Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection. Septic shock and multiple organ dysfunction
syndrome (MODS) often occur in patients with sepsis (1,2). Despite
decades of research, the mortality rates of patients with sepsis
remain high and sepsis is one of the major reasons for deaths of
patients in ICU (3). Therefore, it
is necessary to diagnose sepsis timely and accurately in order to
reasonably evaluate the severity and prognosis of patients with
sepsis. In addition to the uncontrolled inflammation reactions,
oxidative stress is an important factor in the occurrence and
progression of sepsis. In sepsis, the inflammatory response and
oxidative stress often interact as both the cause and effect,
forming a vicious cycle and eventually resulting in damage to
tissue and multiple organs (4,5).
Therefore, studies on oxidative stress in patients with sepsis can
be beneficial in furthering the understanding of the diagnosis,
evaluation and treatment of sepsis. Insulin-like growth factor-1
(IGF-1) functions to maintain cell survival and proliferation and
anti-apoptosis mechanisms. Studies have shown that IGF-1 also has
anti-inflammation and anti-oxidative effects against stress
injuries (6,7). Studies have also demonstrated that
IGF-1 is regulated by oxidative stress. Notably it has also been
demonstrated that IGF-1 levels in blood samples are reduced in the
acute phase for critical patients (8). However, it remains unclear whether
IGF-1 has an anti-oxidative stress role in sepsis and the
association between IGF-1 and the severity or prognosis of patients
with sepsis is also ambiguous.
Micro (mi)RNAs, a type of small non-coding RNAs, are
also being increasingly investigated in studies about critical
diseases, and bioinformatics studies have shown that IGF-1 may be a
target gene of miRNA-1 (9,10). Therefore, this study was not only
designed to investigate whether serum IGF-1 levels could predict
the severity and prognosis in patients with sepsis, but we also
tried to determine the association between IGF-1 and miRNA-1 in
patients. H2O2 has previously been proven to
induce oxidative injury in cells (11,12);
therefore, in vitro we used H2O2 to
simulate oxidative stress in order to reveal the underlying
mechanism involved in miRNA-1 and oxidative stress.
Materials and methods
Patients and study design
A total of 64 patients (age 48.9±7.3 years; 35 male,
29 female) with sepsiswere enrolled between June 2012 and July 2014
and were used as the research subjects. The diagnostic criteria for
sepsis were in accordance with the definitions provided by the
American College of Chest Physicians and the Society of Critical
Care Medicine (13). A total of 35
healthy volunteers (age 48.2±4.9 years; 25 males, 10 females) who
underwent physical examination at the hospital during the same
period were included as healthy control subjects. Exclusion
criteria were: Aged <18 years old; suffering from cancer;
pregnancy; patients with severe chronic diseases of the heart,
liver, kidney or lung; and patients died within 24 h of
hospitalization. After patients with sepsis were enrolled in the
Intensive Care Unit (ICU), their age, sex, medical history,
diagnosis and sepsis-related organ failure assessment (SOFA) score
were recorded, their vital signs were monitored, and blood gas
analysis was performed. Conventional treatment was performed, with
a 28-day follow-up period. All patients with sepsis were divided
into three subgroups depending on the severity (sepsis, severe
sepsis or septic shock). Sepsis complicated by organ dysfunction is
referred to as severe sepsis, while sepsis complicated by
hypotension refractory to adequate volume resuscitation in the
absence of an alternate cause is termed septic shock (14).
All patients were divided into a survival group or
death group according to their 28-day survival. This study was
approved by Medical Ethics Committee of Zhejiang Provincial
People's Hospital and all subjects gave written informed
consent.
Detection of IGF-1 levels by
radioimmunoassay
Venous blood samples (3 ml) were collected from
patients with sepsis within 12 h after diagnosis and the subjects
in the healthy control group. After standing for 4 h at room
temperature, the samples were centrifuged at 2,500 × g for 15 min
at room temperature to separate the serum. Serum IGF-1 content was
detected by a radioimmunoassay kit (DSL-10-9400; Diagnostic Systems
Laboratories, Inc., Webster, TX, USA) in strict accordance with the
manufacturer's protocol using a radioimmunoassay system (M3687;
Beijing Midwest Technology Co., Ltd., Beijing, China).
Detection of miRNA-1 levels by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNA was extracted from serum specimens using a
MiRcute miRNA Extraction-Separation kit (Beijing Tiangen Biotech
Co., Ltd., Beijing, China) according to the manufacturer's
instructions. After the purity and concentration was measured, RT
of the miRNA to cDNA was performed using a One Step PrimeScript
miRNA cDNA synthesis kit (Dalian Takara Co., Ltd., Dalian, China).
RT was performed in a T100 Thermal Cycler PCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the reaction conditions
were prepared according to the instructions. The obtained cDNA was
amplified by qPCR using SYBR Premix Ex Taq II (Dalian Takara
Co., Ltd.). qPCR was performed in a Thermal Cyclers Dice real time
PCR system (Bio-Rad Laboratories, Inc.). Thermal reaction
conditions were in accordance with the manufacturer's instructions:
Each 15 µl of reaction mixture was incubated for 10 sec at 95°C and
then amplified for 40 cycles. Each cycle had two steps; 95°C for 5
sec and 60°C for 30 sec. The miRNA-1 upstream primer sequence was
5′-GCGTGGAATGTAAAGAAGTAT-3′. Data were normalized by cel-miR-39 as
reference, its upstream primer sequence was
5′-GGAGGTTAATGCTAATTGTGATAG-3′. Relative expression levels of
miRNA-1 were expressed as 2−ΔΔCq (15).
Cell culture
Human alveolar epithelial cells (A549 cell line) and
human embryonic kidney cells (HKC cell line) were purchased from
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All
cells were cultured in Dulbecco's modified Eagle medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in an environment
containing 5% CO2.
Cell exposure to
H2O2 and cell death detection via ELISA
A549 cells and HKC cells were prepared into cell
suspensions, planted in 96-well plates at 1×104
cells/well, and routinely cultured for 24 h. Cells were exposed to
100 µM H2O2 for 48 h to simulate oxidative
stress. To confirm the protective effect of IGF-1, some cells
received treatment with 30 ng/ml IGF-1 while exposed to
H2O2. The number of DNA fragments of
apoptotic cells was detected via a cell death detection ELISA kit
(Roche Diagnostics GmbH, Basel, Switzerland) using an ELX808
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA),
which reflects the apoptotic conditions of the cells.
