Introduction
Obesity is considered an epidemic in numerous
countries worldwide and is linked to insulin resistance, which
constitutes a principal risk factor for type 2 diabetes (1–3).
Previous studies have shown that inflammation is a key
pathophysiological process linked to obesity, insulin resistance
and type 2 diabetes (4,5). Insulin resistance is also associated
with chronic low-grade inflammation in vivo, which is
largely mediated by activated innate immune cells (6).
Resistin is a cysteine-rich, 12.5-kDa protein that
was first identified as a mediator of insulin resistance in obese
mice (7–10). Resistin promotes both inflammation
and insulin resistance associated with energy homeostasis
impairment (11). However, the key
molecular mechanisms mediating its effects are unknown. Previous
studies have investigated the function of resistin in mouse obesity
and diabetes models, and have implicated resistin in the
pathogenesis of obesity-mediated insulin resistance and type 2
diabetes (3,12,13).
Moreover, it has been reported that resistin is closely associated
with inflammation (14–16). Resistin regulates the synthesis and
secretion of proinflammatory cytokines such as tumor necrosis
factor α (TNF-α), interleukin-6 (IL-6) and IL-1β in macrophages via
a nuclear factor κB (NF-κB)-dependent pathway (17–20), but
the specific receptor of resistin in vivo has not yet been
identified. A previous study reported that the resistin-induced
inflammatory response may involve the Toll-like receptor 4 (TLR4)
signaling pathway (21). However,
another study reported that the inflammatory response induced by
resistin has no direct link with the TLR4 pathway (22). Other previous reports have stated
that resistin is related to the insulin-like growth factor 1
receptor and receptor tyrosine kinase-like orphan receptor 1 (ROR1)
(22–24). However, the specific receptor of
resistin and its signaling pathway have not yet been identified
in vivo.
NOD1 and NOD2 are cytosolic pattern recognition
receptors in vivo. Some innate immune receptors are
regulated by endogenous molecules, such as TLRs and NODs, which are
capable of inducing ‘aseptic inflammation’ (3). NOD1 and NOD2 induce the recruitment of
receptor-interacting serine/threonine-protein kinase 2 (RIP2),
which promotes NF-κB-mediated proinflammatory gene expression when
exposed to signal molecules (25).
Previous research showed that activation of NOD1 or NOD2
contributes to insulin resistance and a proinflammatory response
(26–28). On this basis, the current study
explored the relationship between resistin and NOD receptors. The
results showed that resistin increased NOD2 expression in RAW 264.7
cells, but had no effect on the expression of NOD1. Resistin also
promoted the secretion of inflammatory cytokines via the NF-κB
signaling pathway. These findings may contribute to identifying a
specific resistin receptor and understanding the underlying
mechanisms of resistin in chronic inflammation and insulin
resistance.
Materials and methods
Cell culture and stimulation
RAW 264.7 mouse monocyte cells (American Type
Culture Collection, Manassas, VA, USA) were grown in Dulbecco's
modified Eagle medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal calf serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin, 100
mg/ml streptomycin (both Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) and 2 mmol/l L-glutamine at 37°C under
5% CO2. Cells were incubated with resistin (PeproTech EC
Ltd., London, UK) at concentrations of 50, 100, 150 and 200 ng/ml
for 3, 6, 12 and 24 h to evaluate the impact of resistin treatment
on NOD pathways in RAW 264.7 cells. Cells were stimulated by
muramyl dipeptide (20 µg/ml; Sigma-Aldrich; Merck KGaA) or
diaminopimelic acid (20 µg/ml; Sigma-Aldrich; Merck KGaA) as a
positive control group, and a control group without any treatment
was also included. For inhibitor treatment, the specific inhibitor
of NF-κB, parthenolide (Sigma-Aldrich; Merck KGaA), was used. The
cell culture medium was replaced with fresh medium containing
inhibitor (25 µM) and incubated for 1 h prior to resistin (200
ng/ml) treatment.
Small interfering RNA (siRNA)
silencing
siRNA duplexes targeting the mouse NOD2 gene (NOD2
siRNA) and non-targeting siRNA (control siRNA) were purchased from
Guangzhou Ribobio Co., Ltd. (Guangzhou, China). Transfection of
NOD2-siRNA and control siRNA into RAW 264.7 cells was performed
using Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's guidelines. Transient transfections
in RAW 264.7 cells were carried out in 6-well plates, and cells
were seeded overnight at 4×105 cells per well for 6 h.
All transfections were carried out in Opti-MEM (Invitrogen, Thermo
Fisher Scientific, Inc.) medium. Cells were incubated at 37°C for
24 h after transfection prior to analysis. Protein and mRNA
expression levels of NOD2 were quantified in order to detect the
effect of the siRNA.
