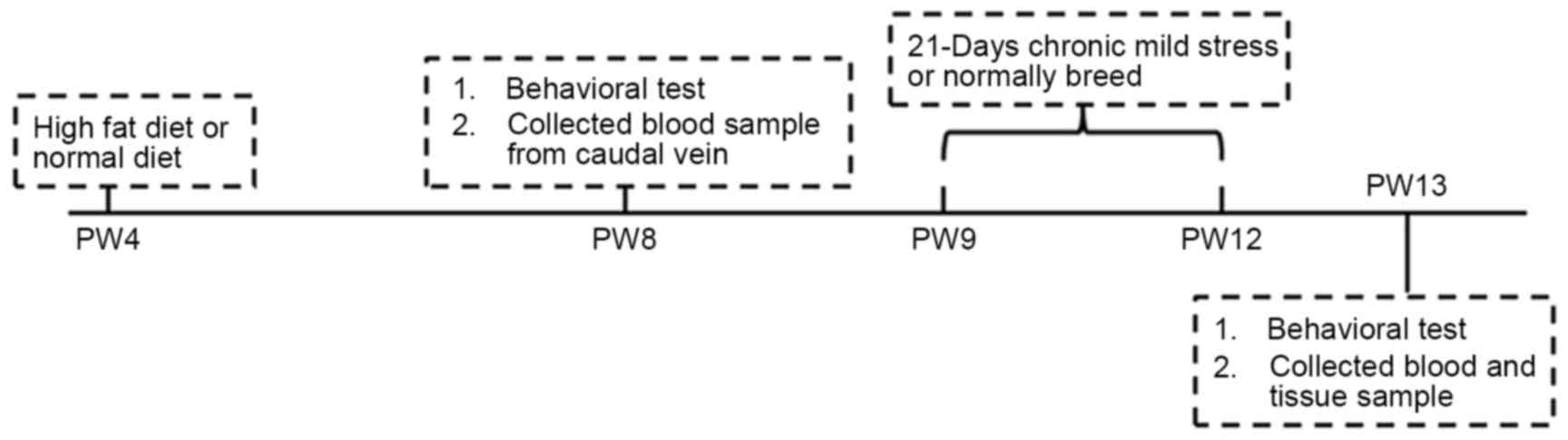
Introduction
A high-fat diet (HFD) is known to cause numerous
metabolic diseases, such as obesity, insulin resistance and fatty
liver. Exposure to a HFD at puberty increases the risk of type 2
diabetes, coronary heart disease and metabolic syndrome (1). Apart from atherosclerosis and obesity,
HFD is also associated with neuroendocrine alterations (2). Obesity, which may develop as a result
of a HFD, is known to increase the risk of behavioral disorders
associated with anxiety in humans; this is particularly pronounced
among females (3). A recent
epidemiological survey revealed that behavioral and emotional
problems during childhood are associated with pre- and postnatal
HFD (4). Likewise, female adult rats
exposed to a HFD for merely 1 week exhibited anxiety and reduced
exploration behavior (5).
A HFD during adolescence has also been shown to
increase the blood corticosterone (CORT) levels and expression of
glucocorticoid receptors (GRs) in the hypothalamus and hippocampus
(6,7). The activity of the
hypothalamic-pituitary-adrenal (HPA) axis is modulated by a
feedback regulation of GCs and adrenocorticotropic hormone (ACTH)
at the levels of the hippocampus, paraventricular nucleus of
hypothalamus and pituitary gland. Verma et al (8) found an increased sensitivity of the
adrenal cortex in women compared to men. In addition, the variance
in ACTH and CORT levels in response to stress are uniformly higher
in female rodents. The HPA axis is a vital neuroendocrine axis with
a negative feedback regulation center in the hippocampus. The
paraventricular nucleus of the hypothalamus directly modulates HPA
axis activity (9). Multiple studies
have demonstrated that HPA axis imbalance contributes to diseases
of the central nervous system (10,11).
Clinical evidence has reveals that patients with depression or
anxiety are also characterized by increased levels of
corticotropin-releasing hormone (CRH) and arginine vasopressin
(AVP) (12). A HFD is also
associated with HPA axis alterations. For instance, maternal HFD
during pregnancy modulates glucocorticoid signaling pathways in
limbic areas, and is associated with the regulation of HPA function
and anxiety in adulthood (13). A
post-weaning HFD until early puberty leads to altered HPA axis
function in female rats, indicating that HFD-induced nutritional
imbalance in young females may lead to neuroendocrine dysfunction,
which in turn may promote the appearance of stress-associated
disorders during adolescence (6).
Chronic mild stress (CMS) is often associated to the pathogenesis
of obesity (14). The ability of CMS
to lead to depression, including the wide range of accompanying
physical, neurochemical and behavioral changes (e.g., anhedonia)
have been well documented (15–17).
Few experimental animal studies on CMS have induced
an obese-like state in the animals. Instead, CMS leads to decreases
in the consumption of highly palatable foods with subsequent loss
in body weight (18–20). Due to the inherent variability of
stress paradigms, it is difficult to map out the hormonal and
neurochemical responses to CMS. In any given CMS protocol, multiple
stressors are used. Furthermore, it is difficult to draw parallels
between animal models of CMS and any type of chronic stress in
humans (21).
Thus far, however, the effects of a HFD from
post-weaning age until adolescence on the HPA axis and behavior
have remained elusive. Moreover, the association between
HFD-induced behavioral changes and HPA axis sensitivity has
remained to be determined. Therefore, the present study used a
female rat model to assess the effects of a post-weaning HFD, CMS
and their combination on the HPA axis as well as their behavior.
Furthermore, the hippocampal mineralocorticoid receptor (MR) and
glucocorticoid receptor (GR) expression levels were determined in
order to assess any possible correlation between the HFD-induced
altered HPA axis sensitivity and behavioral changes.
Materials and methods
Animals and treatment
Animal experiments were performed at the Animal
Center of Yangtze University (Jingzhou, China), which is accredited
by the Association for Assessment and Accreditation of Laboratory
Animal Care International. The study protocol was designed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals and was approved by the Ethical Research Committee of the
Medical College of Yangtze University (Jingzhou, China). Wistar
rats (8–10 weeks; n=8 male and 8 female) were supplied the by
Experimental Center of Hubei Medical Scientific Academy (no.