Detection of miRNA-1 in cells after
H2O2 exposure
A549 cells and HKC cells were treated with 100 µM
H2O2 and, after 48 h, cells were collected
and the miRNA-1 changes in cells were analyzed by RT-qPCR using the
protocol outlined. To normalize the RT-qPCR data, U6 small nuclear
RNA was used as a reference, its upstream primer sequence was
5′-CTCGCTTCGGCAGCACA-3′.
miRNA-1 transfection and detection of
IGF-1 mRNA
A549 cells and HKC cells were prepared into cell
suspension, and inoculated on 96-well plates at a density of 6,000
cells/well. When the confluency of cells reached ~80%, the miRNA-1
mimics were transfected into cells using a
Lipofectamine® 2000 transfection kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The working concentration of miRNA-1
mimics was 50 nM. The unrelated miRNA-1 mimic negative controls
were transfected using the same procedure. Cells were cultured
continuously for 48 h and the transfection effect was validated by
RT-qPCR. IGF-1 mRNA levels in A549 and HKC cells after transfection
were detected by RT-qPCR. Briefly, total RNA in cells was extracted
by TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using a PrimeScript RT
reagent kit (Dalian Takara Co., Ltd., Dalian, China). The RT system
was used in accordance with the manufacturer's instructions. The
synthesized cDNA was amplified using a SYBR Premix Ex Taq II
kit. The following primers were used: IGF-1, upstream
5′-TGCTCTCAACATCTCCCATC-3′ and downstream
5′-GAAGAGATGCGAGGAGGACA-3′; and GAPDH, upstream
5′-ACCACAGTCCATGCCATCAC-3′ and downstream
5′-TCCACCACCCTGTTGCTGTA-3′. The reaction system and program was
used in accordance with the manufacturer's instructions. Relative
expression of target IGF-1 mRNA was analyzed according to the
2−ΔΔCq method
(∆CT=CTIGF-1-CTGAPDH).
Assay for the effect of miRNA-1
transfection on the apoptosis induced by
H2O2
Transfected A549 cells and HKC cells were treated
with 100 µM H2O2, after 48 h. The number of
DNA fragments of apoptotic cells was detected by a cell death
detection ELISA kit.
Statistical analysis
Data analysis was performed using SPSS 19.0 software
(IBM SPSS, Armonk, NY, USA). Measurement data were expressed as the
mean ± standard deviation. For normality data, analysis of variance
was performed for comparison between multiple groups, and unpaired-
t test was performed to compare between two groups. For the
non-normal data, Mann-Whitney non-parametric U-test was performed
to compare between two groups. Spearman correlation analysis was
performed. The survival analysis adopted Kaplan-Meier curves.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline clinical data in patients
with sepsis
A total of 35 healthy subjects and 64 patients with
sepsis were included in this study (Table I). The healthy control group included
25 males and 10 females, with a mean age of 48.2±4.9 years; whereas
the sepsis group included 35 males and 29 females, with a mean age
of 49.6±9.6 years. The patients with sepsis were further divided
into subgroups according to the severity of disease, with 15 cases
in the sepsis subgroup, 30 cases in the severe sepsis subgroup, and
19 cases in the septic shock subgroups. During 28 days of
follow-up, 36 patients survived and 28 succumbed to shock. As shown
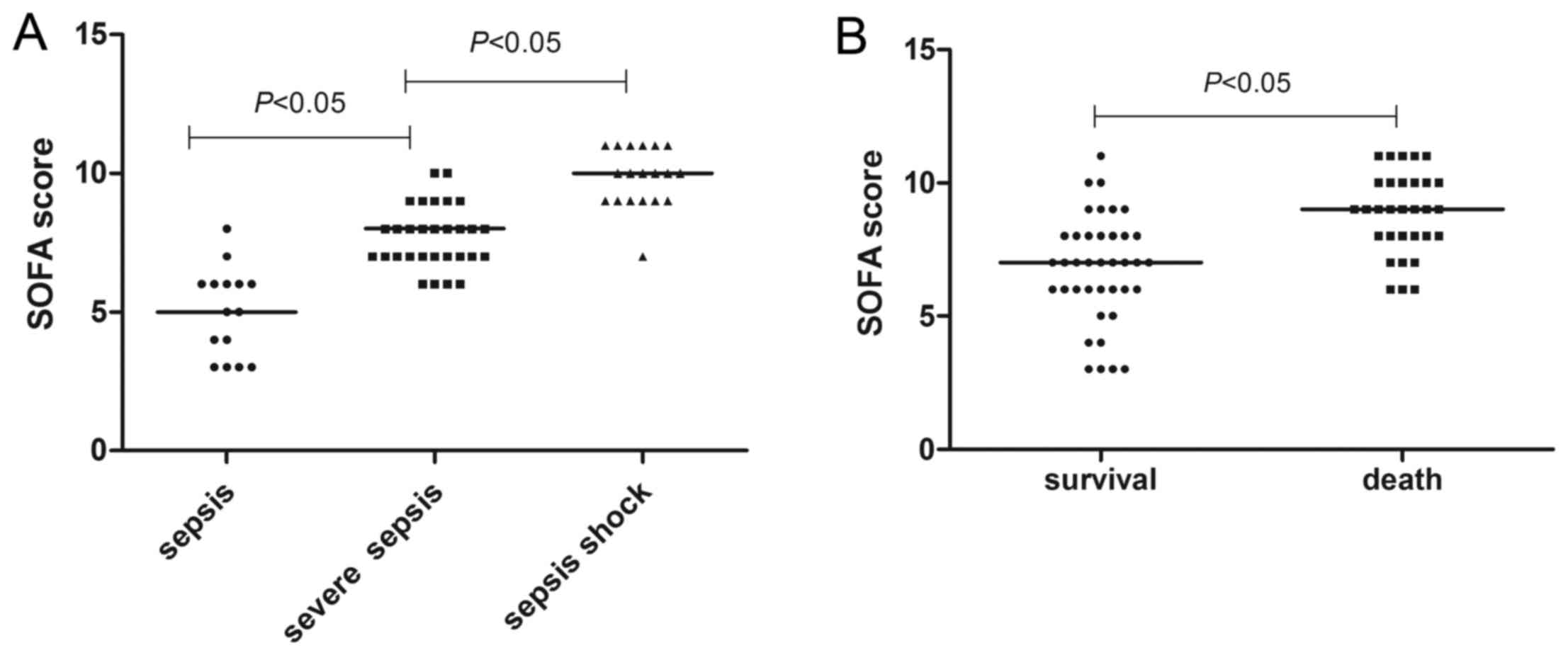
in Fig. 1, the median SOFA score of
patients with septic shock was 10 (7–11), which
was significantly higher than that of the other two subgroups (6
and 7; P<0.05). The median SOFA score of dead patients was
significantly higher than that of the patients who survived
follow-up (9 vs. 7; P<0.05). There were no significant
differences in age, sex and medical history between the healthy
control group and the sepsis group. Furthermore, there were no
significant differences in the age, sex, medical history, infection
site and type of infected bacteria between the sepsis
subgroups.
 | Table I.Baseline data of healthy subjects and
patients with sepsis. |
Table I.