Determination of cytokine levels
The protein levels of IL-6, TNF-α, IL-1β and
resistin were measured by sandwich ELISA (R&D Systems, Inc.,
Minneapolis, MN, USA) using a pair of mouse antibodies, and
expressed in pg/ml.
RNA isolation and quantitative
polymerase chain reaction (qPCR)
Messenger RNA (mRNA) expression levels were
determined by qPCR. Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Total RNA (1 µg) was reverse
transcribed into cDNA using standard reagents (Takara Biotechnology
Co., Ltd., Dalian, China). The cDNA was then submitted to qPCR
analysis using specific primer pairs and an SYBR Premix Ex Taq™ II
kit (Takara Biotechnology Co., Ltd.). Sequences of
promoter-specific primers (Table I)
were designed by Primer Premier 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) and synthesized by BGI Tech
Solutions Co., Ltd. (Copenhagen, Denmark). The reaction conditions
were set to 1 min at 95°C (first segment, one cycle), 5 sec at 95°C
and 30 sec at 62°C (second segment, 39 cycles). Specific
transcripts were confirmed by melting-curve profiles (cooling the
sample to 65°C and heating slowly to 95°C with measurement of
fluorescence) at the end of each PCR cycle using a C1000 thermal
cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Relative
gene expression was defined as a ratio of target gene expression
vs. β-actin gene expression. The results were analyzed using a
2−ΔΔCq assay (29).
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Items | Sense (5′ to 3′) | Antisense (5′ to
3′) |
|---|
| NOD1 |
AGGCATTGAAGGACCACC |
AGCATCTCAGCGAAGCAC |
| NOD2 |
GCTGTCTTGGGATGTGCT |
GGATGAAGGGAGTGAGTGTC |
| RIP2 |
CCTCCTCGTGTTCCTTGGC |
GGTCCTTGTAGGTTTGGTGCT |
| IKKβ |
GTACACCGTGACCGTTGACT |
TCCACTTCGCTCTTCTGCCG |
| Resistin |
CTTCCTTGTCCCTGAACTGC |
ACGAATGTCCCACGAGCC |
| β-actin |
CTGTCCCTGTATGCCTCTG |
ATGTCACGCACGATTTCC |
Western blot analysis
Monoclonal antibodies against NOD1 (3545; 1:1,000),
NOD2 (sc-30199; 1:800), RIP2 (4982; 1:1,000), NF-κB p65 (8242;
1:1,000) and β-actin (BA2305; 1:800) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and horseradish
peroxidase (HRP)-conjugated secondary antibody (BA2305; 1:3,000)
was purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). Total cytoplasmic and nuclear protein was sequentially
extracted using a Cytoplasmic and Nuclear Protein Extraction kit
(BestBio, Inc., Shanghai, China), and protein concentrations were
calculated using bicinchoninic acid assay kits (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Protein lysates were
kept at −20°C until used for western blot analysis. Protein lysates
were fractionated through 7.5–12% SDS-PAGE and transferred to PVDF
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% fat-free milk powder at room temperature for 1 h
and immunoblotted overnight at 4°C with primary antibodies. Next
they there incubated with HRP-conjugated secondary antibody for 1 h
at room temperature. After each step, the membranes were washed 5
times with PBS with Tween for 5 min. Finally, the blots were
developed using the enhanced chemiluminescence (ECL) system (GE
Healthcare Life Sciences).