2009-0004; Hubei, China). The animals were housed under standard
conditions (temperature, 18–22°C; humidity, 40–60%; 12:12 h
light-dark cycle) and allowed access to food ad libitum.
The animals were treated as described previously
(22). Pregnant rats were fed
independently until delivery, and a total of 8 pups from each
litter were kept (4 males and 4 females). On postnatal week (PW) 4,
the pups were separated from their mothers, and 16 female pups (2
female pups from each mother) were assigned to each group. One
group was fed a normal diet (21% kcal from protein, 68.5% kcal from
carbohydrates and 10.5% kcal from fat) and the other group received
a HFD (88.0% corn flour, 11.5% lard and 0.5% cholesterol, which
provided 18.9% kcal from protein, 61.7% kcal from carbohydrates and
19.4% kcal from fat) (23). The HFD
and control groups were then further subdivided into two subgroups
each (n=8 in each): One subgroup of each group was subjected to 21
days of CMS from PWs 9–12, while the second subgroup was not
subjected to stress. Thus, there were a total of four subgroups
(n=8 rats per group): The control, in which the rats were fed
standard chow and received no stress; the HFD group, in which the
rats were fed an HFD; the CMS group, in which the rats were
subjected to CMS; and the CMS + HFD group, in which the rats
received a HFD as well as CMS. Body weights were determined every 2
weeks from PW4-12.
At PW8, all the animals were subjected to the open
field test and electric maze test (Fig.
1). The time interval between the sequential tests was two
days. Behavioral tests were performed between 09:30 am and 15:30
pm. Blood samples were collected from the caudal vein after the
rats were made to fast for 12 h. The rats in the CMS and CMS + HFD
groups were subjected to CMS from PW9-12 according to the procedure
described in a previous study (23).
This included food and water deprivation for 24 h, pinching of the
tail (2 cm from the tail end) for 5 min, treatment with heat at
45°C for 5 min, swimming in cold water at 4–8°C for 4 min followed
by towel drying, reversal of the day and night cycles and social
isolation (single rat per cage) for 24 h. The stressors were
randomly administered and continuously applied at day and night. On
PW13, after the last open field test and electric maze test, all of
the animals were anesthetized with isoflurane (Baxter Healthcare
Co., Deerfield, IL, USA) and decapitated prior to blood sample
collection. Finally, the hippocampi and hypothalami were dissected
and stored at −80°C for subsequent use.
Behavioral tests
Open field test
According to the protocol of a previous study
(24), the open field test was used
to evaluate the locomotor activity and exploratory behavior of the
rats. A plywood box with black walls and the dimensions 120×90×30
cm was divided into 25 equal squares. Each rat was individually
placed in the center of the apparatus at the time of the test for 3
min and their behavior during this time were recorded in detail.
When the hind legs crossed the line of a square, the rat was
considered to have crossed over to the next square (crossing), and
when the forelegs were lifted from the floor, the animal scored one
point. At the end of each open field test, the cage was carefully
cleaned with 75% ethanol and purified water prior to the test for
the next rat, ensuring no possible interference with the
spontaneous behavior of the next animal.
Electric maze test
According to a previously described method (22), rat learning and memory were
determined by using a Y-type electric maze test. In brief, each rat
was placed in the maze and electrical stimulation was performed.
Upon electrical stimulation, direct escape from the starting
position to a safe region was considered as the correct reaction.
The times of correct reaction (TOCR) and total time to escape (TTE)
in 10 tests were utilized to evaluate learning and memory
abilities.
Determination of serum ACTH and CORT levels
The serum ACTH and CORT levels were determined
according to previously described methods (25) using radioimmunoassay (RIA) and ELISA
kits in accordance with the manufacturers' protocols. The rat CORT
ELISA kit was obtained from Assaypro (St. Charles, MO, USA). The
rat ACTH RIA kit was obtained from Beijing North Biotech Institute
(Beijing, China). The gain rate of serum ACTH and CORT levels after
vs. prior to stress were calculated as follows: Gain rate
(%)=[(Concentration after stress-concentration before
stress)/concentration before stress] × 100.
Reverse-transcription quantitative polymerase
chain reaction (RT-qPCR)
RT-qPCR was performed to assess the mRNA expression
levels of MR and GR in the hippocampus as well as CRH and AVP in
the hypothalamus. Oligonucleotide primers used in the present study
for PCR were all synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China) and are listed in Table I.
Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions, and its concentration and purity were
determined with a spectrophotometer. Total RNA was stored in
diethyl pyrocarbonate-treated H2O (DEPC-H2O)
at −80°C. RT-qPCR analysis was performed using a reverse
transcription and qPCR kit (Takara Biotechnology Co., Ltd., Dalian,
China), and reactions were performed and products were analyzed
using an ABI PRISM 7300 instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Single-stranded complementary (c)DNA was
prepared according to the protocol of the Exscript RT reagent kit
(Takara Biotechnology Co., Ltd.). The amplification system had a
total reaction volume of 25 µl containing 2 µl of a 0.1 µg/µl
solution of cDNA template, 0.5 µl of a 10 µmol/l solution of each
primer, 12.5 µl 2X Premix Ex Taq, 0.5 µl 20X SYBR-Green I and 9 µl
DEPC-H2O. The mRNA levels of the housekeeping gene
β-actin were measured as quantitative controls and each sample was
normalized to the specific β-actin mRNA content (26). PCR amplification was performed using
the following protocol: Initial denaturation step at 95°C for 5
min; 40 cycles of 95°C for 5 sec, with various annealing conditions
(Table I) and a step at 72°C for 30
sec if the annealing temperature was <60°C.
 | Table I.Primers and annealing conditions. |
Table I.