Baseline data of healthy subjects and
patients with sepsis.
|
|
| Sepsis patients
(n=64) |
|
|---|
|
|
|
|
|
|---|
|
| Healthy control
(n=35) | Sepsis subgroup
(n=15) | Server subgroup
(n=30) | Septic shock
subgroup (n=19) | P-value |
|---|
| Age (years) | 48.2±4.9 | 47.3±3.6 | 49.5±4.2 | 49.8±6.1 | >0.05 |
| Sex |
|
|
|
| >0.05 |
|
Male | 25 | 7 | 17 | 11 |
|
|
Female | 10 | 8 | 13 | 8 |
|
| Blood culture |
|
|
|
| >0.05 |
|
Negative | – | 3 | 3 | 2 |
|
|
Gram+ bacteria | – | 2 | 11 | 6 |
|
|
Gram− bacteria | – | 8 | 15 | 9 |
|
|
Fungi | – | 2 | 1 | 2 |
|
| Primary infection
site |
|
|
|
| >0.05 |
| Lower
respiratory tract | – | 1 | 3 | 1 |
|
| Upper
respiratory tract | – | – | 2 | 2 |
|
| Urinary
tract | – | 1 | 5 | 2 |
|
|
Abdomen | – | 13 | 20 | 11 |
|
| SOFA score
(range) | – | 6 (3–7) | 7 (4–9) | 10 (7–11) | <0.05 |
| 28-day
survival | – |
|
|
| <0.05 |
|
Yes | – | 10 | 20 | 6 |
|
| No | – | 5 | 10 | 13 |
|
Serum IGF-1 levels correlate with the
severity and prognosis of patients with sepsis
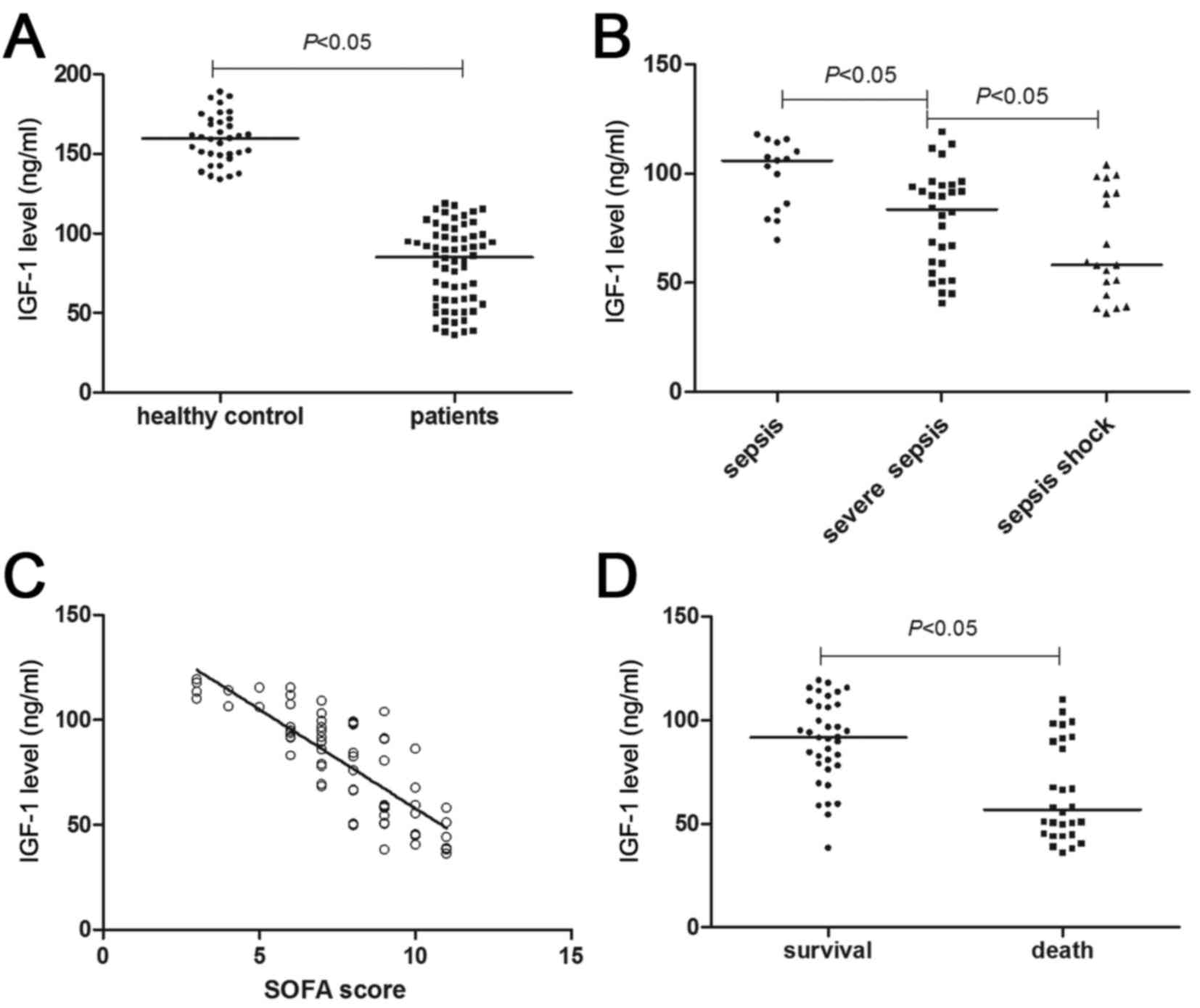
As shown in Fig. 2A,
median serum IGF-1 levels in patients with sepsis and in healthy
controls were 85.28 ng/ml (36.24–119.28 ng/ml) and 159.79 ng/ml
(134.02–188.96 ng/ml), respectively, with a significant difference
detected (P<0.05). In addition, with the aggravation of sepsis,
IGF-1 levels declined. As compared with the patients in the sepsis
and severe sepsis subgroups, patients in the septic shock subgroup
exhibited the lowest IGF-1 levels (P<0.05; Fig. 2B). For all patients with sepsis,
their IGF-1 levels were inversely proportional to their SOFA scores
(r=−0.66; P<0.05; Fig.
