Introduction
Peucedanum japonicum Thunb. (PJ), which
belongs to the Umbelliferae family, has been widely used as
traditional medicine and supplement used to produce food. In recent
decades, studies have indicated that PJ may have antioxidant,
anti-obesity and anti-spasmolytic properties (1–3).
Notably, it was demonstrated that compounds isolated from PJ exert
antagonistic effects on smooth muscle contraction by altering free
cytoplasmic Ca2+ ([Ca2+]i) mobilization (3). Free Ca2+ ions are known to mediate a
diverse range of cellular processes. In particular, the stimulation
of receptor activator of nuclear factor-κB ligand (RANKL) on bone
marrow-derived macrophages (BMMs) elicits [Ca2+]i
mobilization in the form of oscillation (4). RANKL-induced [Ca2+]i
oscillations sequentially activate
Ca2+/calmodulin-calcium/calmodulin-dependent protein kinase
IV/calcineurin-c-fos/nuclear factor of activated T cells,
cytoplasmic 1 (NGATc1) signaling, triggering the final stages of
differentiation into osteoclasts (4–6).
During RANKL-mediated osteoclastogenesis,
[Ca2+]i is simultaneously mobilized from
internal and external Ca2+ stores via numerous signaling
pathways and the suppression of [Ca2+i
oscillation by numerous factors disrupt downstream of
[Ca2+]i oscillations (7–10). This
can be disrupted by natural products, for example, a recent study
by the current authors reported that the constituents of
Glechoma hederacea elicited a transient increase in
[Ca2+]i and suppressed
[Ca2+]i oscillations by modulating
voltage-gated calcium channels (VGCCs), thus inhibiting
RANKL-mediated osteoclastogenesis (11). Furthermore, it was determined that
(+)-cis-4′-O-acetly-3′-O-angeloylkhellactone, a bioactive compound
isolated from PJ, causes relaxation in isolated rat thoracic aorta
by modulating VGCCs (12). These
studies have indicated that PJ and its extracts exert potential
effects on RANKL-mediated osteoclastogenesis and its underlying
mechanisms. In the current study, it was demonstrated that PJ
ethanol extract (PEE) alters RANKL-mediated signaling and
expression of differentiation-related genes by modulating
[Ca2+]i responses, resulting in the
suppression of TRAP-positive multinucleated cells (MNCs)
formation.
Materials and methods
Experimental animals
All experiments were performed with BMMs isolated
from the femur and tibia of C57BL/6J mice purchased from Orient Bio
Inc. (Seongnam, Korea). Mice (C57BL/6 J) were housed, 4 animals per
cage under specific pathogen-free conditions for 3 weeks (12/12 h
light/dark cycles at a temperature of 22±2°C and 50–60% humidity).
A total of 20 mice (male, 6–8 weeks old) weighing 20 g were
sacrificed by brief exposure to 100 % CO2 and cervical
dislocation for all subsequent experiments. All mouse studies were
performed in accordance with protocols approved by the Animal Care
and Use Committee of Wonkwang University (approval no.
WKU16-4).
Reagents
PJ ethanol extract (95%), which was prepared as
described in ‘Preparation of PJ extract’, was used through all
experiments in this study. All cell culture media, fetal bovine
serum (FBS) and supplements were purchased from HyClone (GE
Healthcare Life Sciences, Logan, UT, USA). Recombinant murine
soluble RANKL and recombinant murine macrophage colony-stimulating
factor (M-CSF) were purchased from PeproTech, Inc. (Rocky Hill, NJ,
USA). Nicardipine and U73122, inhibitors of voltage-gated Ca2+
channel (VGCC) and phospholipase C respectively (PLC), were
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Antibodies against phospho-phospholipase C (PLC) γ2 (no. 3874),
cAMP response element-binding protein (CREB, no. 9197) and p-CREB
(no. 9198) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-NFATc1 (no. sc7294), anti-PLCγ2 (no.
sc5238) and anti-c-fos (no. sc253) antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Preparation of PJ extract
The roots of PJ were purchased from a local herbal
company (Kwangmyungdang Medicinal Herbs Co., Ltd., Ulsan, Korea)
and authenticated by Professor G.S. Lee. A voucher specimen
(WKU010107-PJ201305E) was deposited at the Department of Herbology,
College of Korean Medicine, Wonkwang University (Iksan, Korea).
Dried PJ roots (100 g) were ground into fine powder and then
extracted with 1,000 ml 70% aqueous ethanol for 1 h using
ultrasonic extractor (Powersonic 505; Hwashin Tech, Daegu, Korea).
The extract was evaporated under 40 mmHg using a rotary evaporator
(N-1110S-W; Eyela, Tokyo, Japan) and lyophilized using freeze-dryer
(−50°C; ILShin BioBase, Co., Ltd., Dongducheon, Korea). The yield
of the final extract was 12.02% (w/w).
Cell viability assay
Cell viability assays were performed using the
EZ-Cytox Enhanced Cell Viability assay kit (Daeil Lab Service Co.,
Ltd., Seoul, Korea), according to the manufacturer's instructions.
Briefly, BMMs, which act as osteoclast precursors, were plated in
96-well culture plates at a density of 1×104 cells/well with
different concentrations of PEE (0, 2, 5, 10, 25 and 50 µg/ml) for
1 day at 37°C, or were treated with 25 µg/ml PEE under M-CSF
treatment (30 ng/ml) for 4 days at 37°C. All cells were incubated
with 10 µl EZ-Cytox reagent for 4 h at 37°C. Following incubation,
the optical density was measured using a microplate reader
(Sunrise; Tecan Group Ltd., Männedorf, Switzerland) at 450 nm.
In vitro osteoclast
differentiation
Murine osteoclasts were prepared from bone marrow
cells as previously described (13).