Primers and annealing conditions.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Annealing
temperature (°C)/time (sec) |
|---|
| β-actin |
GTTGCCAATAGTGATGACCT |
GGACCTGACAGACTACCTCA | 54/20 |
| CRH |
AGAACAACAGTGCGGGCTCA |
GCTCCGGTTGCAAGAAATTCA | 60/30 |
| AVP |
AAGAGGGCCACATCCGACA |
AGGGCAGGTAGTTCTCCTCCTG | 58/20 |
| MR |
TGCATGATCTCGTGAGTGA |
AAGTTCTTCCTGGCCGGTAT | 62/30 |
| GR |
CACCCATGACCCTGTCAGTC |
AAAGCCTCCCTCTGCTAACC | 61/30 |
Statistical analysis
Data were analyzed using SPSS 17 (SPSS Inc.,
Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La
Jolla, CA, USA). Values are expressed as the mean ± standard error
of the mean. Analysis with Student's unpaired t-test was used to
compare the mean of different groups, as applicable. Analysis with
a paired t-test was used to compare the means of the same group
before and after chronic stress, assuming equal variances.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HFD from PW 4–12 increases rat body
weight gain
As shown in Table
II, there were no significant differences in the body weight
between the HFD, CMS, HFD + CMS and control groups before PW 10.
The body weight in the HFD group was significantly increased at PW
12 compared with that in the control group (P<0.01). The body
weight in the HFD + CMS group at PW 12 as well as that in the HFD
group at PWs 6, 8 and 10 was also increased relative to the control
at the respective time-points; however, the differences were not
statistically significant.
 | Table II.Body weight and weight gain rates in
female rats. |
Table II.
Body weight and weight gain rates in
female rats.
|
| PW 4 | PW 6 | PW 8 | PW 10 | PW 12 |
|---|
|
|
|
|
|
|
|
|---|
| Group | Body weight
(g) | Body weight
(g) | Gain rate (%) | Body weight
(g) | Gain rate (%) | Body weight
(g) | Gain rate (%) | Body weight
(g) | Gain rate (%) |
|---|
| Control | 48.5±1.9 | 76.6±3.2 | 54.3±3.9 | 94.6±4.4 | 96.3±10.4 | 126.8±6.0 | 162.5±12.7 | 176.4±7.6 | 265.4±12.5 |
| HFD | 53.2±2.4 | 81.4±3.8 | 54.9±8.9 | 103.2±4.4 | 96.4±10.9 | 138.4±5.5 | 163.5±13.3 |
210.5±5.9a | 302.6±23.5 |
| CMS | 48.6±1.3 | 78.6±1.6 | 62.2±4.4 | 104.4±3.4 | 95.1±7.5 | 130.8±3.8 | 169.7±8.7 | 170.1±4.1 | 266.5±8.5 |
| HFD+CMS | 54.1±2.2 | 84.5±2.3 | 56.2±4.7 | 104.6±3.3 | 94.7±7.2 | 137.5±7.4 | 155.2±15.5 | 193.9±7.1 | 260.2±11.2 |
Post-weaning HFD and CMS induce
behavioral alterations in female rats
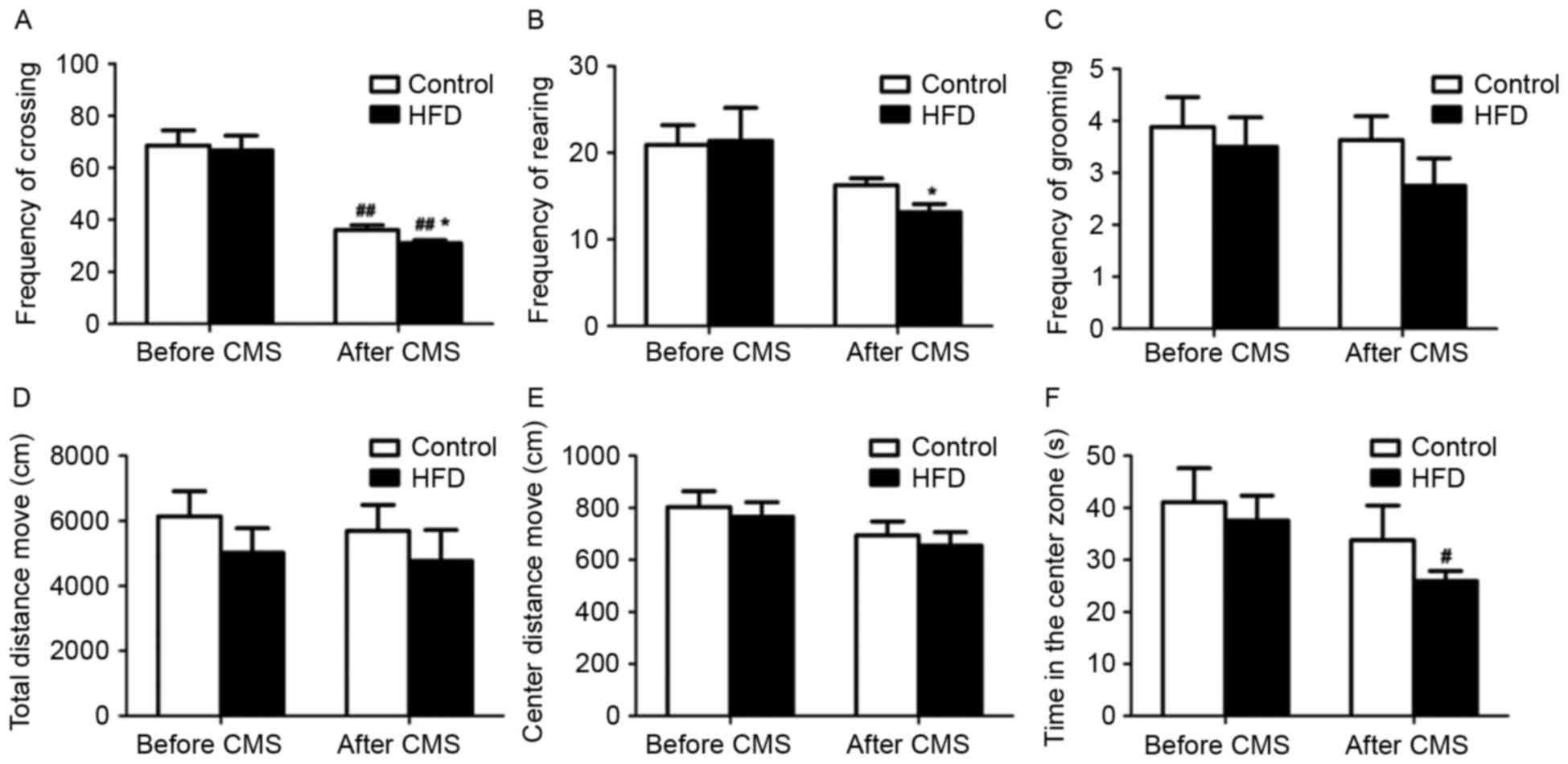
In the open field test, CMS significantly decreased
the frequency of crossing in rats receiving a normal diet or HFD
(P<0.01; Fig. 2A and B).
