperature. Following blocking, cells were incubated overnight at
4°C with corresponding dilutions of the primary antibodies.
Following overnight incubation, the cells were washed in PBS and
incubated with secondary antibody for 1 h at room temperature.
Nucleus counterstaining was performed with 10 µg/ml Hoechst 33285
working solution (861405; Sigma-Aldrich, Buchs, Switzerland) for 60
sec, followed by analysis using a Leica TCS SP8 confocal microscope
(Leica Microsystems GmbH, Wetzlar, Germany). The fluorescence
intensity of PPARγ- and caspase 3-positive hepatocytes was
quantified by automated counting performed using an image analysis
system (ImageJ; National Institutes of Health, Bethesda, MD, USA).
Three fields were selected randomly from each section, and a total
of 100 hepatocytes were evaluated in every field.
Oil Red O staining
Intracellular accumulation of lipid droplets was
observed using Oil Red O staining. Cells were plated on Lab-Tek
Chamber Slides (Nalge Nunc International, Penfield, NY, USA) for 24
h. After 24 h of seeding, cells were treated with unformulated and
β-CD nanoencapsulated Bv extract, respectively, for 48 h. For
staining, the cells were fixed with 4% formaldehyde for 10 min at
4°C. The fixed cultures were incubated with Oil Red O working
solution for 20 min at room temperature. Oil Red O was provided in
a staining kit (04–220923; Bio Optica, Milan, Italy). Nucleus
counterstaining was performed with Mayer's hematoxylin provided in
the same kit (04–220923; Bio Optica). After washing with tap water,
the Permanox slides were mounted. Analysis was performed using an
Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan).
Electron microscopy
For electron microscopy, 1×106 cells were
treated with unformulated and β-CD nanoencapsulated Bv extracts for
48 h. Following the drug treatments, cells were collected by
trypsinization, followed by centrifugation at 240 × g. The
obtained pellet was prefixed in 2.7% glutaraldehyde solution in 0.1
M phosphate buffer for 1.5 h, at 4°C, then washed in 0.15 M
phosphate buffer (pH 7.2) and post-fixed in 2% osmic acid solution
in 0.15 M phosphate buffer for 1 h at 4°C. Dehydration was
performed in acetone, followed by inclusion in the epoxy embedding
resin Epon 812. Blocks 70-nm thick were cut using an LKB
ultramicrotome. The sections were doubly contrasted with solutions
of uranyl acetate and lead citrate and analyzed under a Tecnai 12
Biotwin transmission electron microscope (FEI, Hillsboro, OR,
USA).
Statistical analysis
Statistical analysis of the differences between the
means of various treatment groups was performed using one-way
analysis of variance. STATA statistical software (version 13.0;
StataCorp LP, College Station, TX, USA) was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of unformulated and β-CD
nanoencapsulated Bv extract on CCl4-induced cytotoxicity
in Huh7 cells
To analyze the protective advantage of β-CD
nanoencapsulated Bv extract against CCl4-induced
cytotoxicity, cell viability in the Huh7 cell line was
investigated. The cells were incubated with equivalent doses of
formulated and unformulated Bv extract (5, 7.5 and 10 µg/ml Bv
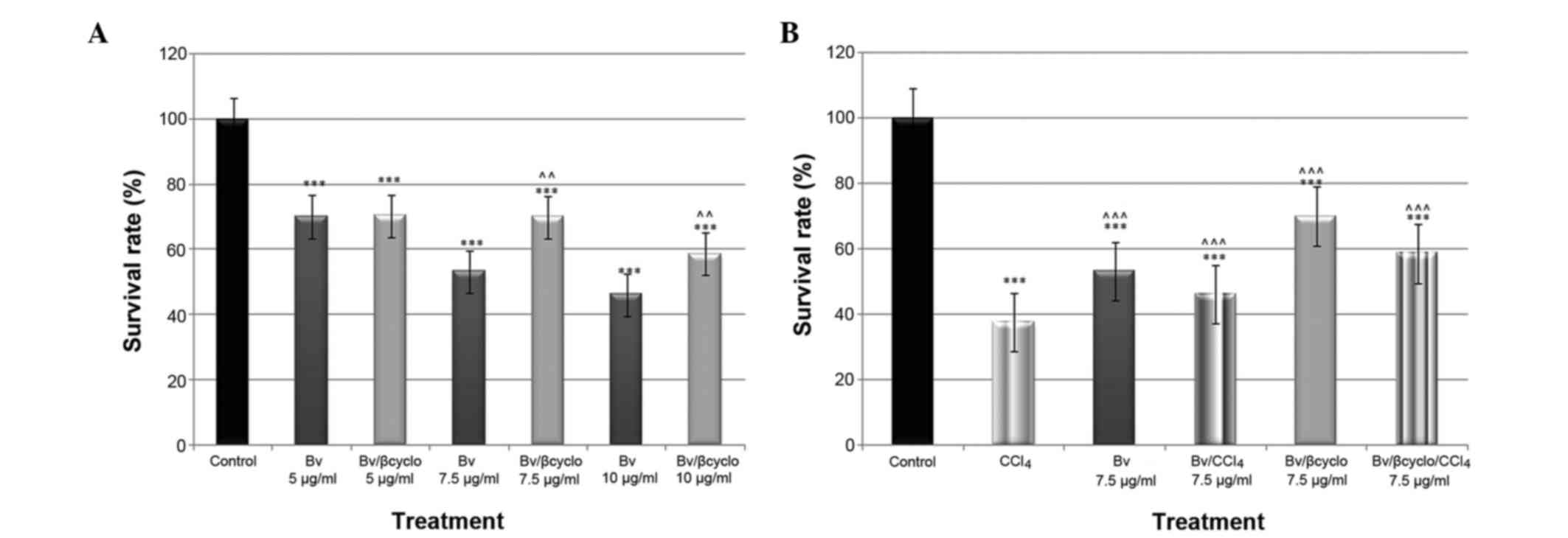
extract) for 48 h. As shown in Fig.