Bone marrow cells were collected from the tibiae and femora of
6–8-week-old mice, following sacrifice (previously described in
‘Experimental animals’) by flushing the marrow space with
phosphate-buffered saline (PBS) and were cultured at 37°C in the
presence of M-CSF (30 ng/ml) for 3 days in α-minimal essential
medium (α-MEM) supplemented with 10% FBS and 5% antibiotics
(Antibiotic-Antimycotic 100x; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Cells attached on Petri dish were replated
on the desired cell culture dish and used as osteoclast precursors
(bone marrow-derived macrophages, BMMs). To generate osteoclasts,
BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) at
37°C for 4 days. Medium was replaced on day 3 with fresh α-MEM
containing M-CSF and RANKL. Cells were fixed in 10% formalin at
room temperature for 10 min and stained for tartrate resistant acid
phosphatase (TRAP) activity. To measure total TRAP activity,
p-nitro phenyl phosphate (Sigma-Aldrich; Merck KGaA) was used as a
substrate of TRAP, and then optical density was measured at an
absorbance of 405 nm using microplate reader. Following the
measurement of total TRAP activity, TRAP positive-multinuclear
cells (TRAP+ MNCs) containing >3 nuclei were counted
using light microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
BMMs treated with or without PEE were cultured with
M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days as described
above. Total RNA was extracted from cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) on days 0, 1, 2, 3 and 4. Thereafter, 1 µg total RNA was
transcribed to first strand cDNA with random primers from the
Maxima Reverse Transcriptase (Thermo Fisher Scientific Inc.)
according to the manufacturer's protocol. qPCR was performed using
the VeriQuest SYBR-Green qPCR Master mix (Affymetrix, Inc., Santa
Clara, CA, USA) and the StepOnePlus Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed with
a preliminary incubation at 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. To control for variation in
mRNA concentrations, all results were normalized to the GAPDH
housekeeping gene. Relative quantitation was performed using the
comparative 2-ΔΔCq method (14). The PCR primers used were as follows:
Mouse TRAP, forward, 5′-CTGGAGTGCACGATGCCAGCGACA-3′ and reverse,
5′-TCCGTGCTCGGCGATGGACCAGA-3′; Oscar, forward,
5′-GGGGTAACGGATCAGCTCCCCAGA-3′ and reverse,
5′-CCAAGGAGCCAGAACGTCGAAACT-3′; Cathepsin K (CTSK) forward,
5′-ACGGAGGCATTGACTCTGAAGATG-3′ and reverse,
5′-GTTGTTCTTATTCCGAGCCAAGAG-3′; dendrocyte expressed seven
transmembrane protein (TM7SF4), forward,
5′-TGGAAGTTCACTTGAAACTACGTG-3′ and reverse,
5′-CTCGGTTTCCCGTCAGCCTCTCTC-3′; ATPase H+ Transporting V0 Subunit
D2 (ATP6V0D2), forward, 5′-TCAGATCTCTTCAAGGCTGTGCTG-3′ and reverse,
5′-GTGCCAAATGAGTTCAGAGTGATG-3′; NFATC1, forward,
5′-CTCGAAAGACAGCACTGGAGCAT-3′ and reverse,
5′-CGGCTGCCTTCCGTCTCATAG-3′; and GAPDH, forward,
5′-TGCCAGCCTCGTCCCGTAGAC-3′ and reverse,
5′-CCTCACCCCATTTGATGTTAG-3′. PCR was repeated three times with
three replicates.
Western blot analysis
Following culture of BMMs with M-CSF (30 ng/ml) and
RANKL (100 ng/ml) in the presence or absence of PEE, cells were
washed with cold PBS and lysed in 100 µl of
radioimmunoprecipitation assay buffer (25 mM Tris-HCl, pH 7.6, 150
mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 1
mM phenylmethylsulfonyl fluoride, protease-inhibitor cocktail
(Roche Diagnostics GmbH, Mannheim, Germany) and phosphatase
inhibitor tablets (Thermo Fisher Scientific, Inc.). Cell lysates
were separated by centrifugation at 14,000 × g for 10 min at 4°C,
then the supernatants were collected for western blot analysis.
Total lysates (30 µg) were subjected to 10% SDS-PAGE and then
transferred to polyvinylidene fluoride membranes (GE Healthcare
Life Sciences). Each membrane was blocked at 4°C for 2 h with 5%
skim milk in TBS with Tween-20 (50 mM Tris-HCl, pH7.6, 150 mM NaCl
and 0.1% Tween-20), then incubated with the primary antibodies,
described in ‘Reagents’ (1:1,000) at 4°C overnight. Horseradish
peroxidase-conjugated immunoglobulin G (1:5,000; nos. sc2004 and
sc2005; Santa Cruz Biotechnology, Dallas, TX, USA) as a secondary
antibody, incubated for 1 h at room temperature. Immunoreactive
proteins were detected using an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
[Ca2+]i
measurement
[Ca2+]i was determined with the
Ca2+-sensitive fluorescence dye Fura2-acetoxymethyl ester
(Fura2-AM, TEFLabs Inc, Austin, TX, USA), as described previously
(11). Briefly, isolated BMMs were
plated on cover glass when they had reached ~80% confluence (6×105
cells/35-mm dish) the day before the experiment. Cells were loaded
with Fura2-AM for 50 min. Then, the cover glass containing cells
was transferred to a perfusion chamber and perfused with
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer
(10 mmol/l HEPES, 140 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l
MgCl2, 1 mmol/l CaCl2 and 10 mmol/l glucose,
adjusted to pH 7.4 and 310 mOsm). Cells were briefly washed with
HEPES buffer and continuously perfused with HEPES buffer. Each of
the indicated compounds were diluted in HEPES buffer and perfused
for a designated time. Under continuous perfusion with HEPES
buffer, intracellular fluorescence was excited at dual wavelengths
(340 and 380 nm) and the emitted fluorescence (510 nm) was captured
using a charge coupled device camera (Andor Technology Ltd,
Belfast, UK). Captured images were digitized and analyzed using
MetaFluor software (version 7.8.3.0; Molecular Devices, Inc.,
Downington, PA, USA), with data expressed as the ratio of
fluorescence intensities (F340/F380). Indicated chemical compound
(PEE, nicardipine, and U73122) was respectively diluted in the
HEPES buffer and then perfused on cells for the designated time. To
remove extracellular Ca2+ ions, CaCl2 in HEPES buffer
was replaced with same concentration of ethylene glycol-bis
(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA; Sigma
Aldrich; Merck KGaA) and presented as ‘Ca2+-free’.