Furthermore, after CMS, the frequency of crossing and rearing in
the rats receiving a HFD was lower compared to that in rats
receiving a normal diet (P<0.05). However, there were no
significant changes in the frequency of grooming, total distance
moved and distance moved from the center between the HFD and HFD +
CMS groups (Fig. 2C-E). Of note, CMS
significantly reduced the time spent in the central zone of the
maze by rats in rats receiving an HFD (P<0.05; Fig. 2F).
The rats were subjected to the electric maze test to
assess animal behavior after stress. Prior to CMS, there were no
differences in reaction time and the TTE between the HFD and
control groups. However, after CMS, the TOCR was significantly
decreased (P<0.05) and the total TTE was slightly increased in
the HFD group relative that in the control group (Table III). The TOCR of both the control
and HFD groups were significantly decreased after CMS (P<0.01).
However, the change in the HFD group after CMS was greater than
that in the control. The total TTE did not markedly change.
 | Table III.TOCR and TTE of the female rats in
the electric maze test prior to and after CMS. |
Table III.
TOCR and TTE of the female rats in
the electric maze test prior to and after CMS.
|
| Before CMS | After CMS |
|---|
|
|
|
|
|---|
| Group | TOCR (count) | TTE (sec) | TOCR (count) | TTE (sec) |
|---|
| Control | 7.62±0.37 | 65.98±4.96 |
6.00±0.76a | 78.44±4.91 |
| HFD | 7.12±0.29 | 64.92±9.34 |
4.75±1.03a,b | 87.21±5.12 |
Post-weaning HFD and CMS affect serum
ACTH and CORT levels in female rats
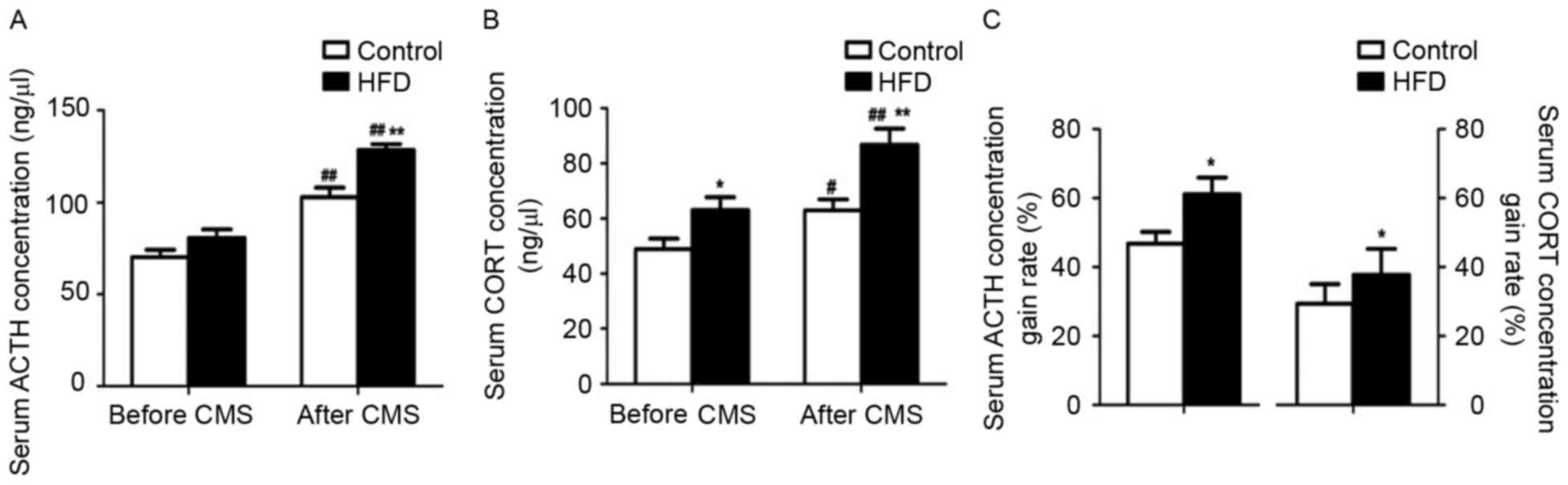
Prior to CMS, the serum ACTH levels were similar
between the control and HFD groups, while the CORT levels were
increased in the HFD group (P<0.05). After CMS, the group
receiving the HFD showed higher serum ACTH and CORT levels than the
group on a normal diet (P<0.01; Fig.
3A and B). Furthermore, in the rats receiving a normal diet as
well as a HFD, the ACTH (P<0.01) and CORT (P<0.05 and
P<0.01) levels were elevated after CMS, while the CMS-induced
increases in the levels of these hormones were considerably higher
in the HFD rats compared with those in the rats receiving a normal
diet (P<0.05; Fig. 3C).
Post-weaning HFD and CMS affect
hypothalamic CRH and AVP mRNA levels in female rats
The AVP and CRH mRNA levels in the hypothalamus were
significantly increased in the HFD + CMS group (P<0.05 and
P<0.01, respectively) compared to those in the control group
(Fig. 4A and B). In addition, the
CRH mRNA levels in the hypothalamus were significantly increased in
the HFD group compared with those in the control group (P<0.01).
