Introduction
The incidence of invasive fungal infections (IFIs)
has markedly increased in recent decades owing to increase in the
number of immunocompromised patients (1,2). Despite
the development of new antifungal drugs, the mortality rate of IFIs
remains high (2). Candida
species remain a major cause of IFIs, resulting in significant
morbidity and mortality in health care settings (1,3,4). Moreover, the number of candidemia cases
caused by non-albicans Candida species has been increasing
in recent years (5–7). It is known that the treatment of some
Candida species is difficult because of their innate or
acquired resistance to antifungal agents (8–10). For
instance, Candida krusei is intrinsically resistant to
fluconazole (1,11). Although its prevalence remains low
among yeast infections (2–5%), its intrinsic resistance to
fluconazole is the reason C. krusei infections have the
highest mortality rate (30–60%) compared to other Candida
species (1,10,12).
Antimicrobial peptides (AMPs) are currently being
studied extensively to assess their use as a new class of
antimycotics because they possess broad-spectrum antimicrobial
activities and may possibly thwart resistance (13–15).
Chromogranin A is a major soluble protein of the adrenal medullary
chromaffin granules and neurons, and is conservative in mammals
(16,17). Vasostatin-1, corresponding to the 1st
to 76th amino acids of bovine chromogranin A, is a natural
antimicrobial peptide derived from the processing of bovine
chromogranin A, and abundant naturally secrets (18). Research has demonstrated that the
C-terminal moiety of bovine vasostatin-1 has potent antifungal
activity. The disulfide bridge loop of Cys17-Cys38 in vasostatin-1
was crucial for its antibacterial activity but not necessary for
its antifungal activity (19). To
further explore the antifungal activity of the vasostatin-1 derived
peptide and avoid aggregation, CGA-N46 which corresponded to human
CGA residues 31–76 was synthesized. Our previous studies elucidated
that CGA-N46 peptide had specific anti-Candidal activity,
especially showed the highest antagonistic activity to C.
krusei, without hemolytic activity on human erythrocytes in
vitro (20). CGA-N46 inhibited
the growth of Candida species by decreasing intracellular
reactive oxygen species levels and mitochondria membrane potentials
while also inhibiting DNA synthesis (21).
However, little is known about the in vivo
activity of CGA-N46 in the treatment of candidiasis in
immunocompromised hosts. In this study, candidemic mice was used to
investigate the effects of CGA-N46 on immune cells and organ
tissues with that of terbinafine was compared.
Materials and methods
CGA-N46 preparation
CGA-N46 were synthesized by solid-phase peptide
synthesis method. Peptide purification was performed using
high-performance liquid chromatography (HPLC). The mass of the
peptide was confirmed via mass spectrometry. Final purity of the
peptides was determined to be 90% by analytical HPLC.
Experimental animals
Specific-pathogen-free female and male Kunming (KM)
mice (n=144), 4-weeks-old, weighing approximately 22–27 g, were
purchased from the Laboratory Animal Center of Zhengzhou
University, Zhengzhou, China. They were housed in an
air-conditioned room maintained at 26°C with a 12 h light/12 h dark
cycle in filter-top cages and fed ad libitum a diet of
laboratory chow and tap water (22).
Candida species are the pathogens of opportunistic
mycosis. They are easy to infect immuno-compromised patients.
Hereby, to get immuno-compromised animals, all mice received
intraperitoneal injections of cyclophosphamide at a dose of 200
mg/kg/day for 3 days after one week of adaptive feeding. The number
of leukocytes was counted 24 h after cyclophosphamide
administration. When the leukocyte count reached less than
1,000/ml, suspension, containing 105 CFU/ml fresh C. krusei
in a 0.2 ml volume of 0.9% sodium chloride (NaCl), was slowly
administered to each mouse via intraperitoneal injection. The
inoculum concentration was confirmed by plating serial dilutions of
the suspension onto SD agar medium plates.
Strain culture
Candida krusei API-600010 was supplied by the
Department of Hematology, Peking University First Hospital,
Beijing, China. The C. krusei culture was prepared using a
method described by Petraitiene et al (23). with modest amendments. A loop of
fresh C. krusei cells from fresh Sabouraud's (SD) agar slant
was transferred into 10 ml of fresh SD broth medium and centrifuged
(220 rpm at 30°C for 12 h). The concentration of cells was adjusted
using a hemocytometer, which was confirmed through a quantitative
culture using a 10-fold serial dilution. An inoculum of fresh C.
krusei suspension (105 CFU/ml) in a 0.2 ml volume of 0.9% NaCl
was administered slowly to each mouse via intraperitoneal
injection. The inoculum concentration was confirmed by plating
serial dilutions of the suspension onto Sabouraud's agar medium
plates.