1A, the unformulated extract presented a dose-dependent
significant cytotoxicity (P<0.001), whereas the formulated one
did not. Due to the fact that the dose of 7.5 µg/ml extract
presented moderate toxicity, it was chosen for the assessment of
antioxidant activity. Following co-exposure of the cells to
CCl4 and 7.5 µg/ml Bv nanoencapsulated extract, the cell
viability was 1.25 fold higher compared with that of cells
co-exposed to CCl4 and non-formulated Bv extract
(Fig. 1B).
Effcts of unformulated and β-CD
nanoencapsulated Bv extract on CCl4.-induced Huh7 cell
apoptosis
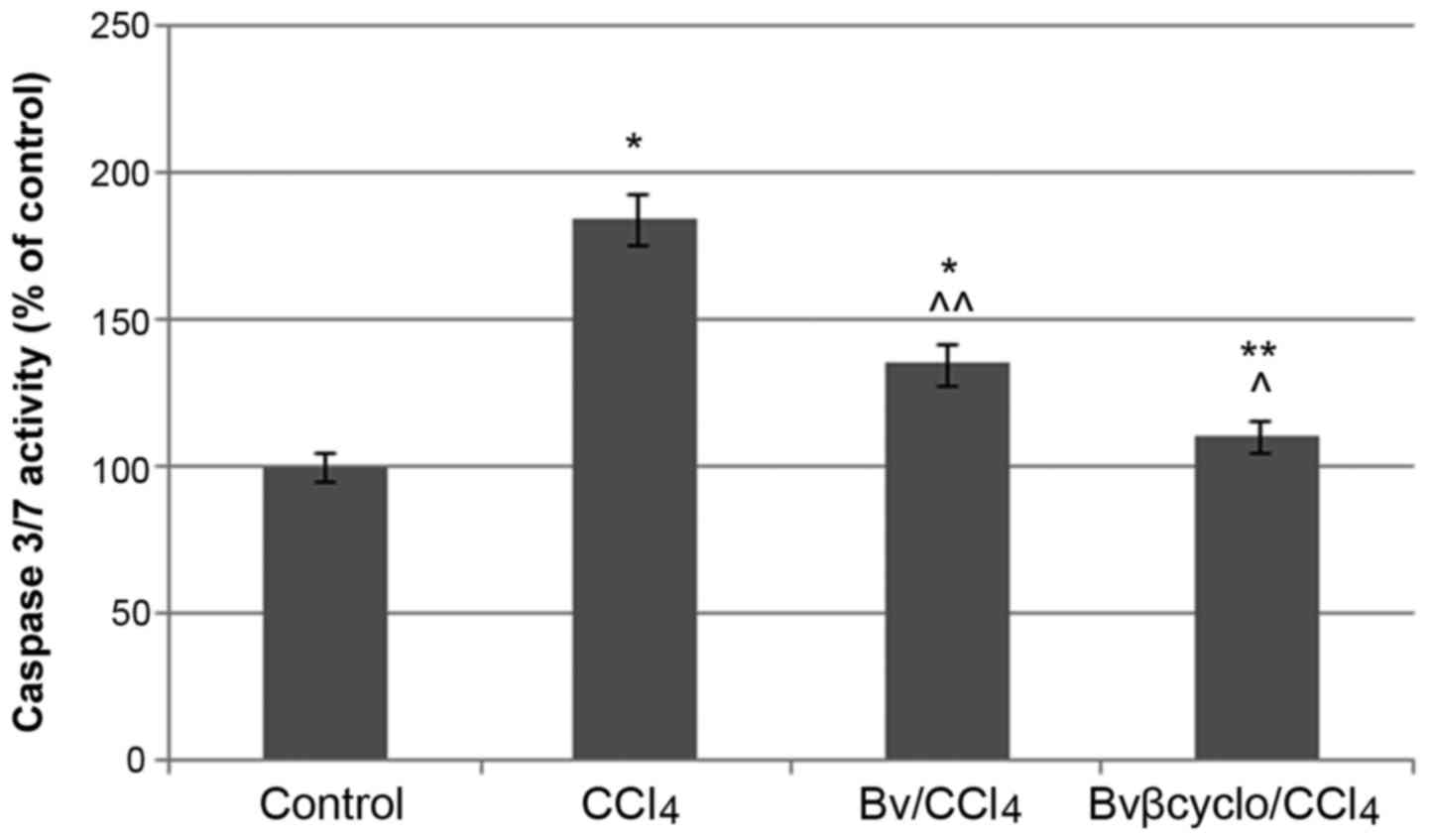
In order to investigate the anti-apoptotic effects
of Bv extracts, the caspase-3/7 activity (Fig. 2) as well as caspase-3 expression in
control and experimental variants (Fig.
3) were evaluated. The caspase-3/7 activity increased
significantly (P<0.05) by ~80% in the CCl4-treated
cells compared with the control, whereas co-treatment with
unformulated and formulated BV extract decreased it by 50 and 70%,
respectively.