Statistical analysis
Data are presented as the mean ± standard deviation
of results from at least three independent experiments. Statistical
differences were analyzed using one-way analysis of variance
followed by Tukey's post hoc test. SPSS 14.0 software (SPSS Inc.,
Chicago, IL, USA) was used to analyze results and P<0.05 was
considered to indicate a statistically significant difference.
Results
PEE reduces RANKL-mediated
TRAP+ MNC formation in a dose-dependent manner
As indicated in previous studies, [Ca2+]i
mobilization stimulated by RANKL is a key factor for triggering the
final stage of differentiation of osteoclasts (9,10,15).
Considering that it has been previously demonstrated that the
constituents of PJ caused antagonistic effects on acetylcholine-
and histamine-induced [Ca2+]i increase in isolated
guinea pig ileum (3), it was
proposed by the current authors that PJ may affect
osteoclastogenesis by regulating [Ca2+]i
mobilization.
Firstly, the cytotoxicity of PEE on BMMs was
evaluated. BMMs were treated with PEE in a dose- and time-dependent
manner and cell viability was assessed. PEE treatment did not exert
a cytotoxic effect on BMMs regardless of the concentration of PEE
or incubation duration (Fig. 1A and
B). Subsequently, the effect of PEE on RANKL-mediated
TRAP+ MNC formation was evaluated using a standard in
vitro osteoclast culture method. Isolated BMMs were
simultaneously treated with RANKL and various concentrations of PEE
(0, 5, 10, 25 and 50 µg/ml), and were incubated for 4 days.
Following incubation for 0, 1, 2, 3 and 4 days, TRAP+
MNC formation and total TRAP activity were measured in cells
(Fig. 1C). Compared with the
control, the number of TRAP+ MNCs was significantly
reduced following PEE treatment in a dose-dependent manner
(P<0.05 for 5 and 10 µg/ml PEE, P<0.01 for 25 and 50 µg/ml
PEE; Fig. 1D). Marked reduction of
TRAP activity in PEE-treated cells was also observed (Fig. 1E). These results suggest that PEE
negatively regulates RANKL-mediated osteoclastogenesis in a
dose-dependent manner without inducing cytotoxicity.
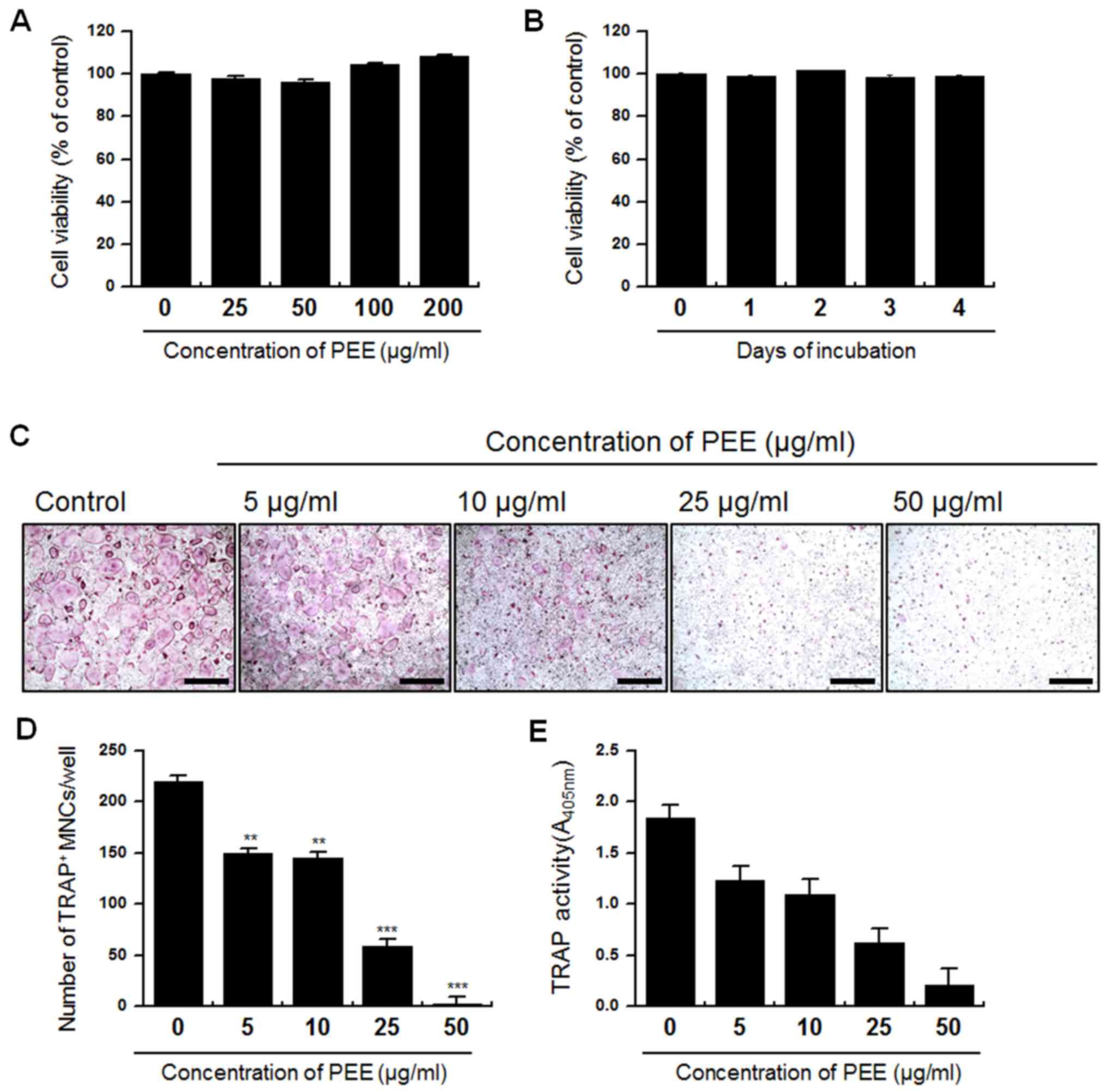 | Figure 1.Effect of PEE on the viability of BMMs