Furthermore, CMS significantly increased the CRH mRNA levels in the
hypothalamus compared with those in the control group (P<0.05;
Fig. 4B). Finally, the CRH mRNA
levels in the hypothalamus were higher in the HFD+CMS group than
those in the CMS group (P<0.05).
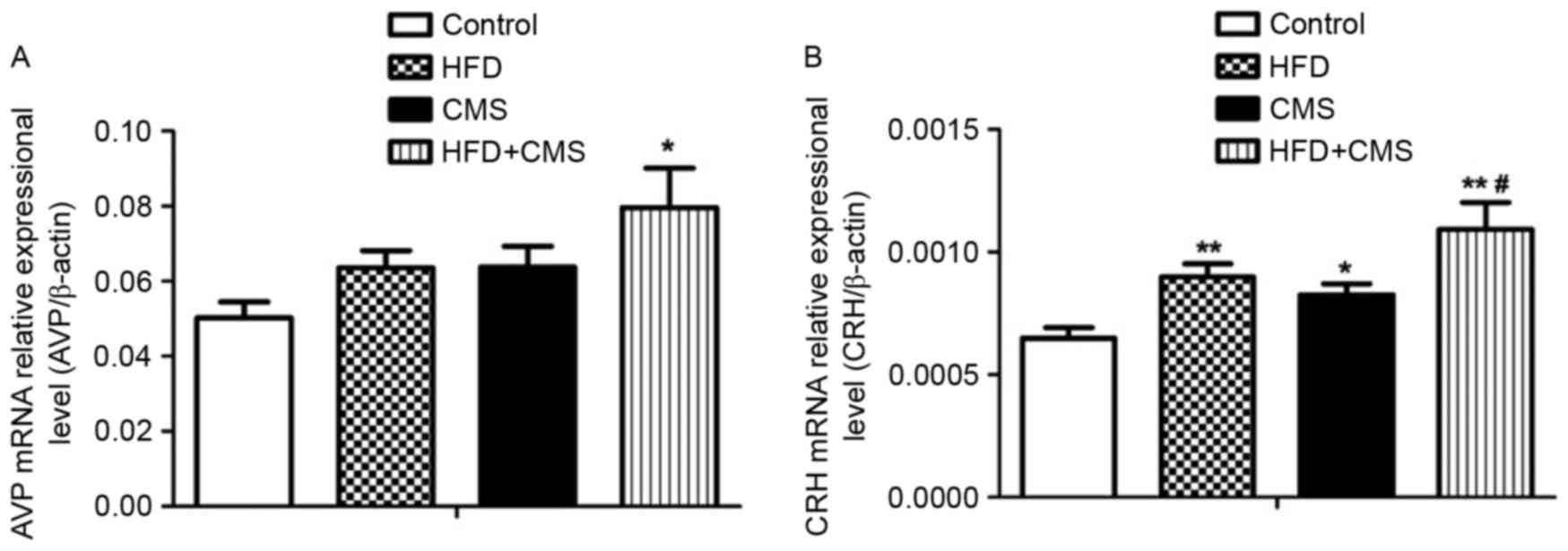 | Figure 4.Effects of a post-weaning HFD on gene
expression levels of hypothalamic (A) CRH and (B) AVP in the
control, HFD, CMS and CMS + HFD groups at PW 13. Values are
expressed as the mean ± standard error of the mean (n=8).
*P<0.05 and **P<0.01 vs. control; #P<0.05 vs.
CMS. Groups: Control, rats receiving normal diet and not subjected
to CMS; CMS, rats receiving normal diet and subjected to CMS (at PW
9–12); HFD, rats receiving HFD (24% fat by weight from PW 4–12) but
not subjected to CMS; CMS + HFD, rats receiving a HFD and subjected
to CMS. CRH, corticotropin-releasing hormone; AVP, arginine
vasopressin; HFD, high-fat diet; CMS, chronic mild stress; PW,
postnatal weeks. |
Effects of post-weaning HFD and CMS on
hippocampal MR and GR mRNA expression levels in female rats
No significant changes were observed in the
hippocampal MR and GR mRNA levels between the HFD and control
groups (Fig. 5A and B). Among all of
the groups, no significant changes in the hippocampal MR mRNA
expression were detected. However, the GR gene expression levels in
the hippocampus were significantly lower in the HFD + CMS group
than in the HFD and CMS groups (all P<0.05; Fig. 5B); in addition, the MR/GR ratio in
the HFD + CMS group was overtly increased (P<0.05; Fig. 5C).
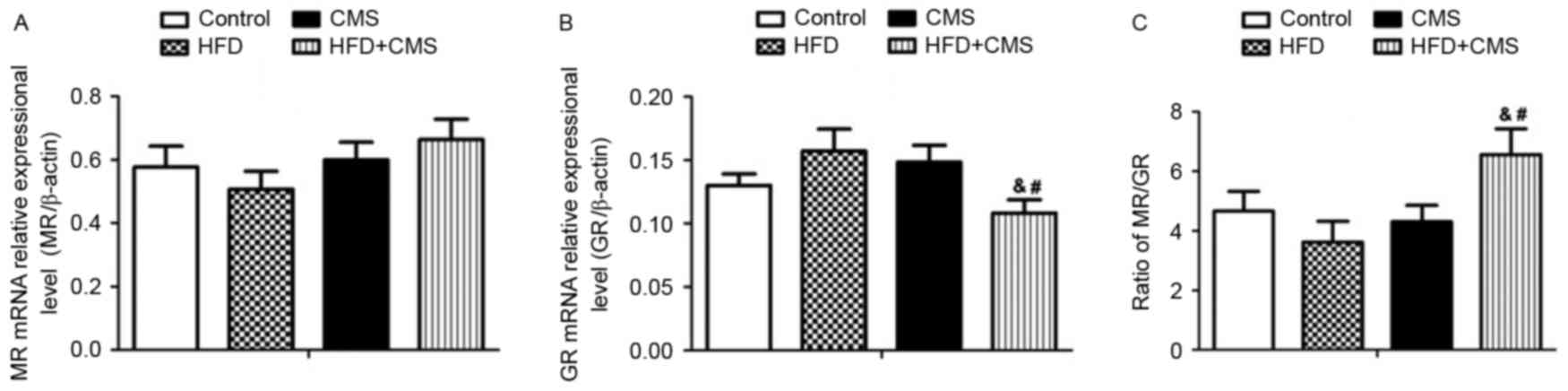 | Figure 5.Effects of post-weaning HFD on
hippocampal expression levels of (A) MR, (B) GR and (C) the ration
of MR/GR in the control, HFD, CMS and CMS + HFD groups at PW 13.