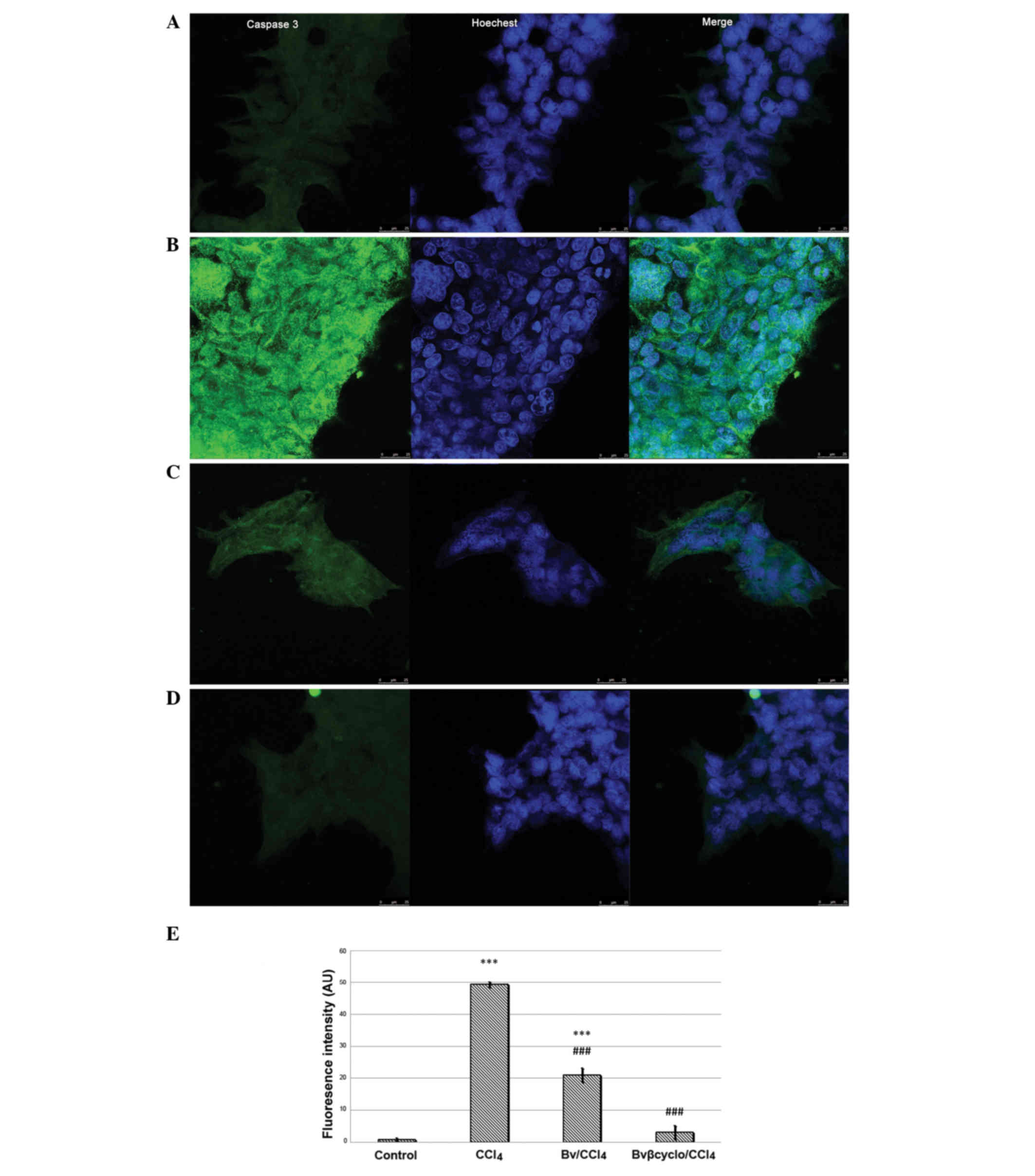
Analysis of confocal microscopy images showed that
caspase-3 immunostaining was significantly increased in
CCl4-treated cells compared with control cells. Under
co-exposure to CCl4 with both formulated and
unformulated Bv extract, signals were significantly reduced
(P<0.001) compared with those in the CCl4-treated
cells, and were reduced most strongly with the formulated Bv
extract (P<0.001; Fig. 3).
Effects of unformulated and β-CD
nanoencapsulated Bv extracts on CCl4.-induced fatty
accumulation in Huh7 cells
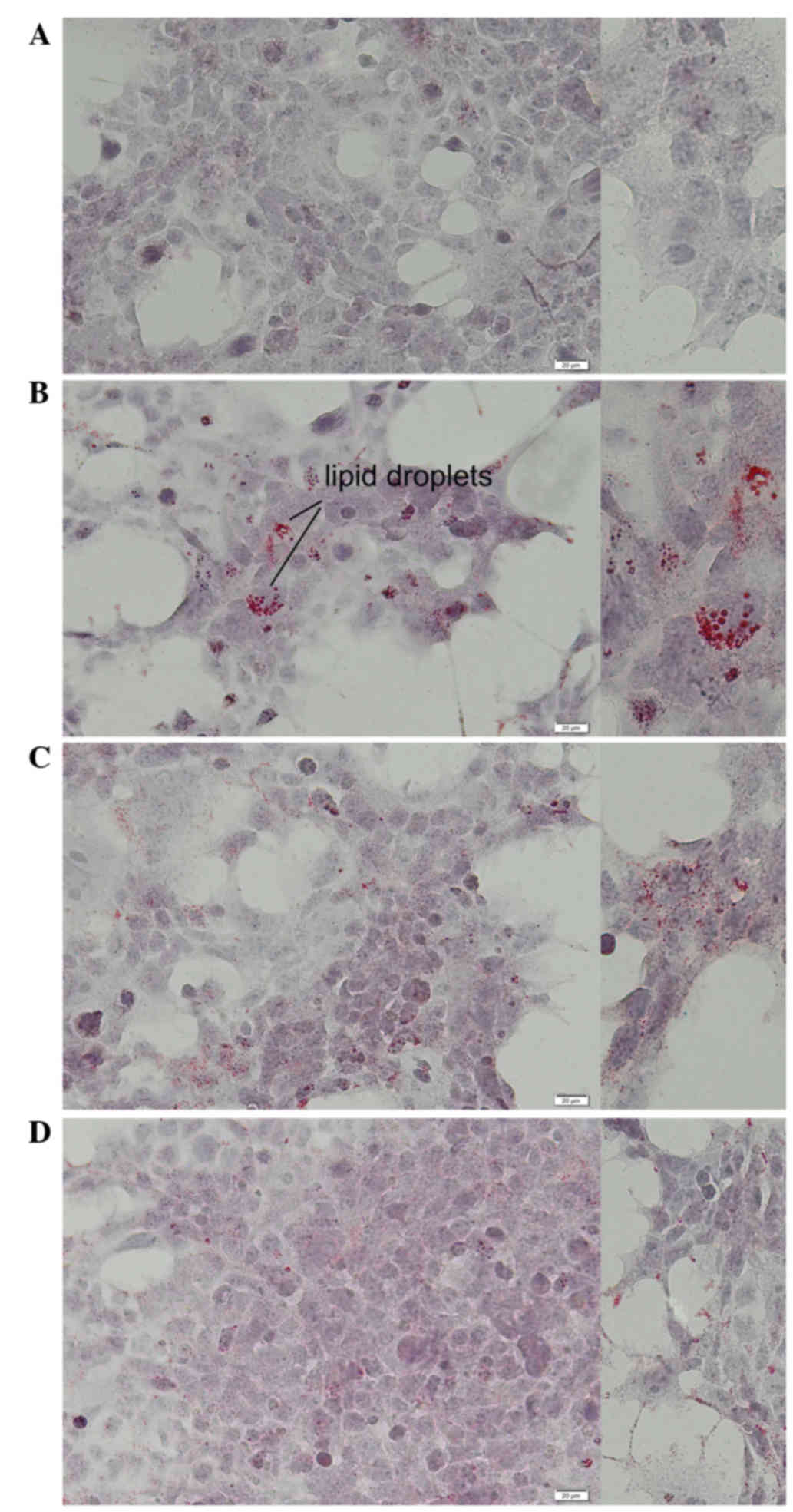
Light microscopic evaluation of Huh7 cells stained
with Oil Red O showed normal morphology of Huh7 cells and few
accumulated intracellular lipid droplets in the control (Fig. 4A). Intracytoplasmic lipids increased
significantly in the CCl4-treated cells without Bv
extract, compared with the control (Fig.
4B). After 48 h of co-treatment with CCl4 and either