and TRAP+ MNC formation. Isolated BMMs were treated with
PEE in a dose- and time-dependent manner. (A) Cells were treated
with various concentrations of PEE (0, 25, 50, 100 and 200 µg/ml)
and incubated for 4 days. (B) Cells were treated with 25 µg/ml PEE
and incubated for various durations (0, 1, 2, 3 and 4 days). (C)
Intracellular TRAP was stained to evaluate the effect of PEE on
osteoclastogenesis. TRAP staining of osteoclasts was conducted
following 4 days culture in the presence of 0, 5, 10, 25 and 50
µg/ml PEE. Scale bar, 200 µm, at ×10 magnification. (D)
Quantification of osteogenesis. TRAP+ MNCs with >3
nuclei were classed as mature osteoclasts. (E) TRAP activity was
measured with a TRAP solution assay at day 4. Absorbance was
measured at 405 nm. Data are presented as the mean ± standard
deviation and are representative of at least three experiments.
**P<0.05, ***P<0.01 vs. 0 µg PEE. PEE, Peucedanum
japonicum Thunb. ethanol extract; BMM, bone marrow-derived
macrophage; TRAP, tartrate resistant acid phosphatase; MNC,
multinuclear cell. |
Suppression of differentiation-related
gene expression by PEE
TRAP, Oscar, Ctsk, TM7SF4, ATP6V0D2 and NFATC1 are
known to be differentiation-related marker genes that are crucial
for cell motility, fusion and bone resorption (4,16–20). To
determine whether PEE regulates differentiation at the gene level,
RANKL-stimulated BMMs were treated with PEE and incubated for 0, 1,
2, 3 or 4 days prior to RT-qPCR. The level of endogenous expression
of the differentiation-related marker genes was subsequently
evaluated. The results indicated that treatment of PEE on
RANKL-stimulated BMMs markedly inhibited RANKL-mediated mRNA
expression of marker genes including TRAP, Oscar, Ctsk, TM7SF4,
ATP6V0D2 and NFATc1, compared with RANKL-only treated BMMs
(Fig. 2). This indicates that PEE
affects and modifies the differentiation-mediating signaling
pathway in the early stages of osteoclastogenesis.
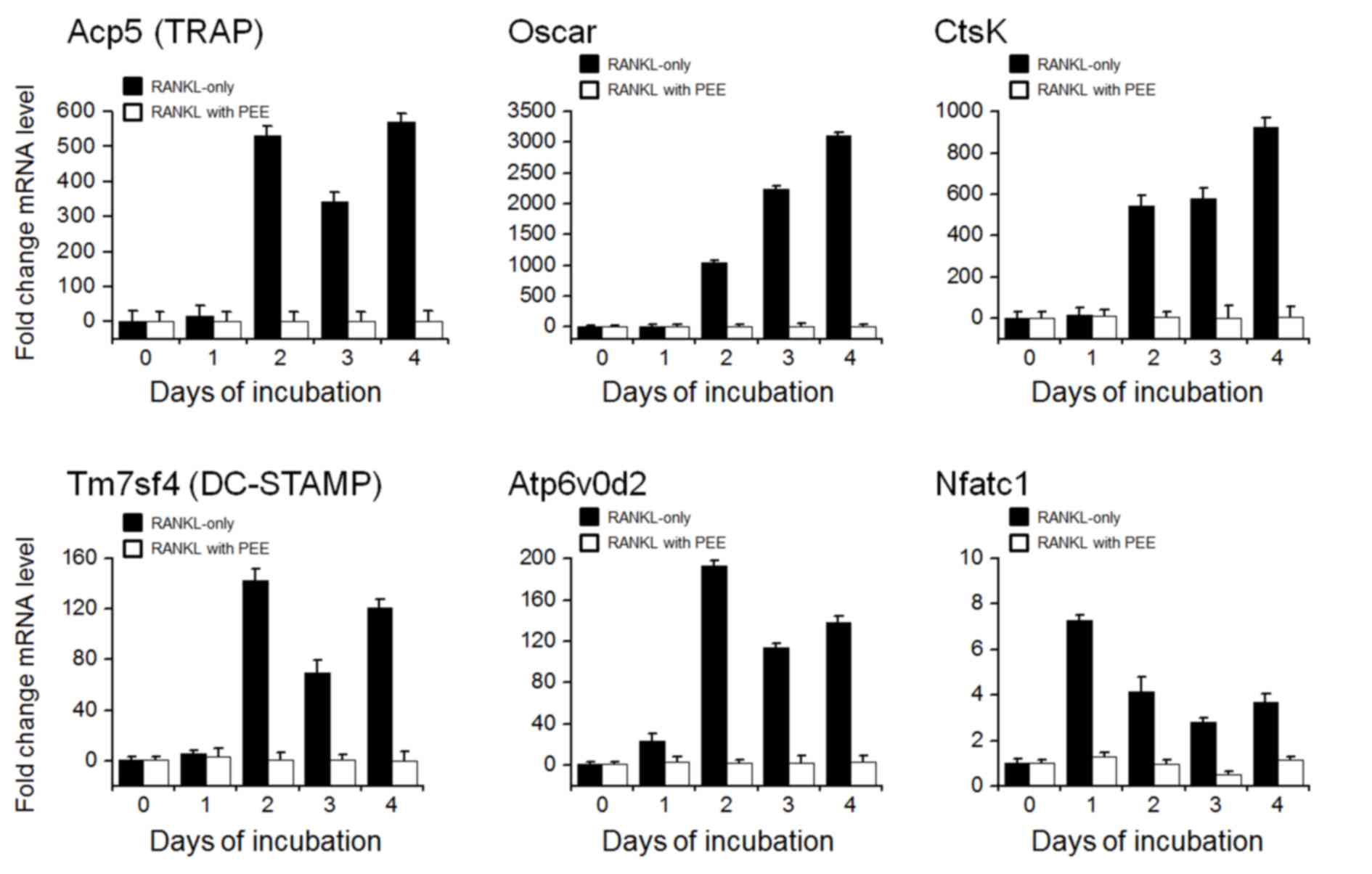 | Figure 2.Expression of osteoclast
differentiation marker genes. Bone marrow-derived macrophages were
cultured with or without 25 µg/ml PEE for 0, 1, 2, 3 or 4 days.