CGA-N46 administration
Administration of the compounds (0.9% NaCl, CGA-N46
and terbinafine) was initiated 24 h after inoculation of C.
krusei. The mice were randomly divided into four groups:
Control group, intraperitoneal injection of 200 µl 0.9% NaCl, n=36;
30 mg/kg/day CGA-N46 group, intraperitoneal injection of CGA-N46
with concentration in the blood of mice was MIC against C.
krusei (0.1 mM), n=36; 60 mg/kg/day CGA-N46 group with
concentration in the blood of mice was 2-folds of MIC against C.
krusei (0.2 mM), n=36; and terbinafine group, intraperitoneal
injection of terbinafine 3.8 mg/kg/day according to the usage of
introduction, n=36. All mice were injected one time each day and
subjected to 2 weeks of injections.
The animals were monitored daily for mortality and
body weight loss. They were humanely euthanized prior to the end of
the experiments or when specific signs appeared (inability to reach
food and water, lethargy or decreased mental alertness, labored
breathing, inability to remain upright) and are not expected to
survive until the next scheduled evaluation.
Sampling
After 3, 5, 7, 10, 12 and 14 days of treatment, mice
from the control and treatment groups were euthanized by
intraperitoneal injection of pentobarbital sodium (60 mg/kg). Fresh
peripheral blood samples were collected to quantify blood cells.
After euthanasia, the weights of the mice's thymuses and spleens at
5, 10, 12 and 14 days of treatment were measured. The thymus and
spleen indices were calculated in the following manner: Organ index
= organ weight (mg) / body weight (g).
Triplicate samples were collected at each
experiment.
Tissues from their livers, spleens, lungs, and
kidneys were removed, and washed thoroughly with 0.9% NaCl to
remove residual blood. Afterwards, the tissue samples were cut into
pieces (5×5 mm) and fixed with 4% (v/v) paraformaldehyde solution
(24). The paraformaldehyde solution
was replaced with fresh paraformaldehyde solution every 24 h and
this was repeated thrice for tissue sample preparation.
Histopathological observation of
tissue sections
Tissue sections were prepared according to the
method developed by Ding (25) with
a little modification. Briefly, the fixed tissue specimens were
dehydrated and embedded in paraffin wax and then stored at 28°C for
more than 12 h. Next, we cut serial paraffin sections (4 µm) and
stored them at 65°C for more than 30 min. The sections were
immersed in xylol for three consecutive washes (5 min each) to
remove paraffin. Then, the sections were hydrated with a series of
alcohol washings of descending strength (100, 95, 85, 80 and 70%),
ending with de-ionized water. The histological paraffin sections
were then stained with hematoxylin and eosin. Changes in
organizational structure were visualized with an Olympus BX51
microscope set at ×100 and ×400 magnification.
Statistical analysis
Experimental data were analyzed using the SPSS 18.0
statistical program (SPSS, Inc., Chicago, IL, USA) to perform a
one-way analysis of variance followed by the Duncan test. The
results are reported as mean ± standard error of the mean (SEM).
Differences between each two groups were considered to be
statistically significant at P<0.05.
Ethics statement
All experimental procedures described in this work
were performed in strict accordance with the guidelines suggested
for the care and use of laboratory animals formulated by the Animal
Ethics Committee of Zhengzhou University, which approved this
protocol (Permit no. 2015-0002). All the injections were performed
after animals were temporarily anesthetized with 1–5% isoflurane
(administered by bell jar and maintained by nose cone). All the
surgeries were performed under sodium pentobarbital anesthesia (60
mg/kg), and all efforts were made to minimize suffering.