2C). These results suggested that a decline in IGF-1 may
predict a more severe condition of sepsis. For the prognosis, the
median IGF-1 level of patients in the death group was significantly
lower than that of the survival group (91.75 vs. 56.84 ng/ml;
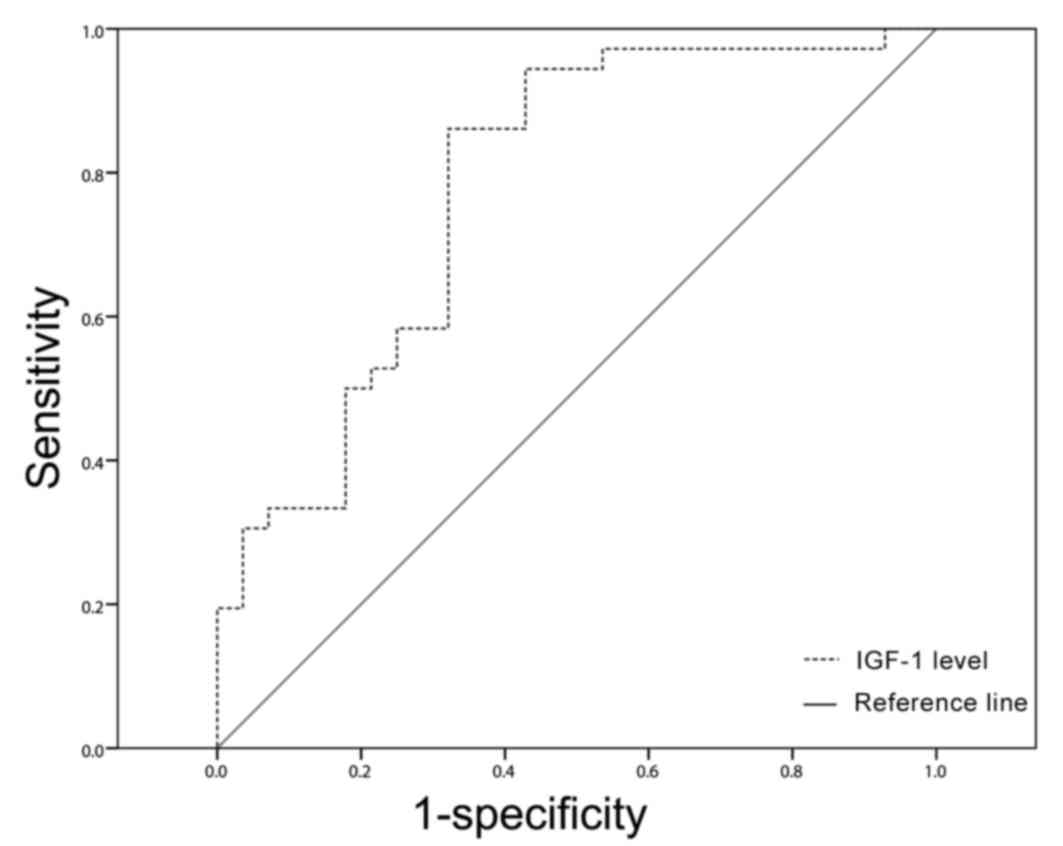
P<0.05; Fig. 2D). The area under
the receiver operating characteristic curve for predicting the
mortality of patients with sepsis using IGF-1 was 0.779 (95%
confidence interval: 0.661–0.897), and the cut-off point was 68.10
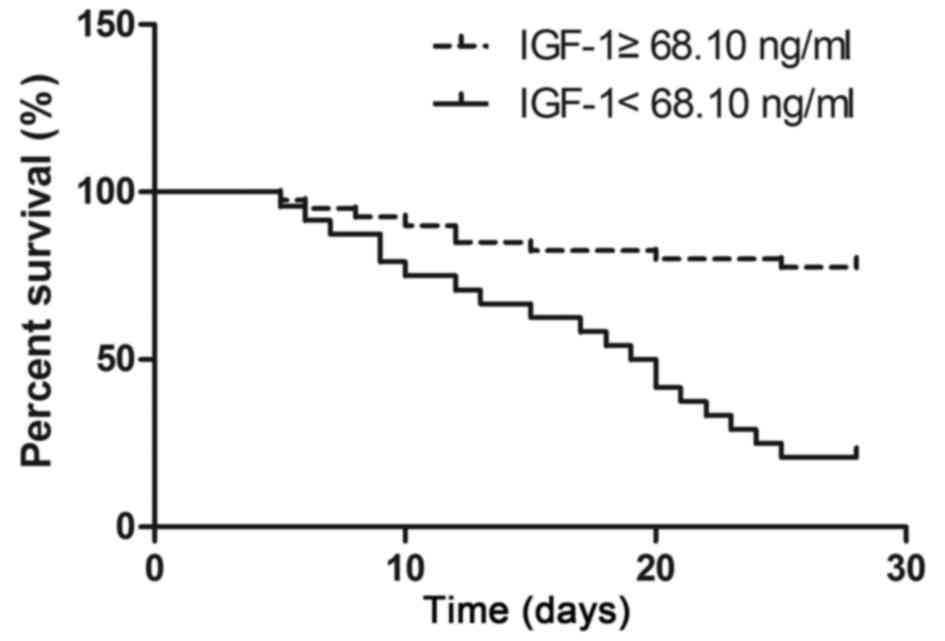
ng/ml (Fig. 3), which was used to
divide all patients with sepsis into either low and high IGF-1
level groups. Kaplan-Meier survival analysis showed that the 28-day
survival rate of patients in the low IGF-1 level group was
significantly lower than in the high IGF-1 level group (21.7 vs.
77.5%; P<0.05; Fig. 4).