Data analysis and statistics
Statistical analysis was performed using SPSS 5
software (SPSS, Inc., Chicago, IL USA). Analysis of variance was
used to analyze differences among the groups. Data are expressed as
the mean ± standard error of the mean of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Resistin treatment increases NOD2
expression in RAW 264.7 cells
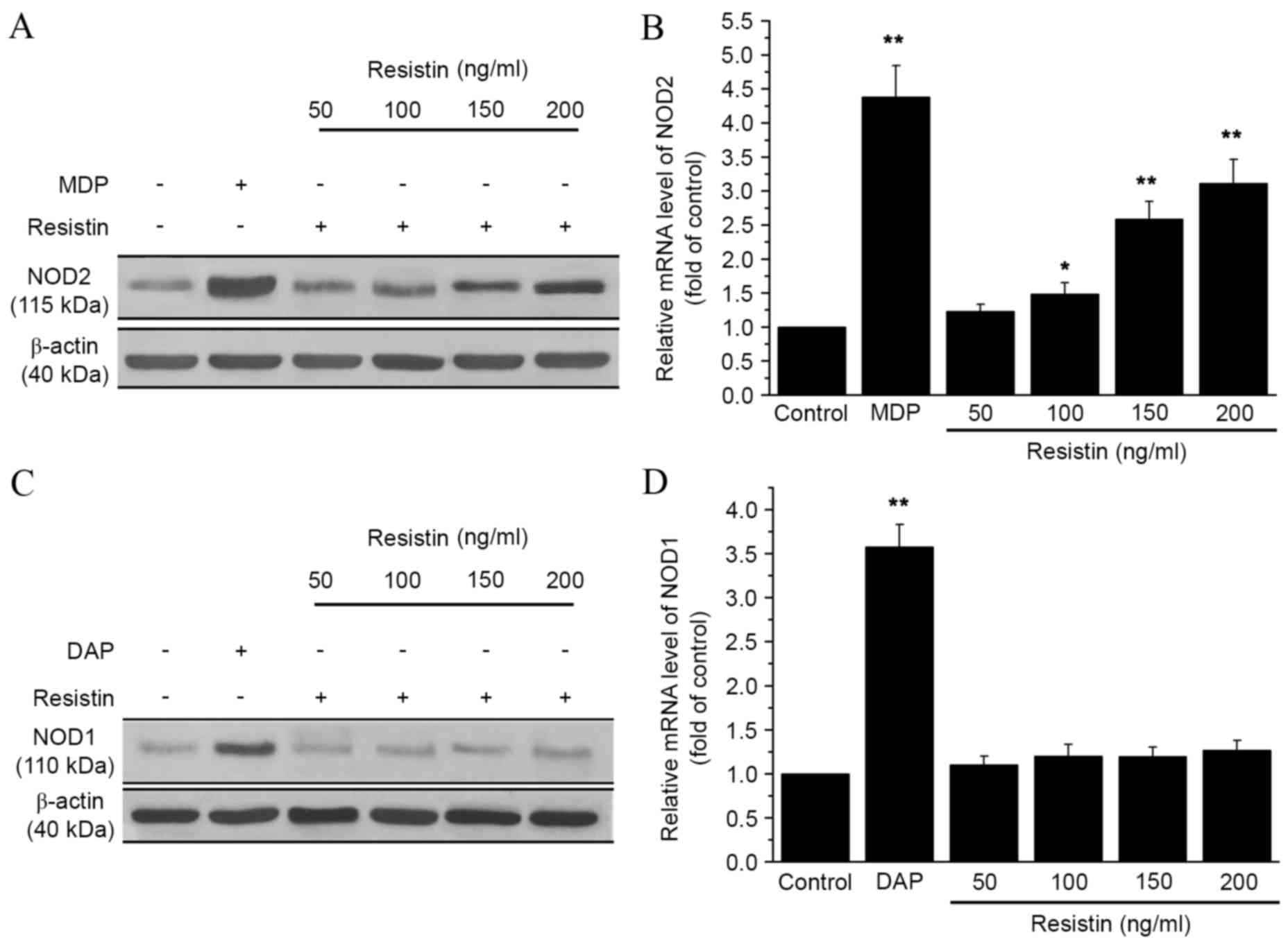
To explore the relationship between resistin and
NOD, the mRNA and protein expression levels of NOD1 and NOD2 were
analyzed in RAW 264.7 cells by qPCR and western blot analysis.
Cells were incubated with different concentrations of resistin (50,
100, 150 or 200 ng/ml). As shown in Fig.
1A, NOD2 protein expression increased following treatment with
resistin, as compared with the control. Furthermore, NOD2 mRNA
expression significantly increased following treatment with
resistin at concentrations of 100, 150 and 200 ng/ml (P<0.05,
P<0.01 and P<0.01, respectively; Fig. 1B), as compared with the control.
However, the expression of NOD1 did not change significantly as
compared with the control. The expression level of NOD2 reached its
peak at 12 h after adding resistin compared with the control group
(P<0.01; Fig. 2). These data
indicated that resistin treatment increased the expression of NOD2,
but not NOD1.