Results
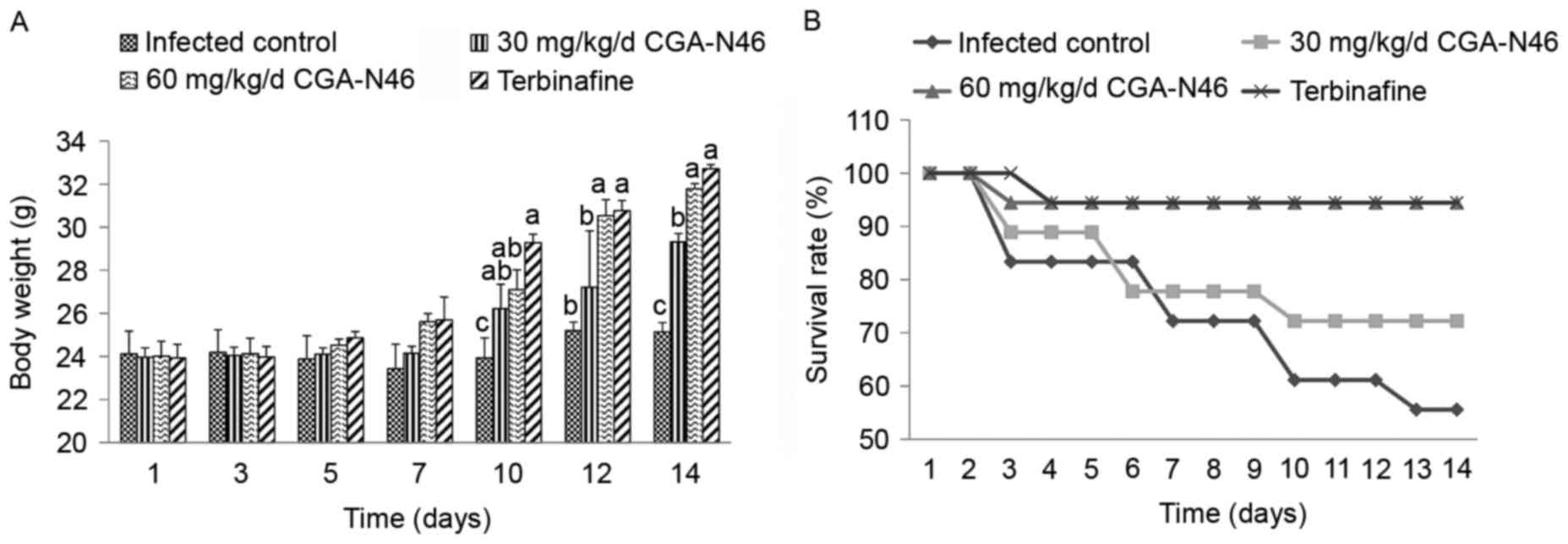
Effects of CGA-N46 on body weight and
mortality
During the treatment period, the body weight of the
survival mice and survival rate of each group were collected and
recorded (Fig. 1). Compared with the
control group, the body weight of the survival mice increased more
quickly after 10 days of treatment. At the 14th day of the
experiment, the average body weight of the control group increased
1.083 g, and the survival rate was 55.56%. Meanwhile, the average
body weight of the 60 mg/kg/day CGA-N46 group increased 7.77 g, and
the survival rate was 94.44%. The effects of CGA-N46 on body weight
and mortality of the infected mice were dose-dependent. The
increase in body weight and the decline in mortality were not
significantly different between the 60 mg/kg/day CGA-N46 and
terbinafine groups.
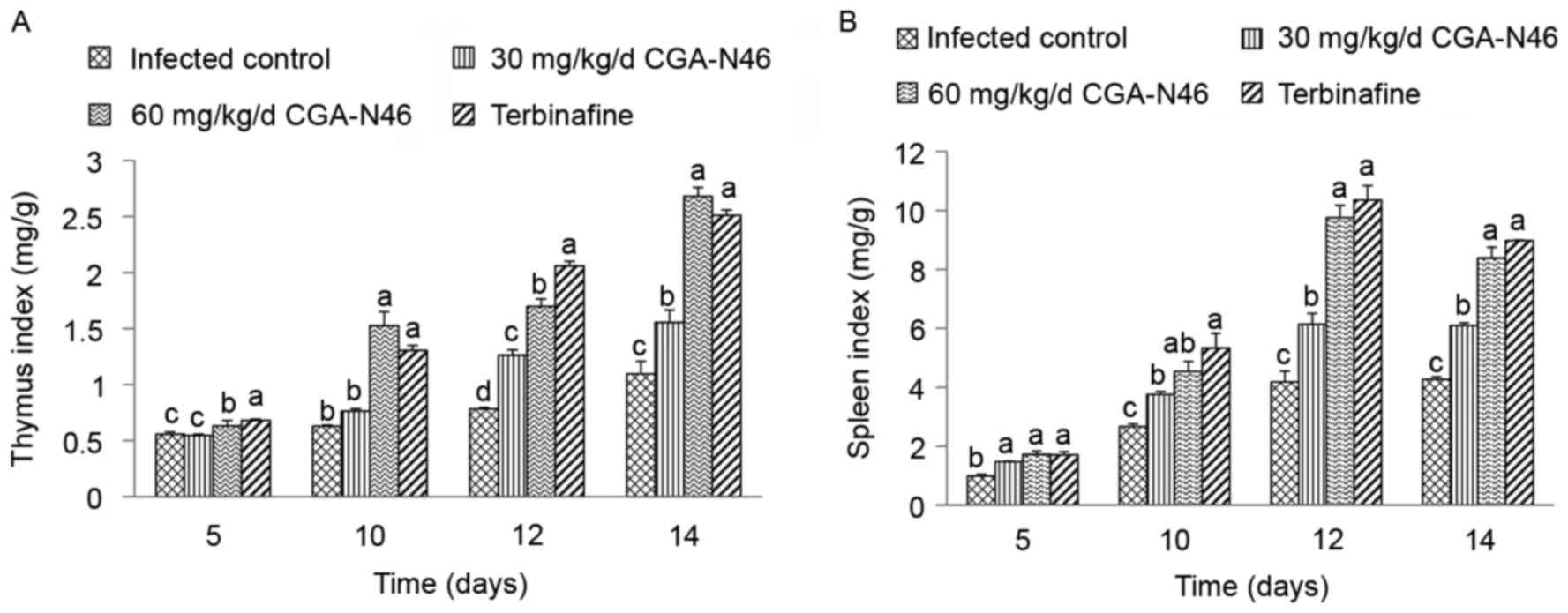
Effect of CGA-N46 on thymus and spleen
indices
The effects of CGA-N46 on lymphatic organs at 5, 10,
12 and 14 days after treatment were studied and the thymus and
spleen indices were recorded. As shown in Fig. 2, the thymus and spleen indices
increased in a time-dependent manner. Also, compared to control,
CGA-N46 significantly increased the thymus and spleen indices in a
dose-dependent manner (P<0.05). Note that the effects of 60
mg/kg/day CGA-N46 on thymus and spleen indices were not
significantly different from the effects of terbinafine.
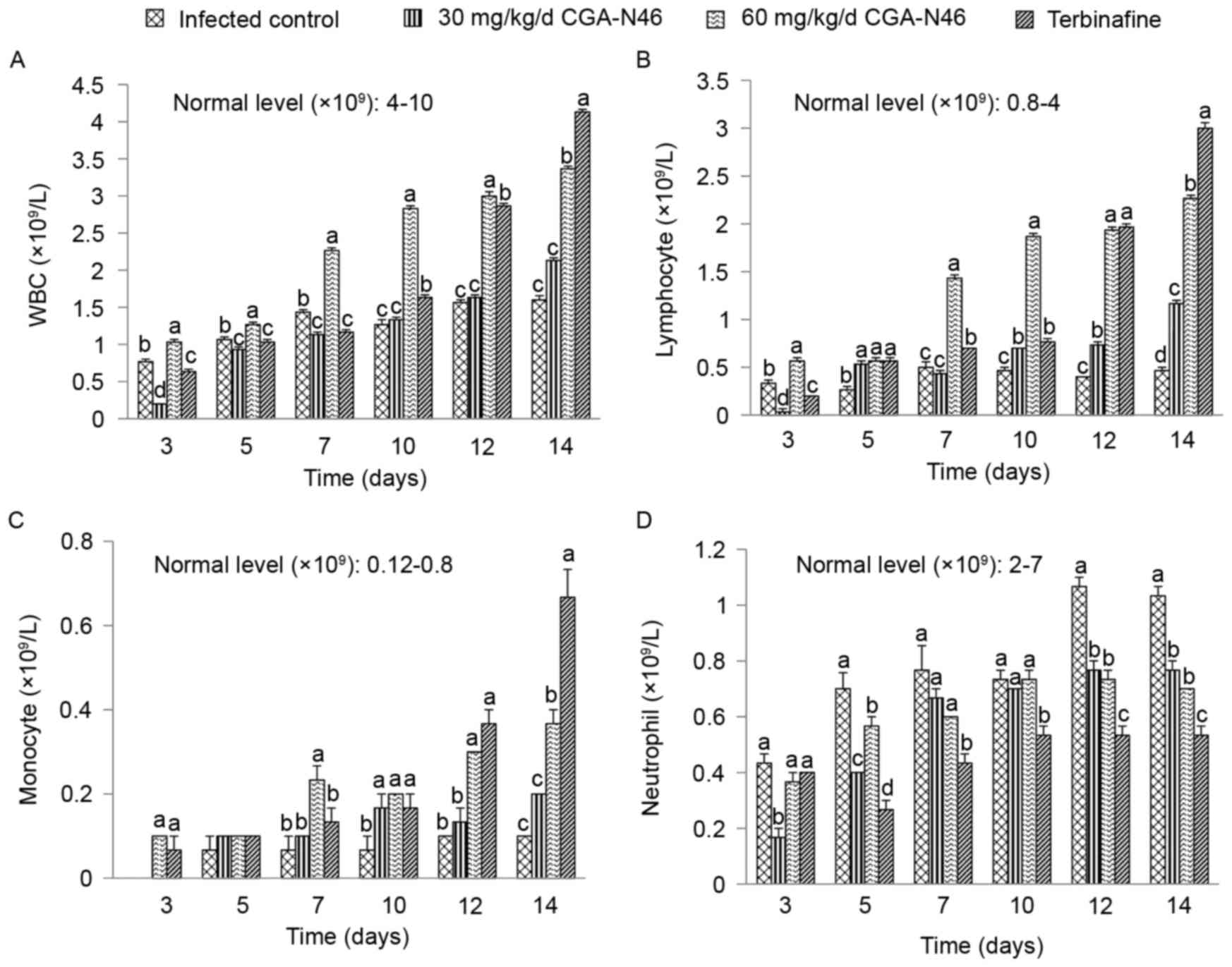
Effect of CGA-N46 on immune cells
The level of white blood cells (WBCs) and
lymphocytes, monocytes, neutrophil granulocytes in peripheral blood