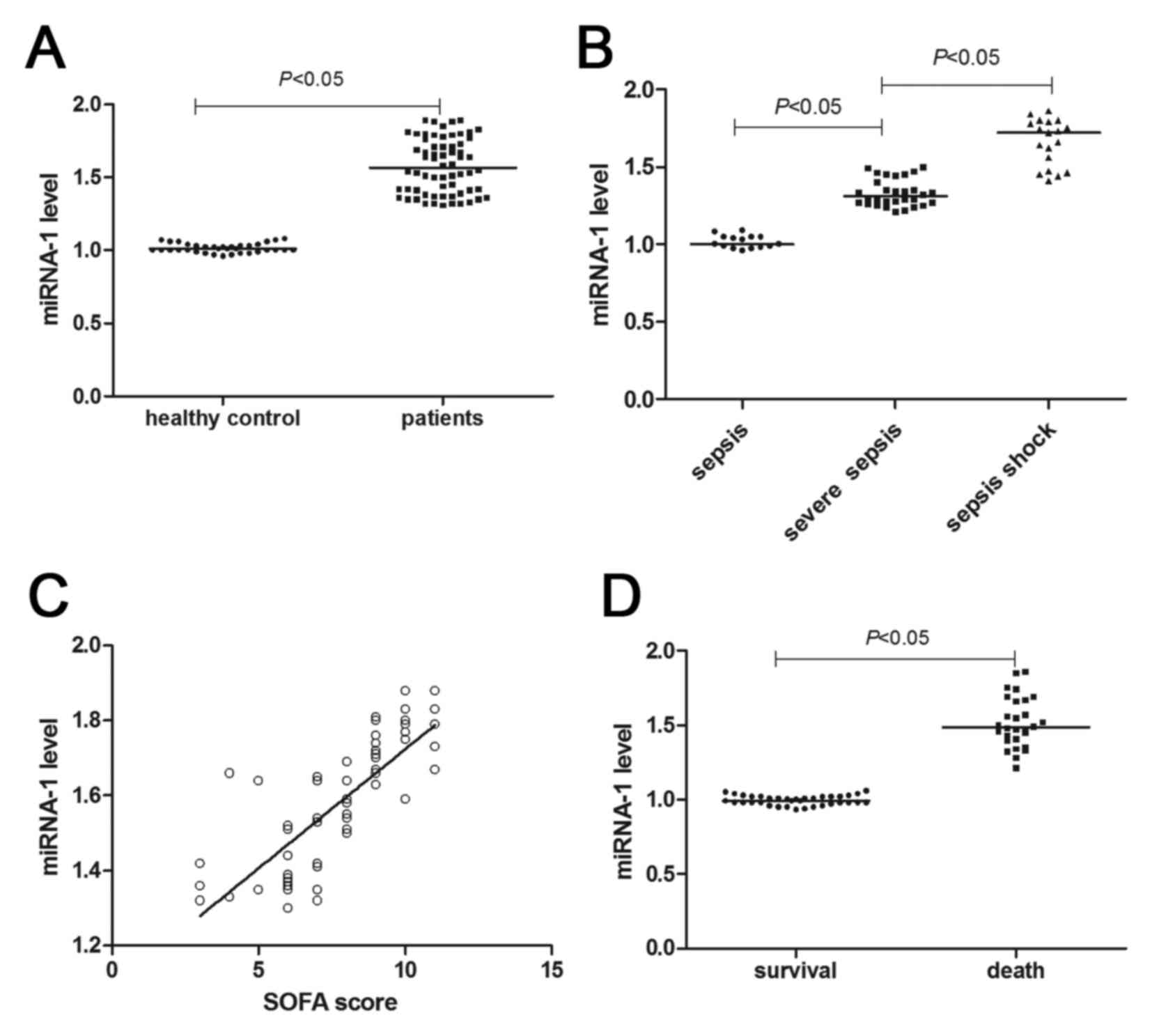
Increased serum miRNA-1 levels in
patients with sepsis are inversely proportional to serum IGF-1
levels
RT-qPCR results (Fig.
5A) showed that miRNA-1 levels of patients with sepsis
significantly increased (~1.5-fold) as compared with the healthy
controls (P<0.05). As shown in Fig.
5B and C, with the aggravation of the septic conditions,
miRNA-1 levels significantly increased (P<0.05). Patient miRNA-1
levels were shown to be directly proportional to the SOFA score
(r=0.606; P<0.05). In addition, the miRNA-1 levels of the
dead patients were higher than that of the patients who survived
follow-up (P<0.05; Fig. 5D).
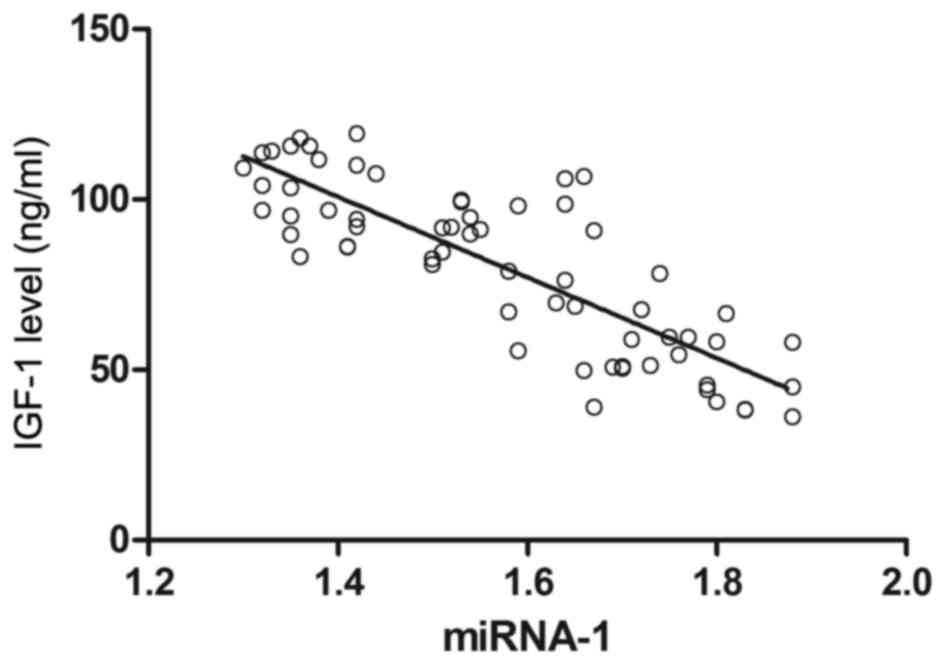
Notably, correlation analysis showed that the serum IGF-1 levels of
patients were inversely proportional to serum miRNA-1 levels
(r=−0.692; P<0.05; Fig. 6).
IGF-1 reduces
H2O2-induced cell death and may be regulated
by miRNA-1
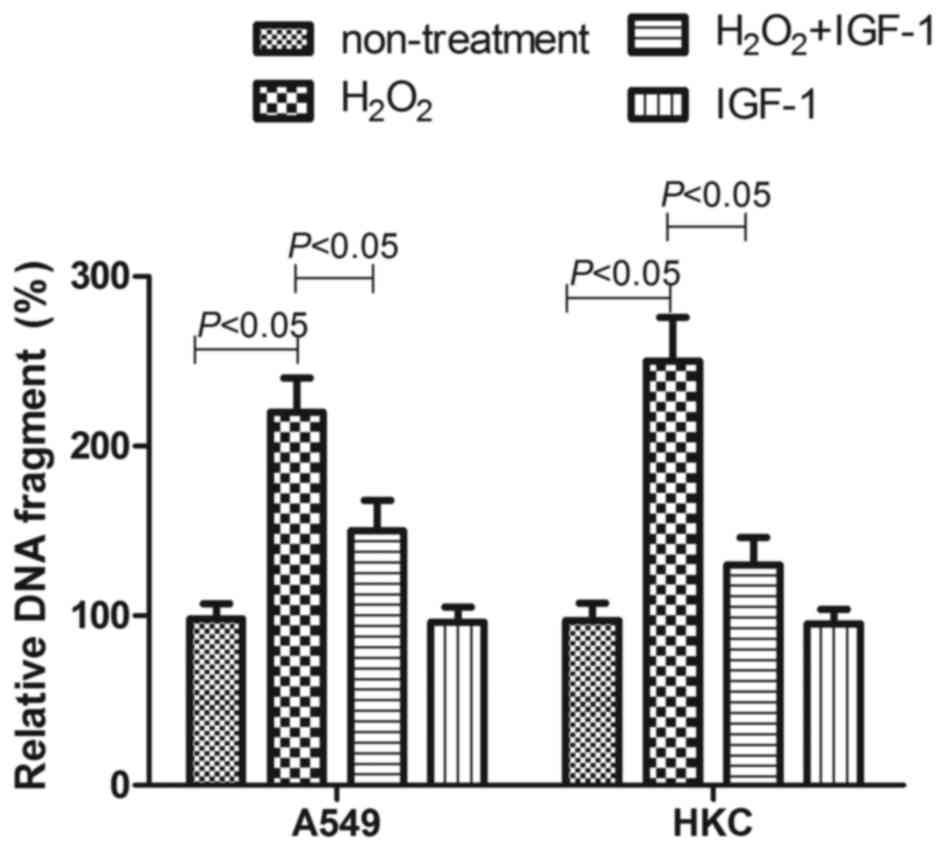
In a preliminary experiment, the oxidative stress
induced by H2O2 was confirmed because
increased ROS levels were detected in cells treated by
H2O2 (data not shown). As shown in Fig. 7, 100 µM H2O2
caused more apoptosis in A549 cells and HKC cells; the relative DNA
fragment percentage in untreated cells was 220±20 and 260±26%,
respectively (P<0.05). However, in 30 ng/ml IGF-1-treated A549
and HKC cells, the H2O2 caused relative DNA
fragment percentages of only 150±18 and 130±16%, respectively
(P<0.05). This indicated that 30 ng/ml IGF-1 treatment may
reduce the apoptosis induced by H2O2.