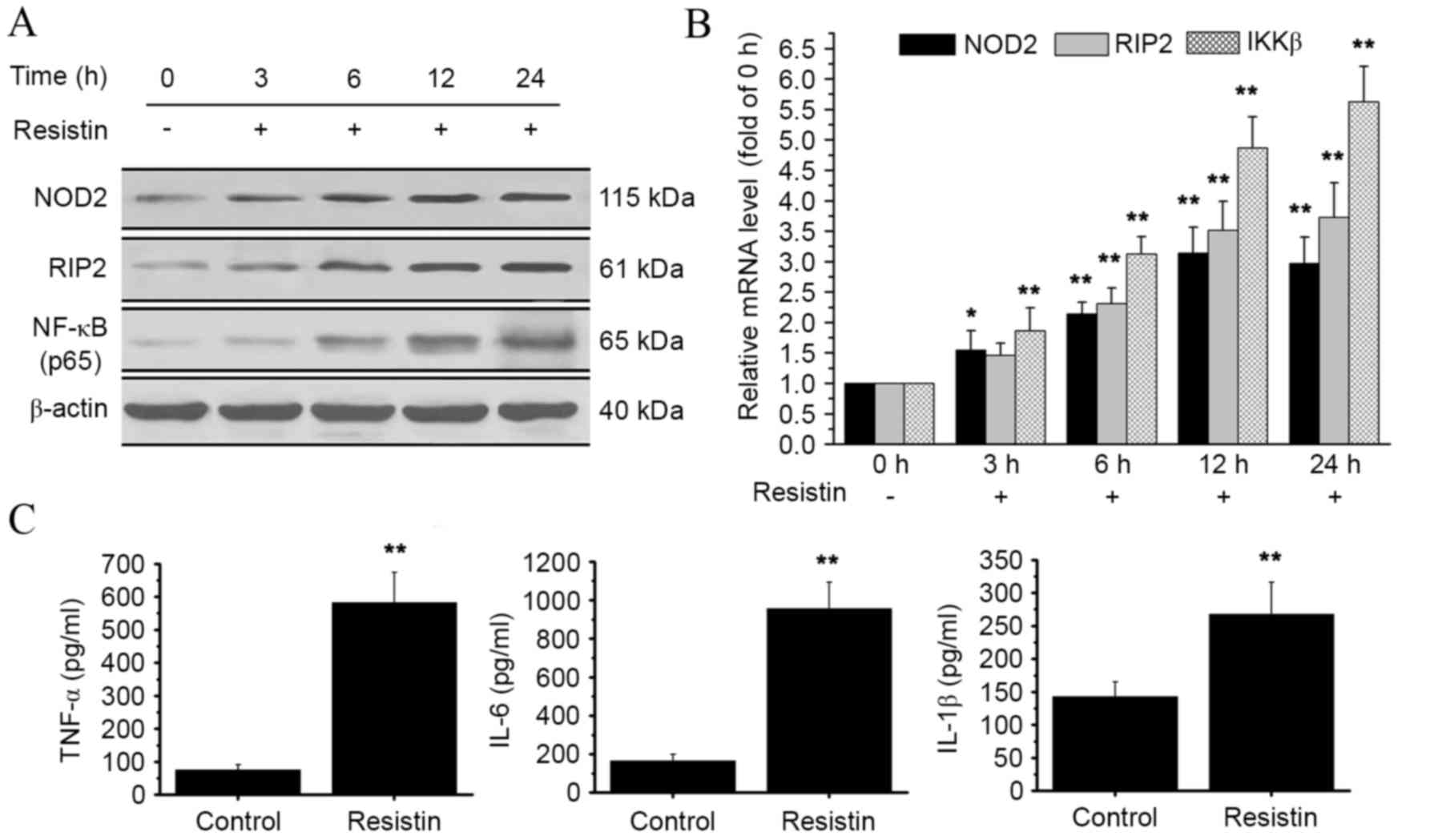 | Figure 2.Effect of resistin treatment on
NOD2-NF-κB pathways. RAW 264.7 cells were treated with resistin
(200 ng/ml) for 0, 3, 6, 12 and 24 h. (A) Protein levels of NOD2,
RIP2 and NF-κB p65 were detected by western blot analysis. The
NF-κB p65 protein was obtained from the nucleus. (B) Relative mRNA
levels of NOD2, RIP2 and IKKβ were analyzed using qPCR at the end
of each experimental period of resistin treatment. mRNA levels at
3, 6, 12 and 24 h were calculated as a fold change of the mRNA
level at 0 h. (C) At the end of the experimental period of resistin
treatment, the cell supernatant was collected and the levels of key
proinflammatory cytokines (TNF-α, IL-6 and IL-1β) were detected by
ELISA. *P<0.05 and **P<0.01 vs. control. NOD2,
nucleotide-binding oligomerization domain-containing protein 2;
NF-κB, nuclear factor-κB; RIP2, receptor-interacting
serine/threonine-protein kinase 2; IKKβ, inhibitor of NF-κB kinase
subunit β; TNF-α, tumor necrosis factor α; IL, interleukin; qPCR,
quantitative polymerase chain reaction. |
Resistin treatment activates
NOD2-NF-κB pathways
To investigate the potential impact of resistin on
NOD2-NF-κB pathways, the expression levels of downstream signaling
molecules, RIP2 and inhibitor of NF-κB kinase subunit β (IKKβ),
were determined by qPCR and western blot analysis. The western blot
data showed an increase in RIP2 protein expression, and an increase
of NF-κB in the nucleus was also observed (Fig. 2A). At the mRNA level, there was a
significant increase in both RIP2 and IKKβ expression at 6, 12 and
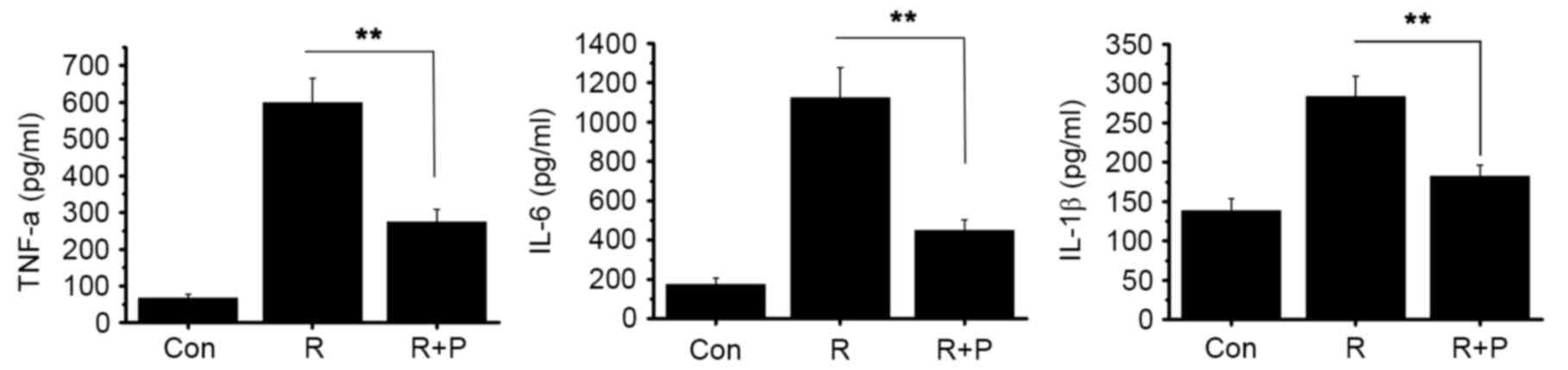
24 h, as compared with the control (P<0.01; Fig. 2B). Furthermore, we evaluated the