samples reflect the effects of CGA-N46 on mice immunity. The
effects of CGA-N46 on immune cells were shown in Fig. 3. The immunosuppressant
cyclophosphamide and Candida albican-infection could reduce
the levels of WBCs. The levels of WBC, lymphocyte and monocyte in
the control group were lower than the normal physiological level
throughout the experiment. CGA-N46 could increase the levels of
WBC, lymphocyte and monocyte in peripheral blood at a
concentration-dependent and time-dependent manner. The immune cell
levels of the CGA-N46 treated mice differed significantly from the
control mice (P<0.05). It is puzzling that neutrophil
granulocyte levels were lower in the treatment groups than in the
control group which need to be further research.
Histopathological observation of
tissues
The histopathology of the mice was examined at the
indicated time points after CGA-N46 administration. The
histopathological changes of the lungs, liver, spleen and kidneys
on the 14th day of CGA-N46 treatment are shown in Fig. 4. The lesions in each tissue of the
control group were more severe than those in the treatment
groups.
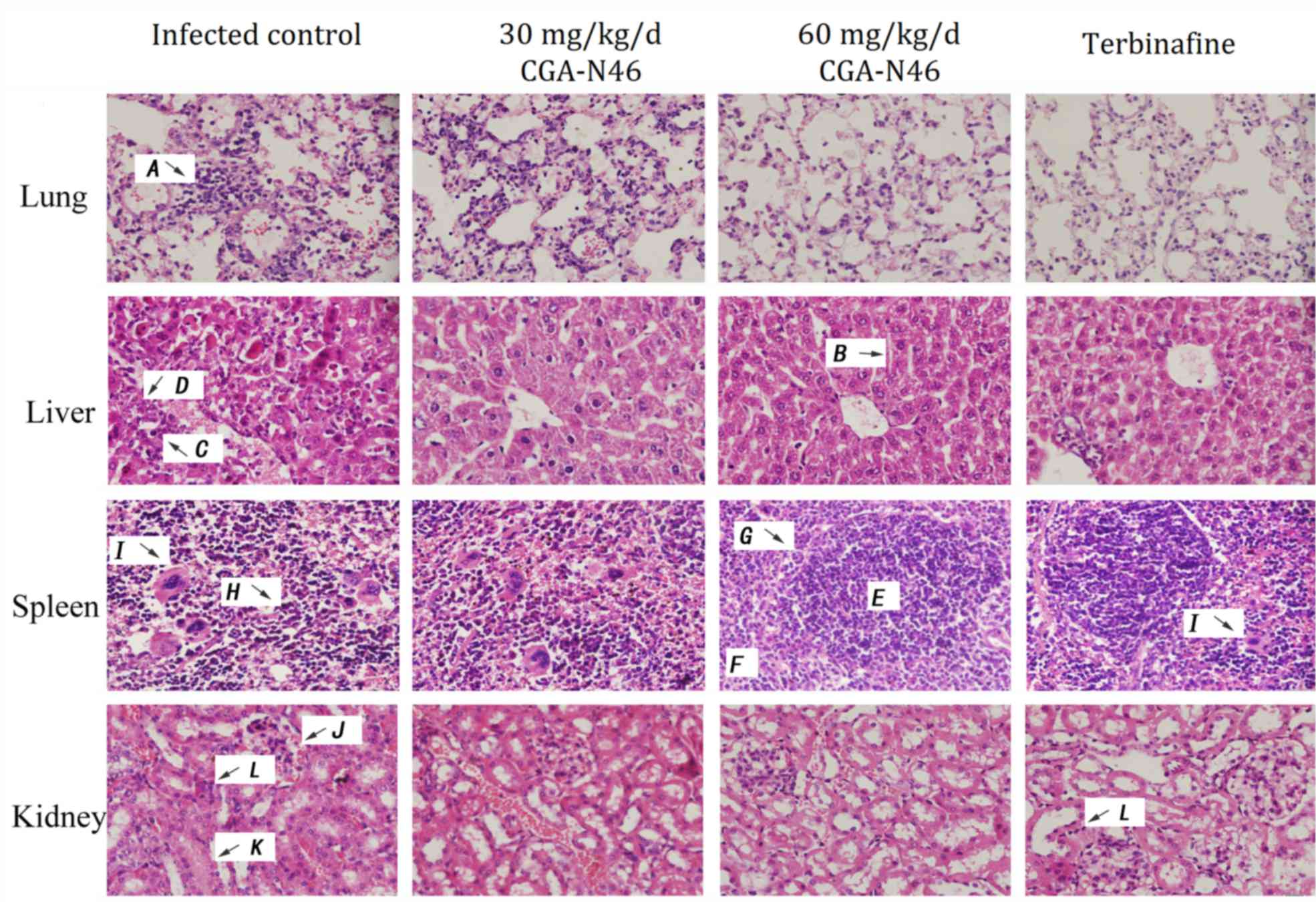 | Figure 4.Histopathological changes of tissues
on day 14 of CGA-N46 treatment. H&E staining. A,
alveolar interstitial lymphocytes infiltration; B, hepatic
cord; C, nuclear pyknosis; D, nuclear dissolution;
E, white pulp; F, red pulp; G, the boundaries
between the red pulp and white pulp; H, structural
disordered splenic corpuscles; I, macrophages; J,
bleeding; K, swelling of the glomerulus; L,
inflammatory cells. |