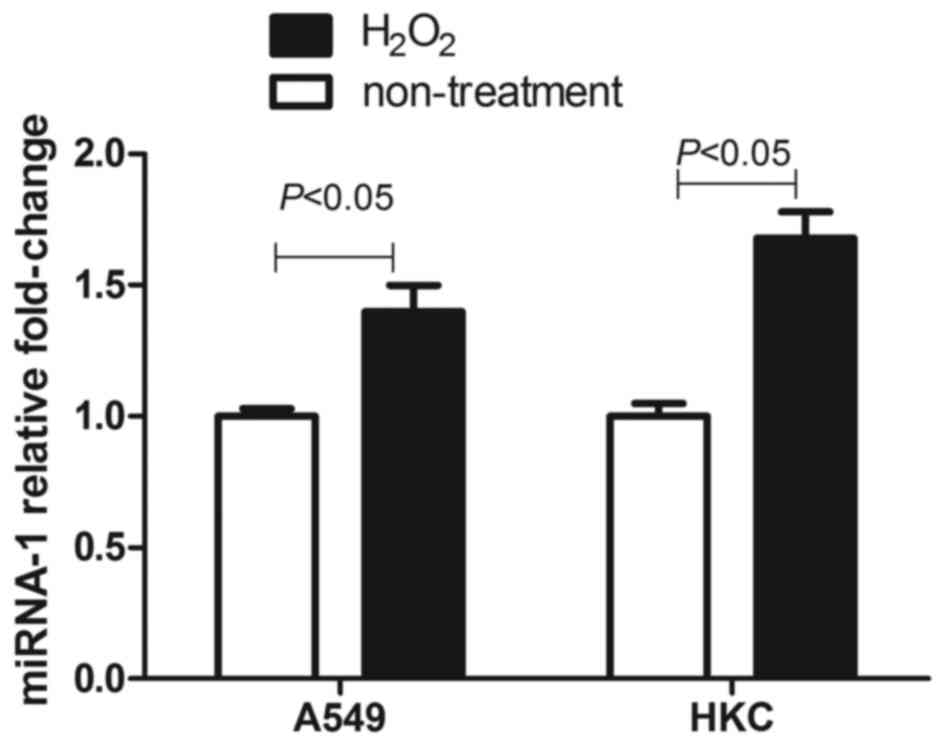
As shown in Fig. 8,
100 µM H2O2 also induced increased expression
of miRNA-1 in A549 and HKC cells, which indicated that oxidative
stress may result in elevated miRNA-1 expression. Furthermore, as
shown in Fig. 9, transfection of
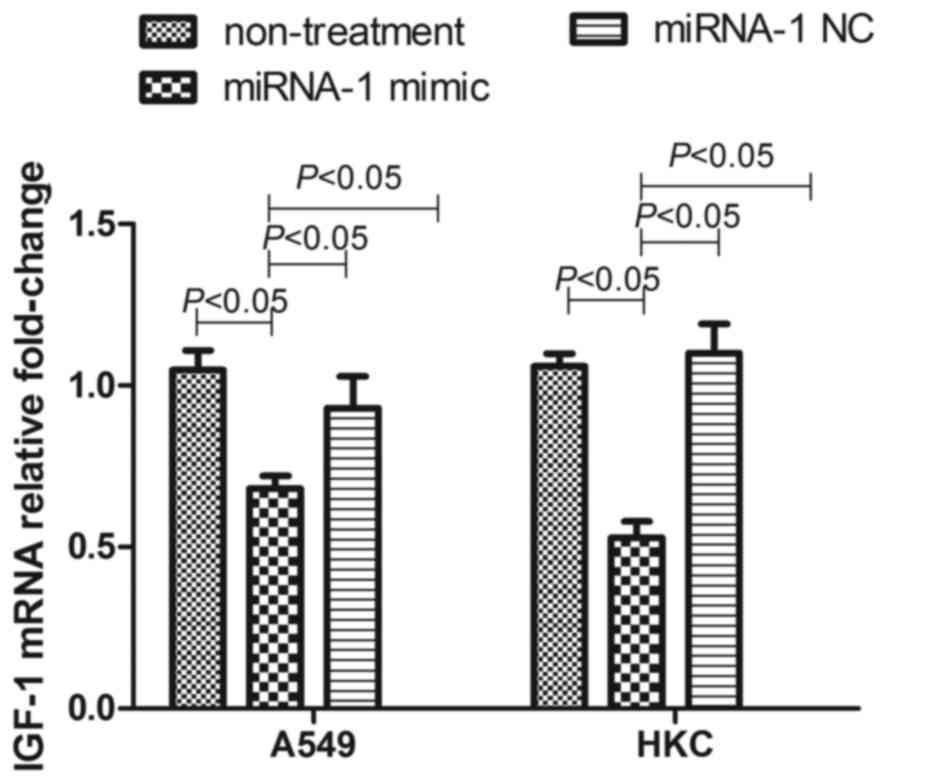
miRNA-1 mimics effectively inhibited the expression of IGF-1 mRNA
in A549 cells and HKC cells; however, the transfection of negative
control did not influence the level of IGF-1 miRNA in cells.