effect of resistin treatment on the expression of three key
proinflammatory cytokines: TNF-α, IL-6 and IL-1β. There was a
significant increase in the protein levels of TNF-α, IL-6 and IL-1β
as compared with the control (P<0.01, Fig. 2C). These data indicate that the
stimulation of resistin activates NOD2-NF-κB pathways.
SiRNA-mediated NOD2 knockdown impairs
resistin-induced activation of NOD2 pathways, but has no effect on
the nuclear translocation of NF-κB
To investigate the potential link between resistin
and NOD2-NF-κB proinflammatory pathways and assess the effect of
NOD2 on resistin signaling, NOD2-siRNA was used to generate
NOD2-deleted RAW 264.7 cells (Fig.
3). Treatment with NOD2-siRNA resulted in a significant
decrease in both NOD2 protein level (P<0.01; Fig. 3A) and NOD2 mRNA level (P<0.01;
Fig. 3C), as compared with treatment
with control siRNA.
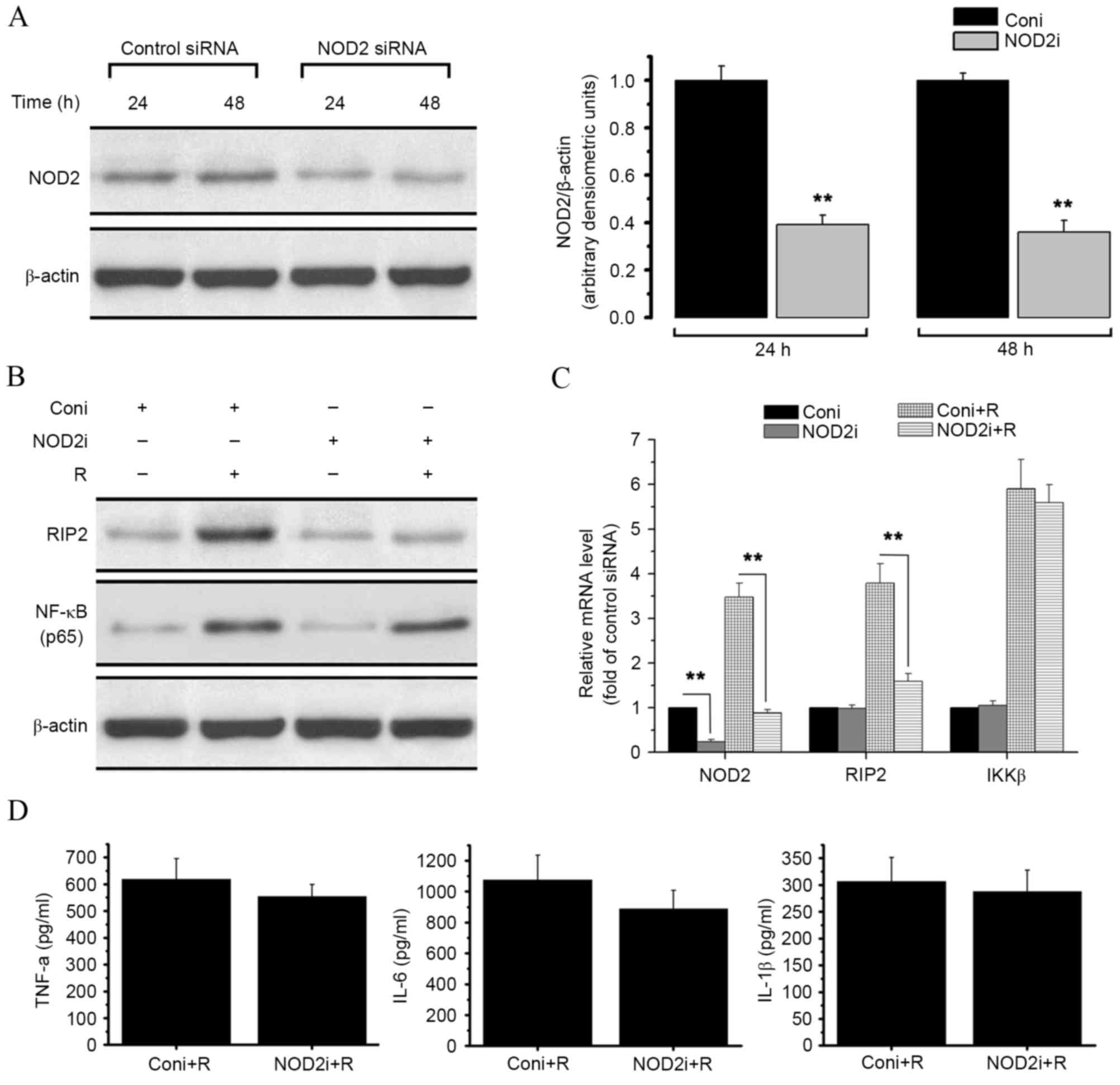 | Figure 3.Effect of siRNA-mediated NOD2
knockdown on resistin signaling in RAW 264.7 cells. RAW 264.7 cells
were treated with control siRNA or specific siRNA duplexes
targeting NOD2. Proteins and RNA were then extracted from the cells
for western blot analysis and qPCR. (A) Western blot analysis of
NOD2 content in control and NOD2 siRNA-treated cells. The results
are expressed as the ratio of NOD2/β-actin. (B) Western blot
analysis of the expression level of RIP2, and the NF-κB p65 protein
level within the nucleus, in the presence or absence of resistin
(200 ng/ml for 24 h) in RAW 264.7 cells titrated with control or
NOD2 siRNA. (C) Relative mRNA levels of NOD2, RIP2 and IKKβ in the
presence or absence or resistin (200 ng/ml for 24 h) in RAW 264.7
cells titrated with control or NOD2 siRNA were measured by qPCR.