Bv extract, HuH7 cells accumulated fewer lipid droplets compared
with cells treated with CCl4 alone (Fig. 4C and D). In addition, the level of
PPARγ expression decreased significantly (P<0.01) in
CCl4-treated cells (Fig.
5), compared with control cells, and this reduction was
attenuated in the cells co-treated with unformulated or formulated
Bv extracts (Fig. 5).
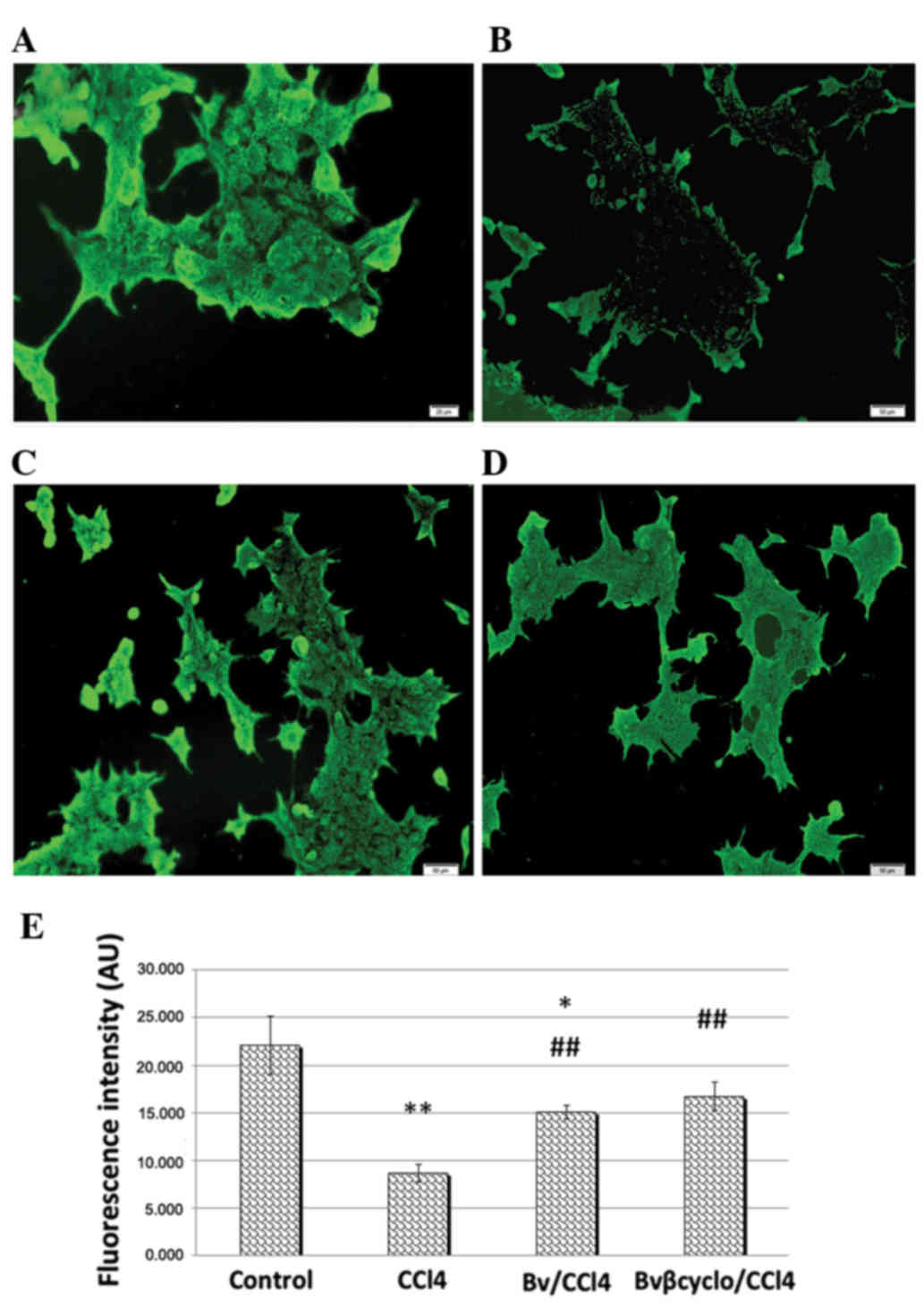 | Figure 5.PPARγ expression after 48 h of
CCl4 exposure. Representative images showing (A) control
Huh7 cells, (B) Huh7 cells treated with 0.1 mM CCl4, (C)
Huh7 cells treated with 0.1 mM CCl4 and 7.5 µg/ml
unformulated Berberis vulgaris extract and (D) Huh7 cells treated
with 0.1 mM CCl4 and 7.5 µg/ml formulated Berberis
vulgaris/β-cyclodextrin complex. PPARγ expression decreased visibly
in CCl4-treated cells, compared with control cells, and
the reduction was attenuated in cells treated with CCl4
and unformulated/β cyclodextrine Berberis vulgaris extracts.