In the control group, the lung lesions were
characterized as severe alveolar interstitial lymphocytes
infiltration. In liver section, no discernable normal lobular
architecture and hepaticcord could be seen, and nuclear pyknosis or
dissolution appeared. In the spleen, the boundaries between the red
pulp and white pulp were unclear, and the structure was severely
disordered. There were no visible splenic corpuscles or obvious
germinal centers, and we observed a large number of macrophage
cells scattered throughout the splenic parenchyma. In the kidney
tissue sections, bleeding, swelling of the glomerulus, interstitial
edema, and inflammatory cell infiltration were observed. While,
CGA-N46 could mitigate the damage caused by C. krusei
infections by reducing inflammatory cell infiltration and
recovering tissues structure in a concentration- and time-dependent
manner. At day 14, 60 mg/kg/day treatment showed more effective
than Terbinafine because, in Terbinifine treatment group, there
were more macrophage cells in spleen tissue and more inflammatory
cells in kidney tissue (Fig. 4).
Discussion
Candidiasis is the mycosis caused by the dimorphic
fungus Candida spieces. In contrast to the other pathogenic fungi,
C. albicans is a member of the normal microbiota within the
gastrointestinal tract, respiratory tract, vaginal area, and mouth.
In healthy individuals, C. albicans does not produce
disease. Growth is suppressed by other microbiota (26). In recent decades, The incidence of
fungal infections is growing worldwide with the increase of
immuno-compromised patients (1,27).
Candida species are important reasons for different clinical