(D) Protein level of TNF-α, IL-6 and IL-1β in each group were
detected by ELISA. **P<0.01 vs. control. NOD2,
nucleotide-binding oligomerization domain-containing protein 2;
NF-κB, nuclear factor-κB; RIP2, receptor-interacting
serine/threonine-protein kinase 2; IKKβ, inhibitor of NF-κB kinase
subunit β; TNF-α, tumor necrosis factor α; IL, interleukin; qPCR,
quantitative polymerase chain reaction; siRNA, small interfering
RNA; coni, control siRNA; NOD2i, NOD2-siRNA; R, resistin. |
As shown in Fig. 2,
treatment of RAW 264.7 cells with resistin increased the expression
of RIP2, NF-κB and IKKβ as well as the release of TNF-α, IL-6 and
IL-1β. However, as shown in Fig. 3B,
NOD2 knockdown reversed the resistin-induced increase in RIP2
protein expression. NOD2 knockdown also significantly decreased the
mRNA expression level of RIP2 after resistin treatment compared
with the control (P<0.01, Fig.
3C). The expression of IKKβ and the nuclear translocation of
NF-κB were not affected (Fig. 3B and
C). No significant changes in cytokine secretion levels were
detected by ELISA assay (Fig.
3D).
Resistin induces a proinflammatory
effect through the NF-κB pathway
The aforementioned data suggested that resistin
induced activation of the NF-κB pathway, and that this was
necessary for a resistin-induced inflammatory response. To further
evaluate the role of the NF-κB pathway in resistin-mediated
effects, parthenolide, an inhibitor of NF-κB, was introduced to the
cell cultures 1 h prior to stimulation with resistin. Inhibiting
NF-κB resulted in significantly decreased resistin-induced protein
expression of TNF-α, IL-6 and IL-1β compared with the control group
(P<0.01, Fig. 4). This suggested
that the NF-κB pathway is involved in resistin signaling.