Original magnification, ×200. (E) Bar chart showing the average
fluorescence intensity of the Huh7 cell immunostaining for PPARγ
expression in experimental variants (A-D). Data are presented as
mean ± standard deviation for triplicates. *P<0.05 and
**P<0.01 vs. the control group; ##P<0.01 vs. the
CCl4-treated group. PPARγ, peroxisome
proliferator-activated receptor γ; Bv, Berberis vulgaris; Bvβcyclo,
Bv complexed with β-cyclodextrin; CCl4, carbon
tetrachloride; AU, absorbance units. |
Effects of unformulated and β-CD
nanoencapsulated Bv extract on CCl4.-induced
ultrastructural injuries of Huh7 cells
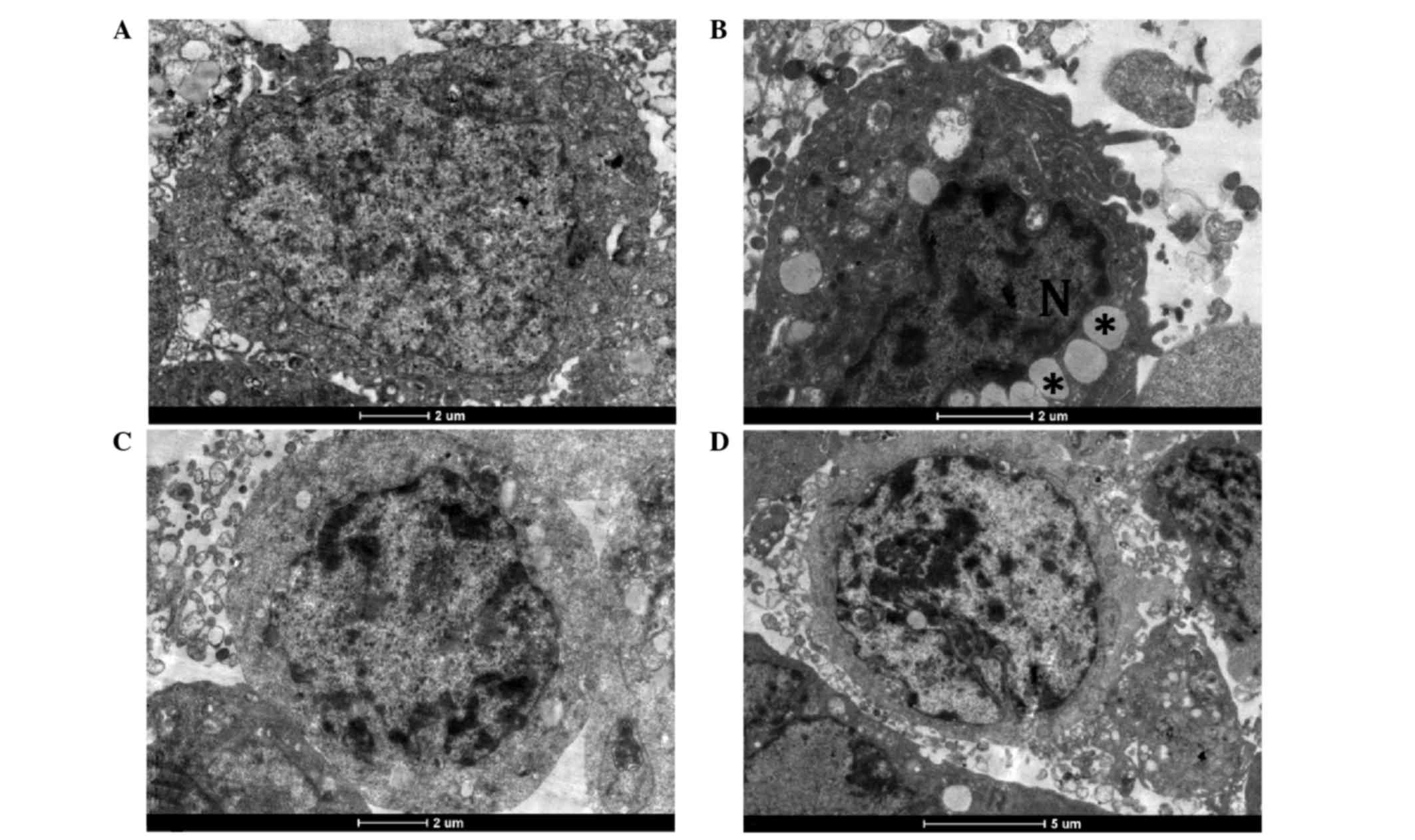
The ultrastructure of Huh7 cells was normal in the
control group. Control cells showed integrated nuclear membranes,
relatively homogeneous chromatin, and organelles with a normal
appearance (Fig. 6A). Following
treatment with 0.1 mM CCl4 for 48 h, Huh7 cells were
characterized by irregular nuclear shapes, chromatin condensation
into dense granules or blocks, vacuolar cytoplasm, and lipid
droplet accumulation, together with altered mitochondria and
rarefied cristae (Fig. 6B).
