Introduction
Cutaneous wound healing is a dynamic process
involving the migration and interaction of cells in the dermis and
epidermis, including fibroblasts and keratinocytes, as well as the
release of chemical mediators from inflammatory processes. In the
early phase of wound healing, fibroblasts migrate into the affected
area and move across the provisional fibrin-based matrix. Since the
provisional fibrin-based matrix is relatively devoid of
fibroblasts, the processes of migration, proliferation and
exracellular mattress production are considered key steps in the
regeneration of functional dermis (1). Moreover, many of growth factors and
cytokines are involved in the regulation of fibroblast migration
(2,3).
Previous studies showed ASCs may contribute to
tissue injury repair. ASCs secrete various growth factors that
manage damaged neighboring cells (4). Conditioned medium from ASC cultures
(ASC-CM) activates dermal fibroblasts and keratinocytes, and can
repair the skin through a paracrine mechanism (1,5). LL-37
is a naturally occurring antimicrobial peptide found in wound beds
that has promoting effects on immune cells (6–9).
Although both ASCs and LL-37 are suggested as wound healing
associators, the relationship between ASCs and LL-37 has not been
clarified. Furthermore, no information is available about the
effect of LL-37 regulated ASCs on the mediation of the fibroblast
migration, which in turn may accelerated wound healing process, and
related mechanism. We hypothesized that LL-37 pretreatment to ASCs
would enhance secretion of active peptides from ASCs participating
in wound healing in a paracrine fashion, and that the resulting
recruitment of human dermal fibroblasts (HDFs) to the wound
microenvironment would support wound healing. To this end, we
investigated the ability of CM from LL-37 treated ASCs to influence
HDFs migration in vitro by up-regulating CXC chemokine
receptor 4 (CXCR4) and SDF-1α expression.
Materials and methods
Cell culture
Human dermal fibroblasts (HDFs) from neonatal
foreskin were obtained from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in a humidified atmosphere
containing 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM)/high glucose (Thermo Fisher Scientific, Pittsburgh, PA, USA)
supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and
10% fetal bovine serum (FBS; Thermo Fisher Scientific) and
maintained at 37°C.
Preparation of adipose stem
cell-conditioned media
Subcutaneous adipose tissue was obtained during
elective surgeries with patient consent; this procedure was
approved by the Samsung Medical Center Institutional Review Board
(IRB). To isolate ASCs, adipose tissue was treated with an equal
volume of a 0.1% collagenase type I (Sigma, St. Louis, MO, USA)
solution for 60 min at 37°C with intermittent shaking. Floating
adipocytes were separated from the stromal vascular fraction by
centrifugation at 1,500 rpm for 10 min. The cell pellet was
suspended in DMEM/low glucose supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin, and cells were plated in
tissue culture dishes. Primary ASCs were cultured for 4–5 days
until they reached confluence and, at this point, were defined as
passage 0. ASCs were used between passages 4 and 6 for experiments.
In some experiments, cells were pretreated with 0.5 µg/ml
neutralizing LL-37 antibody (HyCult Biotechnology, B.V., Uden, The
Netherlands), 10 µg/ml neutralizing SDF-1α antibody (R&D
systems, MN, USA), and/or 100 ng/ml PTX (Sigma) for 60 min before
the addition of human LL-37 (Phoenix Pharmaceuticals, Inc.,
Burlingame, CA, USA). Conditioned media (CM) was collected from ASC
cultures after 48 h and subjected to filtration (0.2 µ filter).
Migration assay
For the transwell migration assay, HDFs cells were
plated in the upper chamber (70,000 cells/upper chamber) of 6.5-mm
diameter, 8-µm pore size transwell inserts (Corning Inc., Acton,
MA, USA) in 24-well plates. Prior to plating cells, ASCs were
treated with hLL-37 (10 ug/ml) in media containing 0.5% FBS for 48
h. The CM from LL-37 treated ASCs was then added to the lower
chamber of 24-well plates. After 24 h, the transwell inserts were
placed in a fresh 24-well plate containing 350 µl of 0.05% crystal
violet, incubated for 20 min, removed, washed by flooding with tap
water until free dye was no longer visible, and allowed to air
dry.