Reverse transcription-quantitative polymerase chain reaction assays
were performed to evaluate the expression levels of osteoclast
differentiation marker genes, ACP5 (TRAP), Oscar, CtsK, TM7SF4
(DC-STAMP), ATP6V0D2 and NFATC1. The expression values represent
three biological replicates and are presented relative to the GAPDH
expression in each sample. PEE, Peucedanum japonicum Thunb.
ethanol extract; TRAP, tartrate resistant acid phosphatase; CtsK,
cathepsin K; DC-STAMP, dendrocyte expressed seven transmembrane
protein; nuclear factor of activated T cells, cytoplasmic 1;
ATP6V0D2, ATPase H+ Transporting V0 Subunit D2. |
PEE elicits a transient
[Ca2+]i increase, which is dependent on both
extracellular and intracellular Ca2+ mobilization
To further investigate the molecular mechanisms
underlying the alteration of osteoclastogenesis caused by PEE, the
intracellular Ca2+ responses to PEE were evaluated. Isolated BMMs
plated on the cover glass were loaded with Fura2-AM, a fluorescent
indicator of free Ca2+. Acute treatment of PEE on BMMs elicited a
transient [Ca2+]i increase, whereas removal of
extracellular Ca2+ reversed this effect (Fig. 3A). To characterize PEE-induced
[Ca2+]i mobilization, cells were pretreated with
nicardipine and U73122, which are inhibitors of VGCCs and PLC,
respectively. The inhibition of VGCCs and PLC resulted in the
suppression of PEE-induced [Ca2+]i increase (Fig. 3B and C). Additionally, PLC
phosphorylation in response to RANKL stimulation was reduced by PEE
treatment in a dose-dependent manner (Fig. 3D). These results suggest that PEE
treatment of BMMs causes [Ca2+]i mobilization that is
dependent on extracellular and intracellular Ca2+ stores.
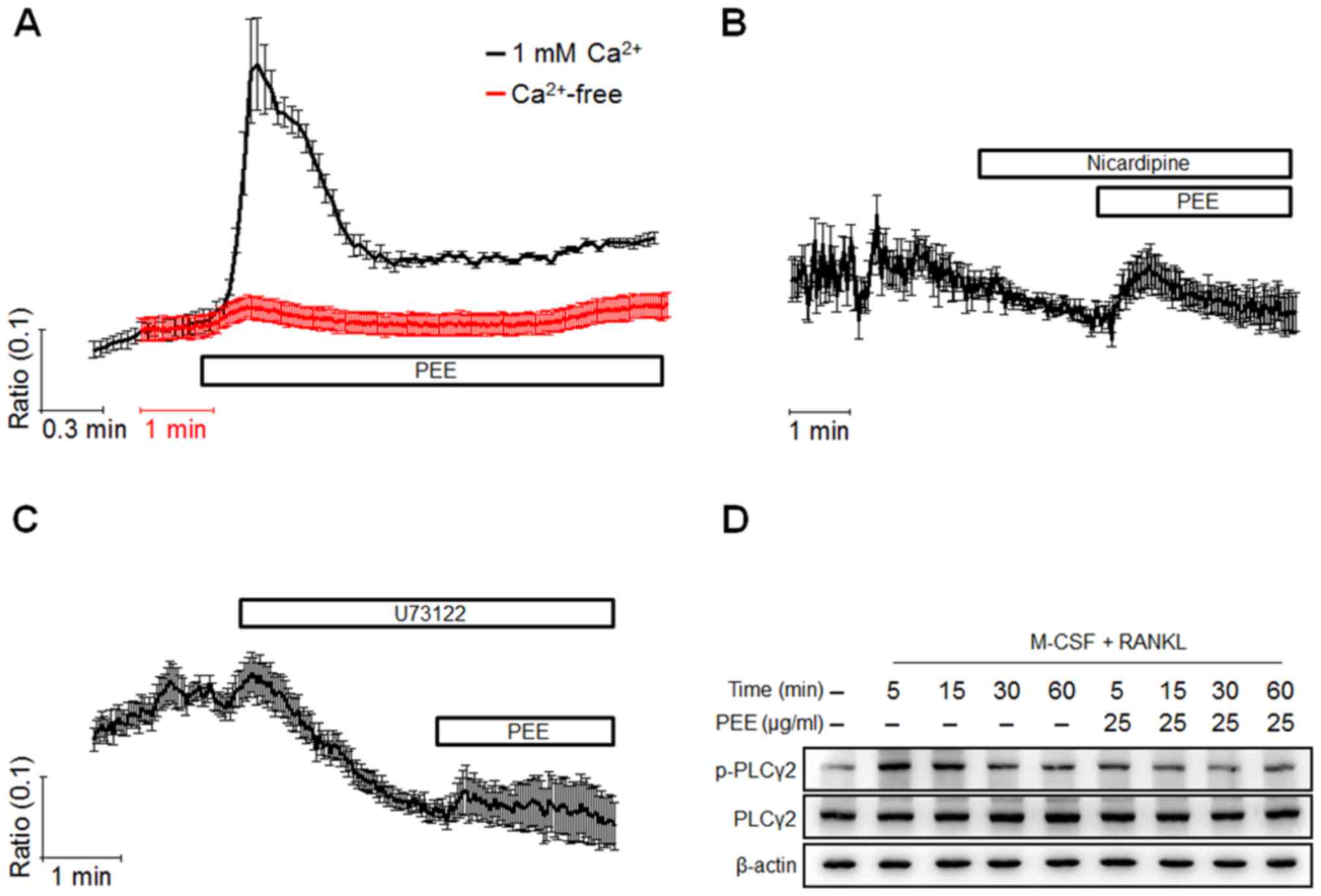 | Figure 3.Characterization of PEE-mediated
Ca2+ responses. Isolated bone marrow-derived macrophages
were seeded and cultured for 24 h in the presence of M-CSF, and
[Ca2+]i mobilization was measured using Fura2
dye on the following day. Cells were initially perfused with HEPES
buffer, and (A-C) each designated compound, including PEE,
nicardipine (10 µM) and U73122 (10 µM), was used to treat cells for
the indicated time. To chelate extracellular Ca2+ ions,
CaCl2 in HEPES buffer was replaced with same
concentration of EGTA and presented as Ca2+ free. Each
trace indicates the mean value ± standard deviation from three or
more independent experiments. (D) The effect of PEE on PLCγ2
activation was evaluated using western blot analysis. Cells were
cultured under the indicated conditions, and whole cell lysate was
used for detecting PLCγ2 activation. The total PLCγ2 was also
evaluated and used as a loading control. PEE, Peucedanum
japonicum Thunb. ethanol extract; RANKL, receptor activator of