manifestations, and they cause significant mortality and morbidity
in health care settings (1,4). Candida species are the pathogens
of opportunistic mycosis. They are easy to infect
immuno-compromised patients. Therefore, normal immunity is
important for fungal infection. Hereby, the effect of CGA-N12 on
the immunity of experimental animals was investigated in present
study.
For effective establishment of C.
krusei-infected mice, cyclophosphamide was administrated at the
dosage of 200 mg/kg/day prior to the inoculation of C.
krusei. The serum leukocyte levels were less than 1,000/ml
after 3 days of administration. Thymus indices, spleen indices and
leukocyte levels in peripheral blood sample of the control group
indicated that the mice's immunity was damage. During the
immunocompromised period, C. krusei could not induce an
immune reaction in the infected mice. Compared to the control,
CGA-N46 had immunomodulatory activities.
Thymus and spleen indices are the two important
indicators of immunity. The indices are directly correlated to
organ functionality and the number of immune cells. For that
reason, they have been widely used to assess immunity responses
(28). CGA-N46 could promote the
immune activity of the thymus and spleen. The effect of CGA-N46 on
thymus indices, spleen indices and immune cells was comparable to
that of terbinafine, which is an effective antifungal drug. CGA-N46
was predicted to exert immuno-modulatory effect in the treatment of
invasive candidiasis in immunocompromised mice, and the effects of
CGA-N46 in vivo were dose-dependent.