Discussion
A previous study has shown that resistin promotes
both inflammation and insulin resistance (11). However, its specific receptor has not
yet been identified, and little is known about the molecular
mechanisms mediating resistin effects. In the current study,
resistin treatment increased the expression of NOD2, RIP2 and IKKβ
and promoted the nuclear translocation of NF-κB. This indicated
that the resistin-induced inflammatory reaction is induced through
the NOD2-NF-κB signaling pathway. It was noted that resistin
treatment had no effect on NOD1 expression. SiRNA-mediated NOD2
knockdown attenuated the expression of NOD2 and RIP2 at both mRNA
and protein level. Furthermore, the inflammatory reaction induced
by resistin was slightly inhibited, which indicated that NOD2-NF-κB
pathway is involved in resistin signaling. These findings suggested
that there may be a synergistic effect between NOD2 and another
receptor for resistin.
Resistin is a member of the resistin-like molecule
(RELM) hormone family, which includes RELMα and RELMβ. Resistin and
RELMβ contain an additional cysteine near the amino terminus, and
crystal structures of both proteins reveal an unusual multimeric
structure. RELMβ is known to contribute to local immune responses
(30). Furthermore, resistin and
RELMβ specifically inhibit insulin action in the liver, resulting
in insulin resistance (13).
Until now, four receptors for resistin have been
reported: TLR4, an isoform of decorin, ROR1 and adenylyl
cyclase-associated protein 1 (CAP1). However, none of these are
considered to be the specific receptor of resistin in vivo
(22). Previous research found no
evidence for a direct interaction between resistin and TLR4 in a
biochemical binding assay (22).
Decorin and ROR1 were only expressed at low levels in human
monocytes and neither expression level increased following resistin
stimulation (22). Therefore, the
inflammatory response induced by resistin was not directly related
to TLR4, ROR1 or decorin. Furthermore, the study found that
resistin could combine with CAP1, but as CAP1 lacks a transmembrane
domain, it is not clear how the signal would be transmitted to the
cell interior (22).
NOD2 is a key receptor in the inflammation reaction,
which has been associated with insulin resistance in previous
studies (26,28). These physiological functions are
similar to those of resistin, therefore the links between resistin
and NODs were investigated in the current study. Resistin treatment
significantly increased the expression level of NOD2, and the
resistin-induced inflammatory response was found to be induced at
least partly through the NOD2-NF-κB signaling pathway.
NF-κB is a key transcription factor in the process
of inflammatory reactions, and many inflammatory cytokines are
regulated by it, including TNF-α, IL-6 and IL-1β (31,32). In
the current study, resistin treatment promoted NF-κB translocation
into the nucleus and significantly increased the levels of key
inflammatory cytokines. Treatment with a specific inhibitor of
NF-κB confirmed that resistin-induced signals are mediated through
NF-κB signaling mechanisms. These findings are consistent with
recent studies, which have reported that NF-κB signaling mechanisms
are essential for the resistin-induced inflammatory response.
In summary, the current study demonstrated that
resistin treatment increases NOD2 expression and that the
inflammatory response induced by resistin involves the NOD2-NF-κB
signaling pathway.
Acknowledgements
This study was supported financially by the Natural
Science Foundation of Science and Technology Department of Sichuan
Province (grant no. 2013NZ0032).
References
|
1
|
Kahn BB and Flier JS: Obesity and insulin
resistance. J Clin Invest. 106:473–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wellen KE and Hotamisligil GS:
Obesity-induced inflammatory changes in adipose tissue. J Clin
Invest. 112:1785–1788. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao L, Kwon MJ, Huang S, Lee JY, Fukase
K, Inohara N and Hwang DH: Differential modulation of Nods
signaling pathways by fatty acids in human colonic epithelial
HCT116 cells. J Biol Chem. 282:11618–11628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferroni P, Basili S, Falco A and Davì G:
Inflammation, insulin resistance, and obesity. Curr Atheroscler
Rep. 6:424–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinberg GR: Inflammation in obesity is
the common link between defects in fatty acid metabolism and
insulin resistance. Cell Cycle. 6:888–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heilbronn LK and Campbell LV: Adipose
tissue macrophages, low grade inflammation and insulin resistance
in human obesity. Curr Pharm Des. 14:1225–1230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mooradian AD: Obesity: A rational target
for managing diabetes mellitus. Growth Horm IGF Res. 11:(Suppl A).