Co-treatment with unformulated and formulated Bv extracts protected
the hepatocyte ultrastructure from CCl4-induced injury;
in the two BV groups the appearance of the Huh7 cells was similar
to that of the control cells (Fig. 6C
and D).
Discussion
Numerous herbs are used in traditional medicine to
prevent and treat liver diseases as a result of hepatotoxicant
action. Barberry root, bark, leaves and fruit have been used in
traditional medicine to treat hepatic disorders, and berberine is
generally thought to be responsible for their beneficial effects
(18). Berberine itself is
considered a potent antioxidant with a wide spectrum of
applications (19); the biological
activities and levels of safety should be established for the whole
Bv extract and its formulations. Another important compound in
barberry is berbamine, a bis-benzylisoquinoline alkaloid, which
inhibits chemically-induced carcinogenesis (20).
To demonstrate the increased protective potential of
β-CD nanoencapsulated Bv extract compared with unformulated Bv
extract against CCl4-induced cytotoxicity, an MTT assay
was performed in the present study. The notable cell mortality
observed with formulated and unformulated extracts could be
attributable to their content of berbamine, which inhibits
Ca2+/calmodulin-dependent protein kinase II in liver
cancer cells (21). CCl4
caused a reduction in cell viability of 62.43% compared with
control. It is likely that a 7.5 µg/ml dose of unformulated or
formulated extract presented a sufficient quantity of berberine to
act as an antioxidant. The β-CD nanoencapsulated Bv extract
displayed 1.25-fold higher protective effects compared with the
unformulated form, probably due to its increased solubility.
Previously, it has been demonstrated that the solubility of
berberine hydrochloride was increased 5.27-fold by cyclodextrin
complexation (22). In addition,
Ziolkowski et al (23) have
shown depletion of mitochondrial cholesterol in the presence
methyl-β-cyclodextrin, which was associated with impaired
bioenergetics and increased resistance to calcium chloride-induced
swelling and subsequently prevented changes associated with early
stage apoptosis.
In the present study 7.5 µg/ml Bv extract/β-CD
complex exhibited no cytotoxicity whereas Kiss et al
(24) showed that significant cell
toxicity was induced by 50 mM β-CD; this difference in cytotoxicity
may be explained by the CD concentration used being 75-fold lower
than that in the previous study.
Apoptosis has been shown to be important in
CCl4-induced hepatotoxicity (25). In the present study, apoptosis was
confirmed by the detection of caspase-3 activation and
ultrastructural analysis. CCl4 induced activation of the
apoptotic signaling pathway via caspase-3, as shown in Figs. 2 and 3, consistent with previous in vivo
studies that have demonstrated a similar apoptotic mechanism for
this toxic agent (26). In addition,
cell death could be initiated by the ability of CCl4 to
arrest Huh7 cells in the G0/G1 phase. Also, it could decrease the
proportion of cells in the S phase, which is associated with
inhibition of DNA synthesis (27).
Co-treatment with Bv/β-cyclodextrin complex significantly increased
(P<0.001) the percentage of viable cells, which suggests that it
reversed the cell cycle by increasing the proportion of cells in
the S phase and inhibited the apoptotic process by suppression of
CCl4-induced caspase-3 activation.
ROS generated by CCl4 exposure can cause
changes in the inner mitochondrial membrane and loss of the
mitochondrial transmembrane potential (28). As a result, cytochrome c and
other mitochondrial proteins are released into the cytosol
(29), activating caspase-9 and
subsequently caspase-3, which cleaves a set of vital proteins and
promotes the apoptotic degradation phase including DNA damage and
morphological changes (30).
Apoptosis was also investigated by electron
microscopy in the present study. The ultrastructural changes that
were observed indicated the apoptosis of Huh7 cells due to the
presence of CCl4, results that are in agreement with
other results on HepG2 hepatocarcinoma cells exposed to fatty acid
ethyl esters (31) or sarsasapogenin
(32). In the present study, both
formulated and unformulated Bv extracts were able to prevent
ultrastructural injuries induced by CCl4 exposure.
Lipid droplets represent intracellular compartments
able to store neutral lipids as triglycerides and cholesteryl
esters. Each lipid droplet is surrounded by a phospholipid
monolayer (33). The accumulation of
neutral lipids (mainly triglycerides and cholesterol esters) into
hepatocyte cytoplasm is associated with hepatic steatosis. This
abnormal accumulation of lipid droplets may result from the release
of free fatty acids (FFAs) by adipocytes, dietary intake,
diminished hepatic export of FFAs, increased de novo
lipogenesis or impaired β-oxidation of FFAs (34). In the present study, a massive
accumulation of lipid droplets in the cytoplasm was observed after
48 h of CCl4 toxic exposure. The high increase in lipid
concentration within the CCl4-treated Huh7 cells can be
attributed to a reduction in fatty acid activation and cholesterol
catabolism (35). Toxic injuries due
to poisoning with compounds like CCl4 are expressed by
fatty infiltration of the liver due to changes in low-density lipid
synthesis (3,5,7). The use
of unformulated and formulated Bv extracts exhibited a very good
protective effect against the formation of lipid droplets due to
CCl4 exposure, with slightly increased protective
effects in the case of formulated Bv extracts.
PPARγ plays an important role as a regulator of
lipid metabolism and is a key mediator of lipid storage and
inflammation (36). The results of
the current study present a number of intriguing observations.