Values are expressed as the mean ± standard error of the mean
(n=8). &P<0.05 vs. HFD group;
#P<0.05 vs. CMS group. Groups: Control, rats
receiving normal diet and not subjected to CMS; CMS, rats receiving
normal diet and subjected to CMS (at PW 9–12); HFD, rats receiving
HFD (24% fat by weight from PW 4–12) but not subjected to CMS; CMS
+ HFD, rats receiving a HFD and subjected to CMS. HFD, high-fat
diet; CMS, chronic mild stress; PW, postnatal weeks; MR,
mineralocorticoid receptor; GR, glucocorticoid receptor. |
Discussion
The high-fat rat diet used in the present study (24%
fat) mimics the HFD consumed by humans (6,7).
Previous studies have shown that women are more prone to mental
diseases after HFD and exhibit a higher sensitivity to CMS than men
(27), which is why only female rats
were assessed in the present study. Fat accumulation is more
pronounced when energy is gained from dietary fat rather than from
carbohydrates or proteins (28). In
the present study, the rat body weight did not show any significant
difference between the HFD and control groups at PW 10, while the
increase was significant at PW 12. While HFD obviously induced the
increase in body weight, it may have also in part been attributed
to enhanced food intake, metabolic disturbance of adipocytes and
energy imbalance, eventually leading to an obesity-like state
(29). Of note, there was no
significant body weight change between the CMS and control groups,
indicating that CMS alone did not induce any changes in weight
gain, indicating that CMS alone without a HFD does not lead to
obesity.
Behavioral alterations are a major symptom of mental
disorders (30,31). These behavioral alterations are
induced by regulatory dysfunction of the cerebral limbic system
(32). A maternal HFD has been shown
to lead to anxiety in offspring, altering the expression levels of
multiple genes via glucocorticoid signaling (33). HFD was shown to have rapid and
long-lasting negative effects on memory in model mice with and
without Alzheimer's disease (34).
The open field test is a classic experimental method for behavioral
evaluation, including locomotor activity and exploratory behavior,
and the electric maze test is used to assess learning and memory.
In the open field test performed in the present study, application
of CMS resulted in a significantly reduced frequency of crossings
in rats with and without HFD as well as a decreasing tendency in
the frequency of rearing, and the time in the center zone in the
HFD group significantly reduced following CMS, suggesting
depression-like symptoms after CMS. The electric maze test revealed
that CMS significantly reduced the TOCR of mice with or without HFD
and increased the TTE, particularly in the HFD group, suggesting
that post-weaning HFD not only predisposed female rats to
psychiatric disorders but also altered learning and memory in them.
Further study is required to confirm whether HFD may alter learning
and memory. Taken together, HFD and CMS appear to act
synergistically to cause depression-like symptoms. Indeed, multiple
studies have suggested that CMS causes depression-like behaviors,
such as alterations of affective processes and impairment of
cognitive performance (35).
The HPA axis has a vital role in the stress response
and is affected by a number of factors (36–38).
Animal experiments have shown that food restriction increased
adrenal gland weight and serum CORT levels after overnight fasting
in rats (36), and enhanced HPA axis
activity resulting in appetite loss and anorexia nervosa (39). Mixed results have been reported by
studies assessing the effects of HFD exposure on CORT during
developmental stages under basal conditions and after stress
challenge (2,40). In the present study, CMS resulted in
higher ACTH and CORT levels and higher expression of CRH gene in
hypothalamus tissue in the HFD group compared to that in the
control group, suggesting that HFD may enhance HPA axis sensitivity
to chronic stress. Considerable evidence suggested associations
between certain psychiatric disorders and HPA axis alterations,
such as elevated glucocorticoid levels (10,41–43).
Furthermore, several psychiatric disorders associated with HPA
dysfunction arise in obese teenagers, who are more prone to develop
depression than adults (44).
Therefore, it can be speculated that behavioral alterations in
female rats with post-weaning HFD may be attributed to
hypersensitivity of the HPA axis induced by HFD.
The hippocampus is the main negative feedback
regulation center of the HPA axis. MR and GR are the steroid
receptors predominantly expressed in the hippocampus. The
hippocampus regulates HPA function in a negative feedback pattern
via the binding of circulating GCs to hippocampal GR and MR. These
two receptors have different roles in HPA axis regulation under
different conditions. Under basal conditions, the hippocampal MR is
mainly responsible for the regulation of the HPA axis by
glucocorticoids, while GR becomes the major receptor for
glucocorticoid under stress (45).
Activation of these receptors can modulate the neuronal system
associated with function including memory, behavior, responses to
stimuli and anxiety (45). MR
harbors a neuroprotective function in the hippocampus (46), while GR is a typical target of
neuronal signaling that is sensitive and vulnerable to GC and its
over-activation would damage hippocampal neurons (45,47),
which may lead to behavioral alterations. For instance, HFD
administered from post-weaning age until puberty was found to
decrease the hippocampal GR protein expression in female rats
(6). In the present study,
hippocampal MR mRNA levels showed an increasing tendency after CMS,
while GR expression was significantly reduced in the HFD group,
increasing the MR/GR ratio. Zhe et al (48) demonstrated that stress induced
downregulation of MR and GR and the MR/GR ratio in the hippocampus.
MR carries neuron-protective function in the hippocampus; the
increase in MR mRNA in hippocampus is involved in negative
feedback. However, to the best of our knowledge, there have been no
reports on the association between the HFD and the hippocampal MR
expression. A change in MR/GR ratio alters the ability to maintain
homeostasis, which, in turn, alters neuronal excitability, stress
responsiveness and behavioral adaptation to a condition of enhanced
vulnerability to disease (49).