nuclear factor κB ligand; M-CSF, macrophage colony-stimulating
factor; PLCγ2, phospholipase C γ2; EGTA, ethylene glycol-bis
(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid. |
PEE treatment of BMMs suppresses
NFATc1 activity by regulating CREB activity
CREB, together with [Ca2+]i mobilization,
is essential in the regulation of RANKL-mediated NFATc1 activity
(21). Thus, Ca2+-CREB-mediated
NFATc1 activation induces expression of numerous genes, including
NFATc1 itself, c-fos and TRAP (4).
The current study evaluated whether PEE affects RANKL-mediated CREB
activation and NFATc1 and c-fos expression. RANKL-stimulated BMMs
were treated with PEE and incubated for 0, 1, 2, 3 or 4 days.
Following incubation, total cell lysates were collected and
subjected to western blot analysis. It was determined that PEE
treatment sequentially suppressed RANKL-elicited CREB
phosphorylation (Fig. 4A) and
inhibited the induction of NFATc1 and c-fos expression (Fig. 4B and C). Notably, phosphorylation of
CREB in PEE-treated sample was observed to increase at day 4
compared with the RANKL-only treated sample. At this point, further
studies are required to elucidate how PEE enhances the
phosphorylation of CREB at day 4. These results demonstrate that
PEE strongly inhibits RANKL-mediated osteoclastogenesis via
disruption of the CREB-NFATc1 signaling pathway.
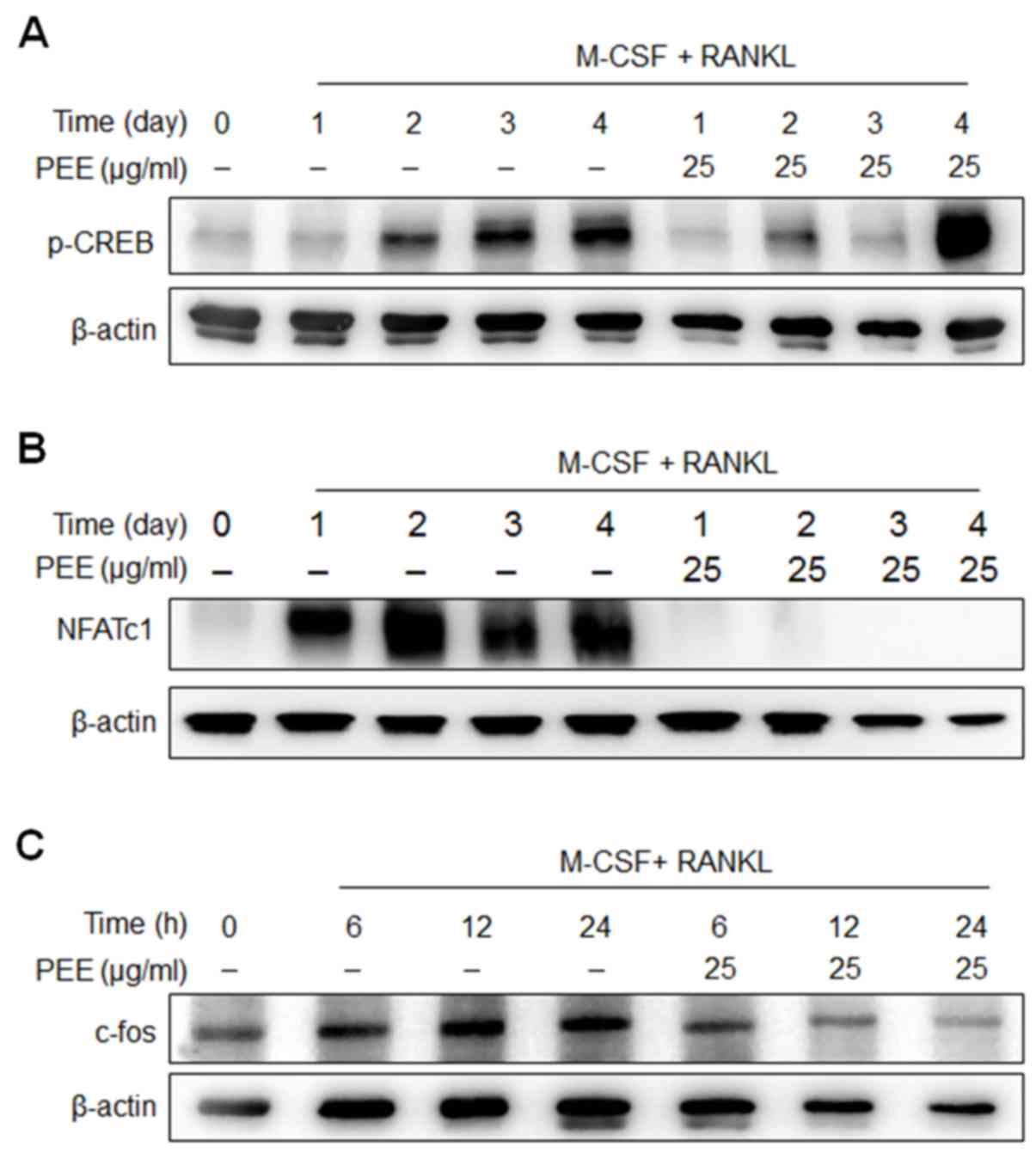 | Figure 4.Effects of PEE on RANKL-induced
intracellular signaling. Bone marrow-derived macrophages were
treated with or without PEE (25 µg/ml) in the presence of RANKL and
M-CSF for the indicated durations (0, 1, 2, 3 and 4 days). Whole
cell extracts were subjected to western blot analysis. (A)
Phospho-CREB, (B) NFATc1 and (C) c-fos were detected with the
specific antibodies. β-actin was used as a loading control. PEE,
Peucedanum japonicum Thunb. ethanol extract; RANKL, receptor
activator of nuclear factor κB ligand; M-CSF, macrophage
colony-stimulating factor; CREB, cAMP response element-binding
protein; NFATc1, nuclear factor of activated T cells, cytoplasmic
1. |
Discussion
Natural extracts have been extensively evaluated in
drug discovery and development owing to their potential therapeutic
benefits. The crude extracts derived from natural products
generally possess components with diverse bioactivities (22). The components from crude extracts
interact with intracellular molecules and alter cellular responses
(23). Notably, it has been reported
that the compounds from PJ exhibit anti-oxidant and
anti-inflammatory properties (1,3), which
are important biological properties for modulating bone remodeling
by regulating osteoclast differentiation. Based on this, the
current study investigated the physiological roles of PJ on the