Splenic macrophages have significantly greater
antifungal activity against the pseudohyphae in the Candida
spp. than that macrophage populations in the liver and lungs
(22,23,29). In
the present study, more macrophages were observed in the spleen
than that in the liver, kidneys, and lungs. Compared with the 60
mg/kg/day CGA-N46 and terbinafine treatment groups, more
macrophages were observed in the splenic tissue sections of the
control group and the 30 mg/kg/day CGA-N46 treatment group. We
predicted that more macrophages existing in the splenic tissue of
the infected control samples and 30 mg/kg/day CGA-N46 treatment
samples may be caused by more C. krusei isolates in them.
Compared the pictures of Terbinafine treatment group with 60
mg/kg/day CGA-N46 treatment group, macrophages were found in the
splenic tissue of Terbinafine treatment group. The result indicated
that CGA-N46 was more effective than Terbinafine.
Acknowledgements
This study was supported by the National Science
Foundation of China (grand nos. 31071922 and 31572264), Project of
science and technology of Henan Province (grand no. 162102310404),
the Fundamental Research Funds for the Henan Provincial Colleges
and Universities in Henan University of Technology (grand no.
2015RCJH03).
Glossary
Abbreviations
Abbreviations:
|
CGA-N46
|
a derived peptide from N-terminus of
human chromogranin A, corresponding to the 31st to 76th amino
acid
|
|
CFU
|
colony-forming unit
|
|
H&E
|
hematoxylin and eosin
|
|
IFIs
|
invasive fungal infections
|
|
mM
|
millimole per liter
|
|
SEM
|
standard error of the mean
|
|
WBC
|
white blood cell
|
References
|
1
|
Scorzoni L, de Lucas MP, Mesa-Arango AC,
Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ
and Zaragoza O: Antifungal efficacy during Candida krusei infection
in non-conventional models correlates with the yeast in vitro
susceptibility profile. PLoS One. 8:e600472013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gedik H, Şimşek F, Kantürk A, Yildirmak T,
Arica D, Aydin D, Demirel N and Yokuş O: Bloodstream infections in
patients with hematological malignancies: Which is more
fatal-cancer or resistant pathogens? Ther Clin Risk Manag.
10:743–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shorr AF, Gupta V, Sun X, Johannes RS,
Spalding J and Tabak YP: Burden of early-onset candidemia: Analysis
of culture-positive bloodstream infections from a large U.S.
database. Crit Care Med. 37:2519–2526; quiz 2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo AL, Tobón A, Restrepo A,
Queiroz-Telles F and Nucci M: Epidemiology of endemic systemic
fungal infections in Latin America. Med Mycol. 49:785–798.
2011.PubMed/NCBI
|
|
5
|
Arendrup MC: Epidemiology of invasive
candidiasis. Curr Opin Crit Care. 16:445–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pemán J, Cantón E, Quindós G, Eraso E,
Alcoba J, Guinea J, Merino P, Ruiz-Pérez-de-Pipaon MT,
Pérez-del-Molino L, Linares-Sicilia MJ, et al: Epidemiology,
species distribution and in vitro antifungal susceptibility of
fungaemia in a Spanish multicentre prospective survey. J Antimicrob
Chemother. 67:1181–1187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pushpanathan M, Rajendhran J, Jayashree S,
Sundarakrishnan B, Jayachandran S and Gunasekaran P: Direct cell
penetration of the antifungal peptide, MMGP1, in Candida albicans.
J Pept Sci. 18:657–660. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliveira VK, Lda S Ruiz, Oliveira NA,
Moreira D, Hahn RC, Melo AS, Nishikaku AS and Paula CR: Fungemia
caused by Candida species in a children's public hospital in the
city of São Paulo, Brazil: Study in the period 2007–2010. Rev Inst
Med Trop Sao Paulo. 56:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leroy O, Gangneux JP, Montravers P, Mira
JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B,
et al: Epidemiology, management, and risk factors for death of
invasive Candida infections in critical care: A multicenter,
prospective, observational study in France (2005–2006). Crit Care
Med. 37:1612–1618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bassetti M, Taramasso L, Nicco E, Molinari
MP, Mussap M and Viscoli C: Epidemiology, species distribution,
antifungal susceptibility and outcome of nosocomial candidemia in a
tertiary care hospital in Italy. PLoS One. 6:e241982011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz P, Sánchez-Somolinos M, Alcalá L,
Rodríguez-Créixems M, Peláez T and Bouza E: Candida krusei
fungaemia: Antifungal susceptibility and clinical presentation of
an uncommon entity during 15 years in a single general hospital. J
Antimicrob Chemother. 55:188–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbas J, Bodey GP, Hanna HA, Mardani M,
Girgawy E, Abi-Said D, Whimbey E, Hachem R and Raad I: Candida
krusei fungemia. An escalating serious infection in
immunocompromised patients. Arch Intern Med. 160:2659–2664. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Hao D and Chen Y, Xu Y, Tan J,
Huang Y, Li F and Chen Y: Inhibitory effects and mechanisms of
physiological conditions on the activity of enantiomeric forms of
an α-helical antibacterial peptide against bacteria. Peptides.