Real time-PCR
Total RNA was extracted using Trizol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA was
synthesized using 2 µg of total RNA and SuperScript II reverse
transcriptase (Invitrogen Life Technologies) according to the
manufacturer's instructions. For Real-Time PCR, quantitative PCRs
(qPCRs) were performed using the 7900 Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA) with the HotStart-IT SYBR-Green
qPCR Master Mix kit (USB Corporation, Camberley, UK). The cycling
profile for Real-Time PCR (50 cycles) was as follows: 95°C for 10
min, 95°C for 15 sec, and 60°C for 60 sec. The primers used were as
follows: GAPDH forward, 5′-ATCACCATCTTCCAGGAGCGA-3′; reverse,
5′-TTCTCCATGGTGGTGAAGACG-3′; for CXCR4 forward,
5′-TCCGTGAAGAAAATGCTAAT-3′; reverse, 5′-GTAGATGACATGGACTGCCT-3′;
SDF-1α forward, 5′-CTAACTCTCTCCCCGACTCCG-3′; reverse,
5′-AAGCAGGGGGACCATTACAC-3′. The comparative quantification cycle
(Cq) method, i.e., 2−ΔΔCq, was used to calculate fold
amplification.
Enzyme-linked immune sorbent assay
(ELISA)
Cells were plated in 6-well plates (300,000
cells/well) and treated with 10 µg/ml hLL-37 in serum-free medium.
After 48 h, conditioned medium was collected for the SDF-1α assay.
The SDF-1α assay was performed as described by the ELISA kit
instructions (Raybiotech, Inc., Norcross, GA, USA) according to the
manufacturer's recommendations.
Fluorescence-activated cell sorting
(FACS)
In order to assess CXCR4 expression, cells were
subjected to surface and intracellular staining with a
FITC-conjugated mouse anti-CXCR4 antibody (R&D Systems, Inc.,
Minneapolis, MN, USA). Briefly, for surface staining, cells were
harvested in trypsin/EDTA and washed in PBS. The cell suspension
was then incubated with a CXCR4 antibody. For intracellular
staining, cells were fixed with 2% paraformaldehyde for 20 min and
then permeabilized with 0.1% saponin and 0.1% sodium azide in PBS
for 20 min. All staining was carried out on ice for 30 min. The
labeled cells were measured with a FACS Calibur (BD Biosciences,
Franklin Lakes, NJ, USA) and analyzed using Win MDI software (Win
MDI version 2.8).
Immunostaining
Cells were seeded on 4-well Lab-Tek II chamber
slides from Nalgene Nunc International (Rochester, USA). After 12
h, the chambers were replaced with conditioned media generated
after 48 h from ASCs. After 18 h, cells were fixed with 4%
paraformaldehyde in PBS for 10 mins and then permeabilized for 5
mins with 0.1% Triton X-100 in PBS. Cells were then blocked with 1%
BSA in PBS at 37°C for 30 mins. Next, the cells were incubated with
anti CXCR4 (sigma) for 30 min at 37°C in the dark, and then washed
twice with PBS. This step was followed by incubation with an Alexa
Fluor® 488 conjugated antibody (Invitrogen) for 30 min
in the dark. A nucleic acid dye (DAPI; 0.5 µg/ml) was then added to
each slide for 5 min to stain the nuclei followed by washing with
PBS. Finally, cells were viewed using an LSM700 confocal microscope
system (Carl Zeiss Inc., Thornwood, NY, USA USA, ×400 objective;
ZEN lite software).
Statistical analysis
Statistical significance was estimated using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Results are shown as the mean
± SD.