Firstly, in the CCl4-treated group intracellular lipid
accumulation was observed, whereas a reduction in PPARγ expression
was evidenced. It has previously been demonstrated that the PPARγ2
isoform regulates intracellular lipid stores (37), whereas PPARγ1 regulates the
expression of inflammatory cytokines through inhibition of nuclear
factor (NF)-κB (37). Studies have
shown a decreased number of PPARγ-positive hepatocytes (26) and an increased number of hepatocytes
expressing NF-κB and tumor necrosis factor (TNF)-α following
CCl4 treatment (38). The
severity of liver lesions has been found to be correlated with a
reduction in the number of hepatocytes expressing PPARγ (38). In addition, results of another study
indicated that the PPARγ1 isoform was suppressed while, in
contrast, PPARγ2 levels remained unchanged and finally peaked at 8
weeks after CCl4 exposure (37). Considering the results of the present
and previous studies, it may be speculated that the decreased
expression of PPARγ due to CCl4 toxic activity could be
followed by an increased inflammatory response mediated via NF-κB
and TNF-α, which leads subsequently to indirect intracellular lipid
accumulation and apoptosis. A recovery of PPARγ expression was
observed following co-exposure with Bv extract, particularly with
nano-encapsulated Bv extract, which may also contribute to the
protective effect against CCl4-induced apoptosis,
whereas protection of the mitochondrial membrane potential by PPARγ
overexpression through the upregulation of anti-apoptotic Bcl-2
family proteins has previously been demonstrated (39).
Formulated and unformulated Bv extract were both
efficient against the anti-proliferative and pro-apoptotic actions
of CCl4 through suppression of CCl4-induced
caspase-3 activation and lipid accumulation. The protective effect
was more pronounced following exposure to the formulated β-CD
extract compared with the unformulated one, which is probably due
to the increased solubility of the complex form, which assured that
mitochondrial bioenergetics were balanced.
In conclusion, Bv extract complexed with β-CD
protects Huh7 cells against apoptosis and cytotoxicity induced by
CCl4 and represents a more potent liver protector
against toxic chemical substances and drugs than does the pure
extract.
Acknowledgements
The present study was supported by strategic grant
POSDRU/159/1.5/S/133391, Project ‘Doctoral and Post-doctoral
programs of excellence for highly qualified human resources
training for research in the field of Life sciences, Environment
and Earth Science’ cofinanced by the European Social Found within
the Sectorial Operational Program Human Resources Development
2007–2013.
References
|
1
|
Fallah HH, Zareei MA, Ziai SA, et al: The
effects of Taraxacum officinale L. and Berberis
vulgaris L. root extracts on carbon tetrachloride induced liver
toxicity in rats. J Med Plants. 9:45–52. 2010.
|
|
2
|
Hermenean A, Popescu C, Ardelean A, Stan
M, Hadaruga N, Mihali CV, Costache M and Dinischiotu A:
Hepatoprotective effects of Berberis vulgaris L. extract/β
cyclodextrin on carbon tetrachloride-induced acute toxicity in
mice. Int J Mol Sci. 13:9014–9034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber LW, Boll M and Stampfl A:
Hepatotoxicity and mechanism of action of haloalkanes: Carbon
tetrachloride as a toxicological model. Crit Rev Toxicol.
33:105–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruch RJ, Klaunig JE, Schultz NE, Askari
AB, Lacher DA, Pereira MA and Goldblatt PJ: Mechanism of
chlorophorm and carbon tetrachloride toxicity in primary cultured
mouse hepatocytes. Environ Health Perspect. 69:301–305. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Videla LA: Oxidative stress signaling
underlying liver disease and hepatoprotective mechanisms. World J
Hepatol. 1:72–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balahoroğlu R, Dülger H, Özbek H and
Bayram I Şekeroğlu: Protective effects of antioxidants on the
experimental liver and kidney toxicity in mice. Eur J Gen Med.
5:157–164. 2008.
|
|
7
|
Manibusan MK, Odin M and Eastmond DA:
Postulated carbon tetrachloride mode of action: A review. J Environ
Sci Health C Environ Carcinog Ecotoxicol Rev. 25:185–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arayne MS, Sultana N and Bahadur SS: The
Berberis story: Berberis vulgaris in therapeutics. Pak J
Pharm Sci. 20:83–92. 2007.PubMed/NCBI
|
|
9
|
Ivanovska N and Philipov S: Study on the
anti-inflamatory action of Berberis vulgaris root extract,
alkaloid fractions and pure alkaloids. Int J Immunopharmacol.
18:553–561. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh A, Duggal S, Kaur N and Singh J:
Berberine. Alkaloid with wide spectrum of pharmacological
activities. J Nat Prod (Gorakhpur). 3:64–75. 2010.
|
|
11
|
Freile ML, Giannini F, Pucci G, Sturniolo
A, Rodero L, Pucci O, Balzareti V and Enriz RD: Antimicrobial
activity of aqueous extracts and of berberine isolates from
Berberis heterophylla. Fitoterapia. 74:702–705. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iizuka N, Miyamoto K, Okita K, Tangoku A,
Hayashi H, Yosino S, Abe T, Morioka T, Hazama S and Oka M:
Inhibitory effect of Coptidis rhizoma and berberine on the
proliferation of human esophageal cancer cell lines. Cancer Lett.
148:19–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo CL, Chi CW and Liu TY: The
anti-inflamatory potential of berberine in vitro and in vivo.
Cancer Lett. 203:127–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kupeli E, Koşar M, Yeşilada E, Hüsnü K and
Başer C: A comparative study on the anti-inflamatory,
antinociceptive and antipyretic effects of isoquinoline alkaloids
from the root of turkish Berberis species. Life Sci.