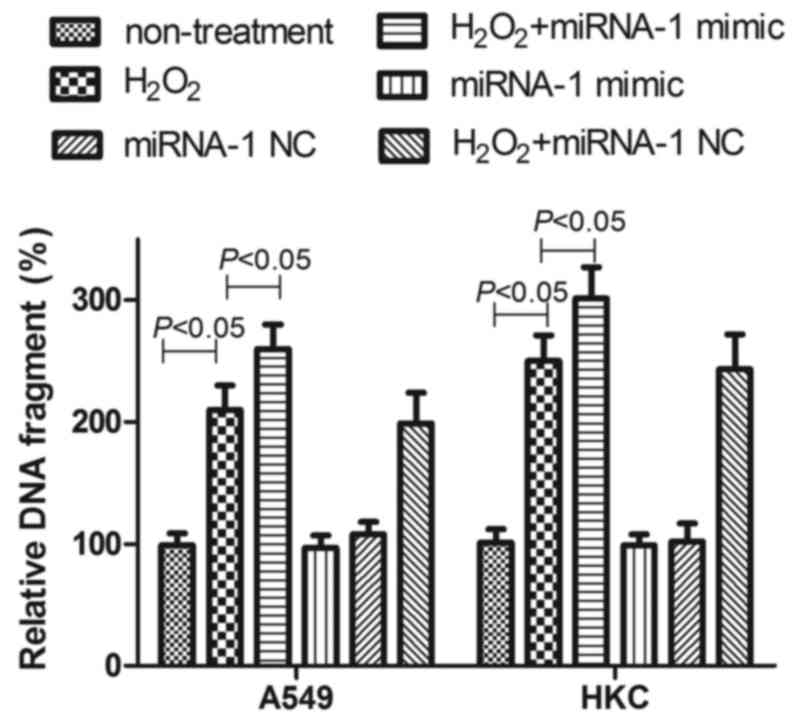
Following transfection of miRNA-1 mimics, A549 cells and HKC cells
exhibited increased sensitivity to exposure to 100 µM
H2O2, manifesting as an increase in
apoptosis. Transfection with negative control had no effect
(Fig. 10). These results suggested
that IGF-1 was able to reduce oxidative stress-induced cell death
and, notably, may be regulated by miRNA-1.
Discussion
In sepsis, excessive reactive oxygen species (ROS)
generated from oxidative stress is the main pathogenic mechanism
for the occurrence of MODS in patients with sepsis, which is
influential of the patient's disease condition and prognosis
(16,17) The mechanism of this process can
simply described as follows: When sepsis occurs, lipopolysaccharide
binding proteins on the surfaces of neutrophils and mononuclear
macrophages bind with endotoxins, which activates the intracellular
xanthine oxidase to release a large number of electrons and produce
excess ROS, which may lead to oxidative stress status (5,18,19).
Excess ROS can cause damage to mitochondria and induce apoptosis,
and more importantly, activate the inflammatory signaling pathways,
and activate complement, resulting in a loss of control of
inflammatory reactions and damage to multiple organs (20,21).
Therefore, the monitoring of oxidative stress-related biomarkers
and anti-oxidative stress therapy may be used as a means for
assessing the disease conditions and treatment of patients with
sepsis.
IGF-1 has been proven to anti-oxidative stress
functions (22,23). Thus, IGF-1 has the potential to
become a biomarker and therapeutic target of sepsis. It has been
demonstrated that IGF-1 levels in critical patients typically
exhibit a declining trend (24).
Therefore, this study focused on the changes in IGF-1 levels in
patients with sepsis. The findings demonstrated that the IGF-1
levels of patients with sepsis were significantly lower than those
of healthy subjects, and continued to decline with the aggravation
of the disease condition. Meanwhile, we found the IGF-1 levels of
patients with sepsis who did not survive the 28-day follow-up were
significantly lower than those of the patients who survived, and
the patients with lower IGF-1 expression exhibited a lower survival
rate. These results indicated that IGF-1 may be used to evaluate
the severity of sepsis in patients and predict their prognosis. It
has previously been have shown that IGF-1 is able to protect cells
from injury induced by oxidative stress (25); although, on the other hand, a higher
oxidative stress level in sepsis patients was also observed in a
series of studies (16,26). Considering these findings, we
speculated that low levels of IGF-1 may not antagonize oxidative
stress, thus, the patients with low IGF-1 expression may suffer
from more oxidative injury when accompanied by severe disease and
poorer prognosis. In vitro experiments have demonstrated
that IGF-1 was able to reduce the intestinal epithelial cell injury
caused by oxidative stress (25). In
pluripotent stem cell experiments, researchers found that IGF-1
prevented oxidative stress-induced apoptosis (7). It is generally accepted that the
mechanism of IGF-1-induced inhibition of oxidative stress injury is
realized through indirect approaches, rather than directly, by
reducing the products of oxidative stress. Possible mechanisms
include: i) Increased expression of the antioxidants, for example,
increased tetrahydrobiopterin expression; ii) increasing the
expression of antioxidant enzymes, such as increased expression of
glutathione peroxidase and superoxide dismutase; and iii)
inhibiting the generation of inflammatory cytokines, such as
reducing interleukin-6 and tumor necrosis factor-α generation, thus
preventing the vicious cycle of inflammation and oxidative stress
(16,22,27).
In order to further explore the mechanism of IGF-1
variation in patients with sepsis, miRNA-1 levels were analyzed in
patients with sepsis, since IGF-1 is the target gene of miRNA-1
according to previous studies (28,29). The
present study demonstrated that patients with sepsis had higher
plasma miRNA-1 levels, which was negatively correlated with the
IGF-1.
Lungs and kidneys are particularly vulnerable to
injury during sepsis (30), hence we
selected A549 and HKC cells to further confirm the miR-1 function
in ROS induced cell damage. H2O2 is able to
induce oxidative stress injury, as reported by various studies
(31,32); therefore, H2O2
treatment was used in the present study to simulate oxidative
stress. In our preliminary experiment,
H2O2-induced oxidative stress was confirmed
via the detection of higher ROS levels in cells treated by
H2O2. As expected, IGF-1 was able to reduce
the cell death caused by H2O2. Notably,
miRNA-1 transfection was able to reduce the expression of IGF-1,
thereby increasing H2O2 damage to cells.
These in vitro experimental data provided more evidence that
the increased expression of miRNA-1 may decrease IGF-1 levels and
antagonize its anti-oxidative stress function.
In conclusion, the findings of the present study
demonstrated that a lower IGF-1 level in patients with sepsis may
predict more severe illness condition and poor prognosis. The
possible mechanism is that the elevated miRNA-1 levels reduce IGF-1
levels, reducing the anti-oxidative stress effect of IGF-1, which
increases the injury caused by oxidative stress in patients with
sepsis.
Acknowledgements
This study was financially supported by a grant from
Department of Health of Zhejiang Province, China (grant no.
2013RCB001).
References
|
1
|
Guerra WF, Mayfield TR, Meyers MS,
Clouatre AE and Riccio JC: Early detection and treatment of
patients with severe sepsis by prehospital personnel. J Emerg Med.
44:1116–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lekkou A, Mouzaki A, Siagris D, Ravani I
and Gogos CA: Serum lipid profile, cytokine production, and
clinical outcome in patients with severe sepsis. J Crit Care.