S79–S83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel SD, Rajala MW, Rossetti L, Scherer
PE and Shapiro L: Disulfide-dependent multimeric assembly of
resistin family hormones. Science. 304:1154–1158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Choi YH, Hartzell DL, Li C,
Della-Fera MA and Baile CA: CNS melanocortin and leptin effects on
stearoyl-CoA desaturase-1 and resistin expression. Biochem Biophys
Res Commun. 311:324–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strausberg RL, Feingold EA, Grouse LH,
Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler
GD, Altschul SF, et al: Generation and initial analysis of more
than 15,000 full-length human and mouse cDNA sequences. Proc Natl
Acad Sci USA. 99:16899–16903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mojiminiyi O and Abdella N: Associations
of resistin with inflammation and insulin resistance in patients
with type 2 diabetes mellitus. Scand J Clin Lab Invest. 67:215–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muse ED, Obici S, Bhanot S, Monia BP,
McKay RA, Rajala MW, Scherer PE and Rossetti L: Role of resistin in
diet-induced hepatic insulin resistance. J Clin Invest.
114:232–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banerjee RR, Rangwala SM, Shapiro JS, Rich
AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, et al:
Regulation of fasted blood glucose by resistin. Science.
303:1195–1198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reilly MP, Lehrke M, Wolfe ML, Rohatgi A,
Lazar MA and Rader DJ: Resistin is an inflammatory marker of
atherosclerosis in humans. Circulation. 111:932–939. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senolt L, Housa D, Vernerová Z, Jirásek T,
Svobodová R, Veigl D, Anderlová K, Müller-Ladner U, Pavelka K and
Haluzík M: Resistin in rheumatoid arthritis synovial tissue,
synovial fluid and serum. Ann Rheum Dis. 66:458–463. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filková M, Haluzík M, Gay S and Šenolt L:
The role of resistin as a regulator of inflammation: Implications
for various human pathologies. Clin Immunol. 133:157–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bokarewa M, Nagaev I, Dahlberg L, Smith U
and Tarkowski A: Resistin, an adipokine with potent proinflammatory
properties. J Immunol. 174:5789–5795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wellen KE and Hotamisligil GS:
Obesity-induced inflammatory changes in adipose tissue. J Clin
Invest. 112:1785–1788. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silswal N, Singh AK, Aruna B, Mukhopadhyay
S, Ghosh S and Ehtesham NZ: Human resistin stimulates the
pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by
NF-kappaB-dependent pathway. Biochem Biophys Res Commun.
334:1092–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tarkowski A, Bjersing J, Shestakov A and
Bokarewa MI: Resistin competes with lipopolysaccharide for binding
to toll-like receptor 4. J Cell Mol Med. 14:1419–1431. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim
J, Lee S, Kim JY, Lee J, Yang HM, et al: Adenylyl
cyclase-associated protein 1 is a receptor for human resistin and
mediates inflammatory actions of human monocytes. Cell Metab.
19:484–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boström EA, Svensson M, Andersson S,
Jonsson IM, Ekwall AK, Eisler T, Dahlberg LE, Smith U and Bokarewa
MI: Resistin and insulin/insulin-like growth factor signaling in
rheumatoid arthritis. Arthritis Rheum. 63:2894–2904. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sánchez-Solana B, Laborda J and Baladron
V: Mouse resistin modulates adipogenesis and glucose uptake in
3T3-L1 preadipocytes through the ROR1 receptor. Mol Endocrinol.
26:110–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inohara N, Koseki T, Lin J, del Peso L,
Lucas PC, Chen FF, Ogura Y and Núñez G: An induced proximity model
for NF-kappa B activation in the Nod1/RICK and RIP signaling
pathways. J Biol Chem. 275:27823–27831. 2000.PubMed/NCBI
|
|
26
|
Tamrakar AK, Schertzer JD, Chiu TT, Foley
KP, Bilan PJ, Philpott DJ and Klip A: NOD2 activation induces
muscle cell-autonomous innate immune responses and insulin
resistance. Endocrinology. 151:5624–5637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao L, Hu P, Zhou Y, Purohit J and Hwang
D: NOD1 activation induces proinflammatory gene expression and
insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol
Metab. 301:E587–E598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schertzer JD and Klip A: Give a NOD to
insulin resistance. Am J Physiol Endocrinol Metab. 301:E585–E586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He W, Wang ML, Jiang HQ, Steppan CM, Shin
ME, Thurnheer MC, Cebra JJ, Lazar MA and Wu GD: Bacterial
colonization leads to the colonic secretion of RELMbeta/FIZZ2, a
novel goblet cell-specific protein. Gastroenterology.
125:1388–1397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok
SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al: Quercetin
suppresses proinflammatory cytokines production through MAP kinases
and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage.
Mol Cell Biochem. 243:153–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: Key elements of proinflammatory
signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|