72:645–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Y, Siu KY, Ye X, Wang N, Yuen MF,
Leung CH, Tong Y and Kobayashi S: Hepatoprotective effects of
berberine on carbon tetrachloride-induced acute hepatotoxicity in
rats. Chin Med. 5:332010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasheed A, Kumanr A and Sravahthi V:
Cyclodextrins as drug carrier molecule: A review. Sci Pharm.
76:567–598. 2008. View Article : Google Scholar
|
|
17
|
Guo L, Dial S, Shi L, Branham W, Liu J,
Fang JL, Green B, Deng H, Kaput J and Ning B: Similarities and
differences in the expression of drug-metabolizing enzymes between
human hepatic cell lines and primary human hepatocytes. Drug Metab
Dispos. 39:528–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutical effects of Berberis
vulgaris and its active constituent, berberine. Phytother Res.
22:999–1012. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang BJ, Xu D, Guo Y, Ping J, Chen LB and
Wang H: Protection by and anti-oxidant mechanism of berberine
against rat liver fibrosis induced by multiple hepatotoxic factors.
Clin Exp Pharmacol Physiol. 35:303–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda K, Hibiya Y, Mutoh M, Koshiji M,
Akao S and Fujiwara H: Inhibition of berberine of cyclooxygenase-2
transcriptional activity in human colon cancer cells. J
Ethnopharmacol. 66:227–233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Z, Li T, Ma X, Wang X, Van Ness C,
Gan Y, Zhou H, Tang J, Lou G, Wang Y, et al: Berbamine inhibits the
growth of liver cancer cells and cancer-initiating cells by
targeting Ca2+/calmodulin-dependent protein kinase II.
Mol Cancer Ther. 12:2067–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S, Wang Y, Shang X, et al: Formulation
of berberine hydrochloride and hydroxypropyl-β-cyclodextrin
inclusion complex with enhanced dissolution and reduced bitterness.
Trop J Pharm Res. 11:871–877. 2012.
|
|
23
|
Ziolkowski W, Szkatula M, Nurczyk A,
Wakabayashi T, Kaczor JJ, Olek RA, Knap N, Antosiewicz J,
Wieckowski MR and Wozniak M: Methyl-beta-cyclodextrin induces
mitochondrial cholesterol depletion and alters the mitochondrial
structure and bioenergetics. FEBS Lett. 584:4606–4610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiss T, Fenyvesi F, Bácskay I, Váradi J,
Fenyvesi E, Iványi R, Szente L, Tósaki A and Vecsernyés M:
Evaluation of the cytotoxicity of β-cyclodextrin derivatives:
Evidences for the role of cholesterol extraction. Eur J Pharm Sci.
40:376–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamel HH, Azza HA, Walaa MSA and Amira HM:
Protective effect of some antioxidants against
CCl4-induced toxicity in liver cells from BRL3A cell
line. J Am Sci. 6:992–993. 2010.
|
|
26
|
Guo XL, Liang B, Wang XW, Fan FG, Jin J,
Lan R, Yang JH, Wang XC, Jin L and Cao Q: Glycyrrhizic acid
attenuates CCl4-induced hepatocyte apoptosis in rats via
a p53-mediated pathway. World J Gastroenterol. 19:3781–3791. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Tao X, Li L, Sun A, Wang Y and
Zhang S: Protective effect of blueberry anthocyanins in a
CCl4-induced injury model in human embryonic liver
cells. Food Agric Immunol. 25:274–286. 2013. View Article : Google Scholar
|
|
28
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saelens X, Festjens N, Van de Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saraste A and Pulkki K: Morphological and
biomedical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aydin HH, Celik A, Deveci R, Karacali S,
Saydam G, Omay Bedii S and Batur Y: Induction of apoptosis by fatty
acid ethyl esters in HepG2 cells. Food Chem Toxicol. 43:139–145.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao W, Pan H, Lu M, Ni Y, Zhang R and Gong
X: The apoptotic effect of sarsasapogenin from Anemarrhena
asphodeloides on HepG2 human hepatoma cells. Cell Biol Int.
31:887–892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujimoto Y, Itabe H, Kinoshita T, Homma
KJ, Onoduka J, Mori M, Yamaguchi S, Makita M, Higashi Y, Yamashita
A and Takano T: Involvement of ACSL in local synthesis of neutral
lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J
Lipid Res. 48:1280–1292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peyrou M, Ramadori P, Bourgoin L and Foti
M: PPARs in liver diseases and cancer: Epigenetic regulation by
microRNAs. PPAR Res. 2012:7578032012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rakic B, Sagan SM, Noestheden M, Bélanger
S, Nan X, Evans CL, Xie XS and Pezacki JP: Peroxisome
proliferator-activated receptor alpha antagonism inhibits hepatitis
C virus replication. Chem Biol. 13:23–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toyoda M, Takagi H, Horiguchi N, Kakizaki
S, Sato K, Takayama H and Mori M: A ligand for peroxisome
proliferator activated receptor gamma inhibits cell growth and
induces apoptosis in human liver cancer cells. Gut. 50:563–567.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morán-Salvador E, Titos E, Rius B,
González-Périz A, García-Alonso V, López-Vicario C, Miquel R, Barak
Y, Arroyo V and Clària J: Cell-specific PPARγ deficiency
establishes anti-inflamatory and anti-fibrogenic properties for
this nuclear receptor in non-parenchimal liver cells. J Hepatol.
59:1045–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Orfila C, Lepert JC, Alric L, Carrera G,
Béraud M and Pipy B: Immunohistochemical distribution of nuclear
factor kappaB and peroxisome proliferated-activated receptors in
carbon tetrachloride- induced chronic liver injury in rats.
Histochem Cell Biol. 123:585–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu JS, Lin TN and Wu KK: Rosiglitazone and
PPAR-gamma overexpression protect mitochondrial membrane potential
and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family
proteins. J Cell Physiol. 220:58–71. 2009. View Article : Google Scholar : PubMed/NCBI
|