32:1488–1495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gopal R, Seo CH, Song PI and Park Y:
Effect of repetitive lysine-tryptophan motifs on the bactericidal
activity of antimicrobial peptides. Amino Acids. 44:645–660. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv Y, Wang J, Gao H, Wang Z, Dong N, Ma Q
and Shan A: Antimicrobial properties and membrane-active mechanism
of a potential α-helical antimicrobial derived from cathelicidin
PMAP-36. PLoS One. 9:e863642014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simon JP and Aunis D: Biochemistry of the
chromogranin A protein family. Biochem J. 262:1–13. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helman LJ, Ahn TG, Levine MA, Allison A,
Cohen PS, Cooper MJ, Cohn DV and Israel MA: Molecular cloning and
primary structure of human chromogranin A (secretory protein I)
cDNA. J Biol Chem. 263:11559–11563. 1988.PubMed/NCBI
|
|
18
|
Lugardon K, Raffner R, Goumon Y, Corti A,
Delmas A, Bulet P, Aunis D and Metz-Boutigue MH: Antibacterial and
antifungal activities of Vasostatin-1, the N-terminal fragment of
chromogranin A. J Biol Chem. 275:10745–10753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lugardon K, Chasserot-Golaz S, Kieffer AE,
Maget-Dana R, Nullans G, Kieffer B, Aunis D and Metz-Boutigue MH:
Structural and biological characterization of chromofungin, the
antifungal chromogranin A-(47–66)-derived peptides. J Biol Chem.
276:35875–35882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li RF, Lu YL, Lu YB, Zhang HR, Huang L,
Yin Y, Zhang L, Liu S, Lu Z and Sun Y: Antiproliferative effect and
characterization of a novel antifungal peptide derived from human
chromogranin A. Exp Ther Med. 10:2289–2294. 2015.PubMed/NCBI
|
|
21
|
Li RF, Yan XH, Lu YB, Lu YL, Zhang HR,
Chen SH, Liu S and Lu ZF: Anti-candidal activity of a novel peptide
derived from human chromogranin A and its mechanism of action
against Candida krusei. Exp Ther Med. 10:1768–1776. 2015.PubMed/NCBI
|
|
22
|
Roilides E, Lyman CA, Sein T, Gonzalez C
and Walsh TJ: Antifungal activity of splenic, liver and pulmonary
macrophages against Candida albicans and effects of macrophage
colony-stimulating factor. Med Mycol. 38:161–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petraitiene R, Petraitis V, Groll AH,
Candelario M, Sein T, Bell A, Lyman CA, McMillian CL, Bacher J and
Walsh TJ: Antifungal activity of LY303366, a novel echinocandin B,
in experimental disseminated candidiasis in rabbits. Antimicrob
Agents Chemother. 43:2148–2155. 1999.PubMed/NCBI
|
|
24
|
Bideskan AR Ebrahimzadeh, Nikravesh MR,
Hassanzadeh Taheri MM and Fazel AR: Lectin histochemical study of
vasculogenesis during rat pituitary morphogenesis. Iran J Basic Med
Sic. 14:161–169. 2011.
|
|
25
|
Ding Y, Tang J, Zou J, She R, Wang Y, Yue
Z, Tian J, Xia K, Yin J and Wang D: The effect of microgravity on
tissue structure and function of rat testis. Braz J Med Biol Res.
44:1243–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prescott ML, Harley PJ and Klein AD:
Microbiology. 5th edition. McGraw-Hill Companies, Inc.; New York,
NY: pp. 9492002
|
|
27
|
Pfaller MA and Diekema DJ: Epidemiology of
invasive mycoses in North America. Crit Rev Microbiol. 36:1–53.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuang SY, Xiao WW, Feng L, Liu Y, Jiang J,
Jiang WD, Hu K, Li SH, Tang L and Zhou XQ: Effects of graded levels
of dietary methionine hydroxy analogue on immune response and
antioxidant status of immune organs in juvenile Jian carp (Cyprinus
carpio var. Jian). Fish Shellfish Immunol. 32:629–636. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erdogan H, Fadillioğlu E, Kotuk M, Iraz M,
Tasdemir S, Oztas Y and Yildirim Z: Effects of ginkgo biloba on
plasma oxidant injury induced by bleomycin in rats. Toxicol Ind
Health. 22:47–52. 2006. View Article : Google Scholar : PubMed/NCBI
|