differentiation of BMMs into osteoclasts.
Previous studies that have investigated crude
extracts derived from natural product have reported that they
exhibit cytotoxic effects against pathogens (24,25). In
the current study, PEE treatment on RANKL-stimulated BMMs
suppressed TRAP+ MNC formation and TRAP activities in a
dose- and time-dependent manner. Furthermore, PEE did not exert
cytotoxic effects on BMMs up to a concentration of 200 µg/ml and
following 4 days of incubation.
Marked suppression of differentiation-related gene
expression indicated that PEE may physiologically act on the
signaling molecules relaying RANKL-mediated osteoclast
differentiation information, resulting in a reduction of osteoclast
differentiation. Notably, the expression of the genes TRAP, Oscar,
CtsK, DC-STAMP, ATP6V0D2 and NFATC1 were completely suppressed by
PEE treatment, indicating that PEE blocks the early events of
RANKL-mediated signaling pathways.
RANKL-RANK interactions elicit Ca2+
responses in the early stages of differentiation (4). RANKL-induced Ca2+ responses
are dependent on the Ca2+ influx from both internal and
external Ca2+ stores (26). The lack of Ca2+ influx
from either store results in the reduction of osteoclastogenesis.
Furthermore, it has previously been reported that extracts of G.
hederacea to cause transient Ca2+ increase via
VGCCs, altering RANKL-mediated differentiation of osteoclasts
(11). Results from the present
study indicate that PEE mobilizes external and internal
Ca2+ through VGCCs and inositol 1,4,5-triphosphate
production. Previous results have indicated that RANKL-mediated
Ca2+ responses are essential in bone homeostasis as they
regulate the activities of CREB, c-fos and NFATc1. In addition, the
results of the current study suggest that PEE markedly
downregulates CREB, NFATc1 and c-fos activity, demonstrating that
PEE modulates the RANKL/RANK signaling axis, which primarily
regulates osteoclast differentiation. Therefore, PEE modifies
RANKL-mediated signal transduction in the early stages of
differentiation, leading to a marked reduction of mature
osteoclasts.
In conclusion, the results of present study indicate
that PEE markedly suppresses RANKL-mediated osteoclastogenesis by
disrupting PLC-Ca2+-CREB/c-fos-NFATc1 signaling and
expression of the genes that determine differentiation. Notably, PJ
ethanol extract exhibited no cytotoxicity on BMMs even at a high
concentration (~200 µg/ml), indicating that PEE physiologically
regulates the RANKL-mediated signaling pathway. Collectively, these
findings suggest the potential therapeutic use of PJ in treating
bone disorders caused by overgrowth of osteoclasts.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant
no. 2011-0030130, NRF-2015R1D1A1A01058272).