29:723–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keegan J and Wira CR III: Early
identification and management of patients with severe sepsis and
septic shock in the emergency department. Emerg Med Clin North Am.
32:759–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brosche T, Bertsch T, Sieber CC and
Hoffmann U: Reduced plasmalogen concentration as a surrogate marker
of oxidative stress in elderly septic patients. Arch Gerontol
Geriatr. 57:66–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karapetsa M, Pitsika M, Goutzourelas N,
Stagos D, Becker A Tousia and Zakynthinos E: Oxidative status in
ICU patients with septic shock. Food Chem Toxicol. 61:106–111.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu Y, Wang C and Cohen A: Effect of IGF-1
on the balance between autophagy of dysfunctional mitochondria and
apoptosis. FEBS Lett. 577:357–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Shelat H and Geng YJ: IGF-1 prevents
oxidative stress induced-apoptosis in induced pluripotent stem
cells which is mediated by microRNA-1. Biochem Biophys Res Commun.
426:615–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Essandoh K and Fan GC: Role of
extracellular and intracellular microRNAs in sepsis. Biochim
Biophys Acta. 1842:2155–2162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montano M: MicroRNAs: miRRORS of health
and disease. Transl Res. 157:157–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX,
Lin SG and Li Y: Glucose induces apoptosis of cardiomyocytes via
microRNA-1 and IGF-1. Biochem Biophys Res Commun. 376:548–552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah G, Zielonka J, Chen F, Zhang G, Cao
Y, Kalyanaraman B and See W: H2O2 generation by bacillus
Calmette-Guérin induces the cellular oxidative stress response
required for bacillus Calmette-Guérin direct effects on urothelial
carcinoma biology. J Urol. 192:1238–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trinh MD, Ngo DH, Tran DK, Tran QT, Vo TS,
Dinh MH and Ngo DN: Prevention of H2O2-induced oxidative stress in
Chang liver cells by 4-hydroxybenzyl-chitooligomers. Carbohydr
Polym. 103:502–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G:
SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ATS/SIS International
Sepsis Definitions Conference. Crit Care Med. 31:1250–1256. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock, 2012. Intensive Care
Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mühl D, Woth G, Drenkovics L, Varga A,
Ghosh S, Csontos C, Bogár L, Wéber G and Lantos J: Comparison of
oxidative stress & leukocyte activation in patients with severe
sepsis & burn injury. Indian J Med Res. 134:69–78.
2011.PubMed/NCBI
|
|
17
|
Apostolova N, Garcia-Bou R,
Hernandez-Mijares A, Herance R, Rocha M and Victor VM:
Mitochondrial antioxidants alleviate oxidative and nitrosative
stress in a cellular model of sepsis. Pharm Res. 28:2910–2919.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santos RS, Silva PL, De Oliveira GP,
Santos CL, Cruz FF, De Assis EF, de Castro-Faria-Neto HC, Capelozzi
VL, Morales MM, Pelosi P, et al: Oleanolic acid improves pulmonary
morphofunctional parameters in experimental sepsis by modulating
oxidative and apoptotic processes. Respir Physiol Neurobiol.
189:484–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Holthoff JH, Seely KA, Pathak E,
Spencer HJ III, Gokden N and Mayeux PR: Development of oxidative
stress in the peritubular capillary microenvironment mediates
sepsis-induced renal microcirculatory failure and acute kidney
injury. Am J Pathol. 180:505–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whelan SP, Carchman EH, Kautza B, Nassour
I, Mollen K, Escobar D, Gomez H, Rosengart MA, Shiva S and
Zuckerbraun BS: Polymicrobial sepsis is associated with decreased
hepatic oxidative phosphorylation and an altered metabolic profile.
J Surg Res. 186:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zapelini PH, Rezin GT, Cardoso MR, Ritter
C, Klamt F, Moreira JCF, Streck EL and Dal-Pizzol F: Antioxidant
treatment reverses mitochondrial dysfunction in a sepsis animal
model. Mitochondrion. 8:211–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukuoka H, Iida K, Nishizawa H, Imanaka M,
Takeno R, Iguchi G, Takahashi M, Okimura Y, Kaji H, Chihara K and
Takahashi Y: IGF-I stimulates reactive oxygen species (ROS)
production and inhibits insulin-dependent glucose uptake via ROS in
3T3-L1 adipocytes. Growth Horm IGF Res. 20:212–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papaconstantinou J: Insulin/IGF-1 and ROS
signaling pathway cross-talk in aging and longevity determination.
Mol Cell Endocrinol. 299:89–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elijah IE, Branski LK, Finnerty CC and
Herndon DN: The GH/IGF-1 system in critical illness. Best Pract Res
Clin Endocrinol Metab. 25:759–767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baregamian N, Song J, Jeschke MG, Evers BM
and Chung DH: IGF-1 protects intestinal epithelial cells from
oxidative stress-induced apoptosis. J Surg Res. 136:31–37. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Dessauer B, Bongain J, Molina V,
Quilodrán J, Castillo R and Rodrigo R: Oxidative stress as a novel
target in pediatric sepsis management. J Crit Care. 26:103.e1–e7.
2011. View Article : Google Scholar
|
|
27
|
Bayram F, Bitgen N, Donmez-Altuntas H,
Cakir I, Hamurcu Z, Sahin F, Simsek Y and Baskol G: Increased
genome instability and oxidative DNA damage and their association
with IGF-1 levels in patients with active acromegaly. Growth Horm
IGF Res. 24:29–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naeem A, Zhong K, Moisá SJ, Drackley JK,
Moyes KM and Loor JJ: Bioinformatics analysis of microRNA and
putative target genes in bovine mammary tissue infected with
Streptococcus uberis1. J Dairy Sci. 95:6397–6408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huat TJ, Khan AA, Abdullah JM, Idris FM
and Jaafar H: MicroRNA expression profile of bone marrow
mesenchymal stem cell-derived neural progenitor by microarray under
the influence of EGF, bFGF and IGF-1. Genom Data. 5:201–205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Griffiths B and Anderson ID: Sepsis, SIRS
and MODS. Surgery (Oxford). 27:446–449. 2009. View Article : Google Scholar
|
|
31
|
García-Nebot MJ, Cilla A, Alegría A and
Barberá R: Caseinophosphopeptides exert partial and site-specific
cytoprotection against H2O2-induced oxidative stress in Caco-2
cells. Food Chem. 129:1495–1503. 2011. View Article : Google Scholar
|
|
32
|
Zhai L, Zhang P, Sun RY, Liu XY, Liu WG
and Guo XL: Cytoprotective effects of CSTMP, a novel stilbene
derivative, against H2O2-induced oxidative stress in human
endothelial cells. Pharmacol Rep. 63:1469–1480. 2011. View Article : Google Scholar : PubMed/NCBI
|