Therefore, it can be speculated that dysregulation of the MR/GR
balance may aggravate HPA axis hypersensitivity, which may be an
important cause of behavioral alterations.
In conclusion, the present study revealed that
post-weaning HFD increased HPA activity in female rats, and the
combination of HFD and CMS enhanced those effects. In addition,
female rats that were fed a HFD exhibited impaired memory and
learning skills following CMS. These effects may be due to
hippocampal MR/GR dysregulation, which further aggravated HPA axis
hypersensitivity.
Acknowledgements
This work was supported by grants from the Science
Foundation of Hubei Provincial Department of Education (grant nos.
Q20151308 and Q20141301), the Yangtze Fund for Youth Teams of
Science and Technology Innovation (grant no. 2016YZYT1128), Hubei
Province Health and Family Planning Scientific Research Project
(grant no. WJ2016-YZ-01), the Medical School of Yangtze University
Foundation (grant nos. YXYQ201401 and YXYQ201405).
References
|
1
|
Peleg-Raibstein D, Luca E and Wolfrum C:
Maternal high-fat diet in mice programs emotional behavior in
adulthood. Behav Brain Res. 233:398–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auvinen HE, Romijn JA, Biermasz NR, Pijl
H, Havekes LM, Smit JW, Rensen PC and Pereira AM: The effects of
high fat diet on the basal activity of the
hypothalamus-pituitary-adrenal axis in mice. J Endocrinol.
214:191–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rofey DL, Kolko RP, Iosif AM, Silk JS,
Bost JE, Feng W, Szigethy EM, Noll RB, Ryan ND and Dahl RE: A
longitudinal study of childhood depression and anxiety in relation
to weight gain. Child Psychiatry Hum Dev. 40:517–526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Connor TG: Prenatal and postnatal
exposure to an unhealthy diet is associated with behavioural and
emotional problems in children. Evid Based Ment Health. 17:382014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soulis G, Papalexi E, Kittas C and Kitraki
E: Early impact of a fat-enriched diet on behavioral responses of
male and female rats. Behav Neurosci. 121:483–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boukouvalas G, Antoniou K, Papalexi E and
Kitraki E: Post weaning high fat feeding affects rats' behavior and
hypothalamic pituitary adrenal axis at the onset of puberty in a
sexually dimorphic manner. Neuroscience. 153:373–382. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boukouvalas G, Gerozissis K and Kitraki E:
Adult consequences of post-weaning high fat feeding on the
limbic-HPA axis of female rats. Cell Mol Neurobiol. 30:521–530.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verma R, Balhara YP and Gupta CS: Gender
differences in stress response: Role of developmental and
biological determinants. Ind Psychiatry J. 20:4–10. 2011.PubMed/NCBI
|
|
9
|
Szabo K and Hennerici MG: The Hippocampus
in Clinical Neuroscience. Front Neurol Neurosci Basel Karger. 2014.
View Article : Google Scholar
|
|
10
|
Li SX, Yan SY, Bao YP, Lian Z, Qu Z, Wu YP
and Liu ZM: Depression and alterations in
hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid
axis function in male abstinent methamphetamine abusers. Hum
Psychopharmacol. 28:477–483. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreno-Ramos OA, Lattig MC and González
Barrios AF: Modeling of the hypothalamic-pituitary-adrenal
axis-mediated interaction between the serotonin regulation pathway
and the stress response using a Boolean approximation: A novel
study of depression. Theor Biol Med Model. 10:592013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holsboer F and Ising M: Central CRH system
in depression and anxiety-evidence from clinical studies with CRH1
receptor antagonists. Eur J Pharmacol. 583:350–357. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki A, De Vega WC, St-Cyr S, Pan P and
McGowan PO: Perinatal high fat diet alters glucocorticoid signaling
and anxiety behavior in adulthood. Neuroscience. 240:1–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
García-Díaz DF, Campion J, Milagro FI,
Lomba A, Marzo F and Martínez JA: Chronic mild stress induces
variations in locomotive behavior and metabolic rates in high fat
fed rats. J Physiol Biochem. 63:337–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stöhr T, Szuran T, Welzl H, Pliska V,
Feldon J and Pryce CR: Lewis/Fischer rat strain differences in
endocrine and behavioural responses to environmental challenge.
Pharmacol Biochem Behav. 67:809–819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: A 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willner P, Muscat R and Papp M: Chronic
mild stress-induced anhedonia: A realistic animal model of
depression. Neurosci Biobehav Rev. 16:525–534. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muscat R and Willner P: Suppression of
sucrose drinking by chronic mild unpredictable stress: A
methodological analysis. Neurosci Biobehav Rev. 16:507–517. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patterson ZR, Ducharme R, Anisman H and
Abizaid A: Altered metabolic and neurochemical responses to chronic
unpredictable stressors in ghrelin receptor-deficient mice. Eur J
Neurosci. 32:632–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Westenbroek C, Ter Horst GJ, Roos MH,
Kuipers SD, Trentani A and den Boer JA: Gender-specific effects of
social housing in rats after chronic mild stress exposure. Prog
Neuropsychopharmacol Biol Psychiatry. 27:21–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patterson ZR and Abizaid A: Stress induced
obesity: Lessons from rodent models of stress. Front Neurosci.
7:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou DR, Wang Y, Zhou L, Chen K, Tian Y,
Song Z, Bao J and Yang QD: Altered angiotensin-converting enzyme
and its effects on the brain in a rat model of Alzheimer disease.
Chin Med J (Engl). 121:2320–2323. 2008.PubMed/NCBI
|
|
23
|
Zhang L, Xu D, Zhang B, Liu Y, Chu F, Guo
Y, Gong J, Zheng X, Chen L and Wang H: Prenatal food restriction
induces a hypothalamic-pituitary-adrenocortical axis-associated
neuroendocrine metabolic programmed alteration in adult offspring
rats. Arch Med Res. 44:335–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xinxing W, Wei L, Lei W, Rui Z, Baoying J
and Lingjia Q: A neuroendocrine mechanism of co-morbidity of
depression-like behavior and myocardial injury in rats. PLoS One.