References
|
1
|
Hisamoto M, Kikuzaki H, Ohigashi H and
Nakatani N: Antioxidant compounds from the leaves of Peucedanum
japonicum Thunb. J Agric Food Chem. 51:5255–5261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nukitrangsan N, Okabe T, Toda T, Inafuku
M, Iwasaki H and Oku H: Effect of Peucedanum japonicum Thunb
extract on high-fat diet-induced obesity and gene expression in
mice. J Oleo Sci. 61:89–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aida Y, Kasama T, Takeuchi N, Chiba M and
Tobinaga S: Pharmacological activities of khellactones, compounds
isolated from Peucedanum japonicum THUNB. and Peucedanum
praeruptorium DUNN. Methods Find Exp Clin Pharmacol. 20:343–351.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim H, Kim T, Jeong BC, Cho IT, Han D,
Takegahara N, Negishi-Koga T, Takayanagi H, Lee JH, Sul JY, et al:
Tmem64 modulates calcium signaling during RANKL-mediated osteoclast
differentiation. Cell Metab. 17:249–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayanagi H, Ogasawara K, Hida S, Chiba
T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al:
T-cell-mediated regulation of osteoclastogenesis by signalling
cross-talk between RANKL and IFN-gamma. Nature. 408:600–605. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YM, Jung HH, Lee SJ, Choi HJ, Kim MS
and Shin DM: TRPM7 is essential for RANKL-induced
osteoclastogenesis. Korean J Physiol Pharmacol. 17:65–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Son A, Kim MS, Jo H, Byun HM and Shin DM:
Effects of Inositol 1,4,5-triphosphate on osteoclast
differentiation in RANKL-induced osteoclastogenesis. Korean J
Physiol Pharmacol. 16:31–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang YM, Kim MS, Son A, Hong JH, Kim KH,
Seo JT, Lee SI and Shin DM: Alteration of RANKL-induced
osteoclastogenesis in primary cultured osteoclasts from SERCA2+/−
mice. J Bone Miner Res. 24:1763–1769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Prasad V, Hyung SW, Lee ZH, Lee
SW, Bhargava A, Pearce D, Lee Y and Kim HH: Plasma membrane calcium
ATPase regulates bone mass by fine-tuning osteoclast
differentiation and survival. J Cell Biol. 199:1145–1158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang JK, Erkhembaatar M, Gu DR, Lee SH,
Lee CH, Shin DM, Lee YR and Kim MS: Glechoma hederacea suppresses
RANKL-mediated osteoclastogenesis. J Dent Res. 93:685–690. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Roh TC, Rho MC, Kim YK and Lee HS:
Mechanisms of relaxant action of a pyranocoumarin from Peucedanum
japonicum in isolated rat thoracic aorta. Planta Med. 68:891–895.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Kim T, Jeong D, Kim N and Choi Y:
The Tec family tyrosine kinase Btk Regulates RANKL-induced
osteoclast maturation. J Biol Chem. 283:11526–11534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koga T, Inui M, Inoue K, Kim S, Suematsu
A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al:
Costimulatory signals mediated by the ITAM motif cooperate with
RANKL for bone homeostasis. Nature. 428:758–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu M, Moreno JL, Stains JP and Keegan AD:
Complex regulation of tartrate-resistant acid phosphatase (TRAP)
expression by interleukin 4 (IL-4): IL-4 indirectly suppresses
receptor activator of NF-kappaB ligand (RANKL)-mediated TRAP
expression but modestly induces its expression directly. J Biol
Chem. 284:32968–32979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumoto M, Kogawa M, Wada S, Takayanagi
H, Tsujimoto M, Katayama S, Hisatake K and Nogi Y: Essential role
of p38 mitogen-activated protein kinase in cathepsin K gene
expression during osteoclastogenesis through association of NFATc1
and PU.1. J Biol Chem. 279:45969–45979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell-specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:176–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim
N, Kang JS, Miyamoto T, Suda T, Lee SK, et al: v-ATPase V0 subunit
d2-deficient mice exhibit impaired osteoclast fusion and increased
bone formation. Nat Med. 12:1403–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrow AD, Raynal N, Andersen TL, Slatter
DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, et al:
OSCAR is a collagen receptor that costimulates osteoclastogenesis
in DAP12-deficient humans and mice. J Clin Invest. 121:3505–3516.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato K, Suematsu A, Nakashima T,
Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K,
Yamaguchi A, Takai T, et al: Regulation of osteoclast
differentiation and function by the CaMK-CREB pathway. Nat Med.
12:1410–1416. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Xu X, Cheng F, Liu H, Luo X, Shen
J, Chen K, Zhao W, Shen X and Jiang H: Virtual screening on natural
products for discovering active compounds and target information.
Curr Med Chem. 10:2327–2342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McChesney JD: Natural products in drug
discovery-organizing for success. P R Health Sci J. 21:91–95.
2002.PubMed/NCBI
|
|
24
|
Nguta JM, Appiah-Opong R, Nyarko AK,
Yeboah-Manu D, Addo PG, Otchere I and Kissi-Twum A:
Antimycobacterial and cytotoxic activity of selected medicinal
plant extracts. J Ethnopharmacol. 182:10–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joray MB, Trucco LD, González ML, Napal
GN, Palacios SM, Bocco JL and Carpinella MC: Antibacterial and
Cytotoxic Activity of Compounds Isolated from Flourensia oolepis.
Evid Based Complement Alternat Med. 2015:9124842015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim MS, Yang YM, Son A, Tian YS, Lee SI,
Kang SW, Muallem S and Shin DM: RANKL-mediated reactive oxygen
species pathway that induces long lasting Ca2+ oscillations
essential for osteoclastogenesis. J Biol Chem. 285:6913–6921. 2010.
View Article : Google Scholar : PubMed/NCBI
|


