9:e884272014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Liu F, Kou H, Zhang BJ, Xu D, Chen
B, Chen LB, Magdalou J and Wang H: Prenatal nicotine exposure
induced a hypothalamic-pituitary-adrenal axis-associated
neuroendocrine metabolic programmed alteration in intrauterine
growth retardation offspring rats. Toxicol Lett. 214:307–313. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao AM, Meynen G and Swaab DF: The stress
system in depression and neurodegeneration: Focus on the human
hypothalamus. Brain Res Rev. 57:531–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woods SC, Seeley RJ, Rushing PA, D'Alessio
D and Tso P: A controlled high-fat diet induces an obese syndrome
in rats. J Nutr. 133:1081–1087. 2003.PubMed/NCBI
|
|
29
|
McAllan L, Keane D, Schellekens H, Roche
HM, Korpela R, Cryan JF and Nilaweera KN: Whey protein isolate
counteracts the effects of a high-fat diet on energy intake and
hypothalamic and adipose tissue expression of energy
balance-related genes. Br J Nutr. 110:2114–2126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nelovkov A, Philips MA, Kõks S and Vasar
E: Rats with low exploratory activity in the elevated plus-maze
have the increased expression of limbic system-associated membrane
protein gene in the periaqueductal grey. Neurosci Lett.
352:179–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoogenboom WS, Perlis RH, Smoller JW,
Zeng-Treitler Q, Gainer VS, Murphy SN, Churchill SE, Kohane IS,
Shenton ME and Iosifescu DV: Limbic system white matter
microstructure and long-term treatment outcome in major depressive
disorder: A diffusion tensor imaging study using legacy data. World
J Biol Psychiatry. 15:122–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Winter SS, Köppen JR, Ebert TB and Wallace
DG: Limbic system structures differentially contribute to
exploratory trip organization of the rat. Hippocampus. 23:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki A, De Vega W, Sivanathan S, St-Cyr
S and McGowan PO: Maternal high-fat diet alters anxiety behavior
and glucocorticoid signaling in adolescent offspring. Neuroscience.
272:92–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knight EM, Martins IV, Gümüsgöz S, Allan
SM and Lawrence CB: High-fat diet-induced memory impairment in
triple-transgenic Alzheimer's disease (3xTgAD) mice is independent
of changes in amyloid and tau pathology. Neurobiol Aging.
35:1821–1832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sahin TD, Karson A, Balci F, Yazir Y,
Bayramgurler D and Utkan T: TNF-alpha inhibition prevents cognitive
decline and maintains hippocampal BDNF levels in the unpredictable
chronic mild stress rat model of depression. Behav Brain Res.
292:233–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Belda X, Ons S, Carrasco J and Armario A:
The effects of chronic food restriction on
hypothalamic-pituitary-adrenal activity depend on morning versus
evening availability of food. Pharmacol Biochem Behav. 81:41–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kokavec A, Lindner AJ, Ryan JE and Crowe
SF: Ingesting alcohol prior to food can alter the activity of the
hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav.
93:170–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Naert G, Ixart G, Maurice T,
Tapia-Arancibia L and Givalois L: Brain-derived neurotrophic factor
and hypothalamic-pituitary-adrenal axis adaptation processes in a
depressive-like state induced by chronic restraint stress. Mol Cell
Neurosci. 46:55–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lawson EA, Holsen LM, Desanti R, Santin M,
Meenaghan E, Herzog DB, Goldstein JM and Klibanski A: Increased
hypothalamic-pituitary-adrenal drive is associated with decreased
appetite and hypoactivation of food-motivation neurocircuitry in
anorexia nervosa. Eur J Endocrinol. 169:639–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shalev U, Tylor A, Schuster K, Frate C,
Tobin S and Woodside B: Long-term physiological and behavioral
effects of exposure to a highly palatable diet during the perinatal
and post-weaning periods. Physiol Behav. 101:494–502. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Csabafi K, Jászberènyi M, Bagosi Z, Liptàk
N and Telegdy G: Effects of kisspeptin-13 on the
hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and
locomotor activity in rats. Behav Brain Res. 241:56–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kenny R, Dinan T, Cai G and Spencer SJ:
Effects of mild calorie restriction on anxiety and
hypothalamic-pituitary-adrenal axis responses to stress in the male
rat. Physiol Rep. 2:e002652014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sarubin N, Nothdurfter C, Schüle C, Lieb
M, Uhr M, Born C, Zimmermannc R, Bühner M, Konopka K, Rupprecht R
and Baghai TC: The influence of Hatha yoga as an add-on treatment
in major depression on hypothalamic-pituitary-adrenal-axis
activity: A randomized trial. J Psychiatr Res. 53:76–83. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McElroy SL, Kotwal R, Malhotra S, Nelson
EB, Keck PE and Nemeroff CB: Are mood disorders and obesity
related? A review for the mental health professional. J Clin
Psychiatry. 65:634–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Kloet ER, Vreugdenhil E, Oitzl MS and
Joëls M: Brain corticosteroid receptor balance in health and
disease. Endocr Rev. 19:269–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Kloet ER, Sutanto W, van den Berg DT,
Carey MP, van Haarst AD, Hornsby CD, Meijer OC, Rots NY and Oitzl
MS: Brain mineralocorticoid receptor diversity: Functional
implications. J Steroid Biochem Mol Biol. 47:183–190. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Quervain DJ, Aerni A, Schelling G and
Roozendaal B: Glucocorticoids and the regulation of memory in
health and disease. Front Neuroendocrinol. 30:358–370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhe D, Fang H and Yuxiu S: Expressions of
hippocampal mineralocorticoid receptor (MR) and glucocorticoid
receptor (GR) in the single-prolonged stress-rats. Acta Histochem
Cytochem. 41:89–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Kloet ER, Oitzl MS and Joëls M:
Functional implications of brain corticosteroid receptor diversity.
Cell Mol Neurobiol. 13:433–455. 1993. View Article : Google Scholar : PubMed/NCBI
|