Results
Conditioned medium from LL-37 treated
ASCs enhances HDF migration activity in vitro
Release of the LL-37 peptide in a wound is a
physiologic response to cutaneous injury. To study the effects of
CM from LL-37 treated ASCs on HDF migration, the migratory response
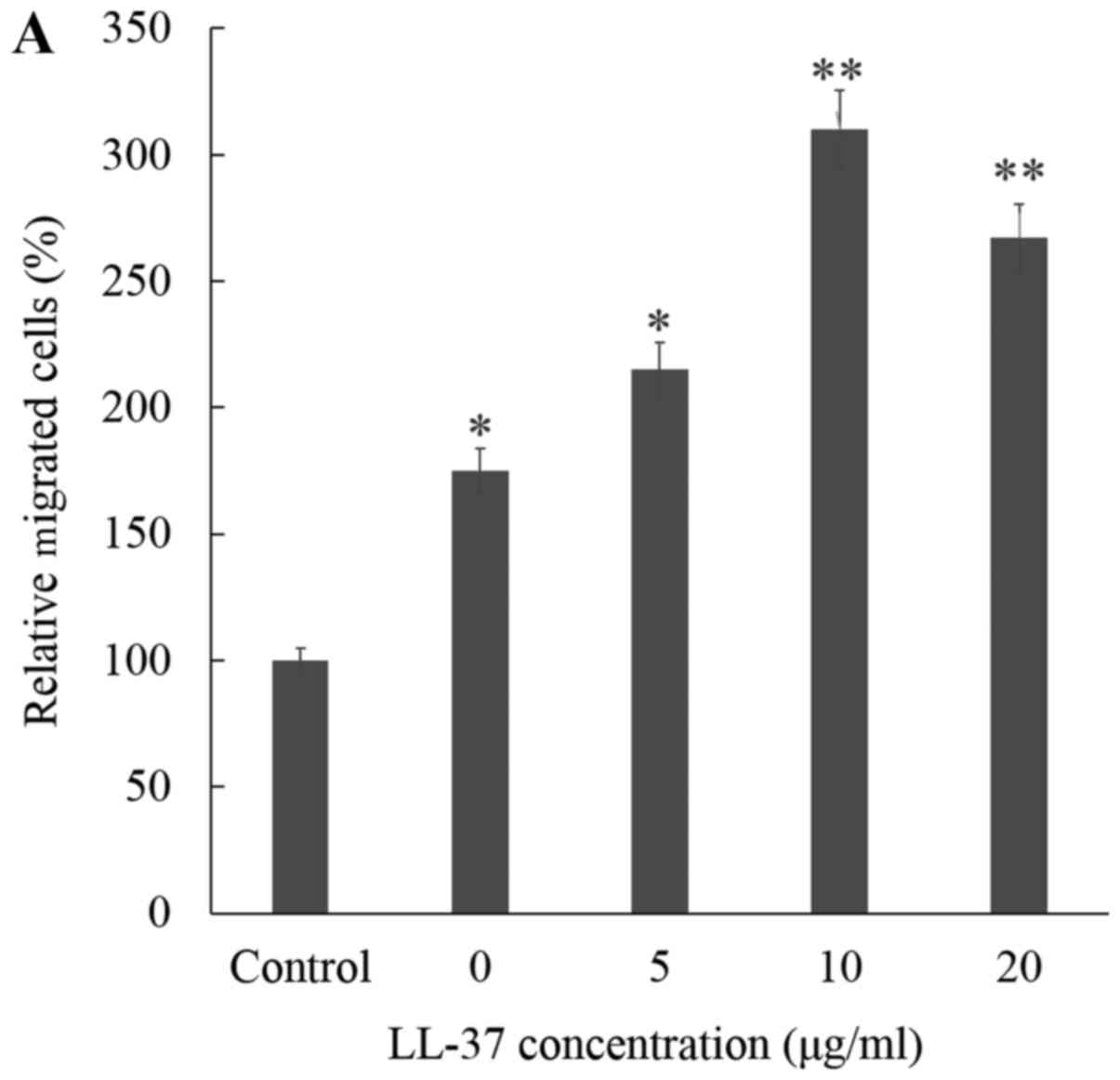
of HDFs was assessed quantitatively by transwell migration assay.
The migration of fibroblasts was markedly increased in CM from
LL-37 treated ASCs, followed in order by CM from untreated ASCs and
then control media. CM from LL-37 treated ASCs was found to induce
HDF migration in a dose-dependent manner (Fig. 1A). The optimal dose of LL-37 for
stimulating ASCs to induce migration in vitro was found to
be 10 µg/ml. In addition, we performed a time course evaluation of
CM from LL-37 treated ASCs-induced cell migration at 12, 18, 24 and
36 h. As shown in Fig. 1B, the level
of HDF migration in the presence of CM from LL-37 treated ASCs
increased in a time-dependent manner, with a maximum difference in
migration observed at 24 h. Together, these results suggested that
CM from LL-37 treated ASCs can enhance HDF migratory response in a
dose- and time-dependent manner.
Conditioned medium from LL-37 treated
ASCs enhances secretion of chemokine SDF-1α
Because SDF-1α/CXCR4 has been shown the recruitment
of HDFs involved in wound healing process, we examined whether
SDF-1α, which is secreted by ASCs, plays a role in CM from LL-37
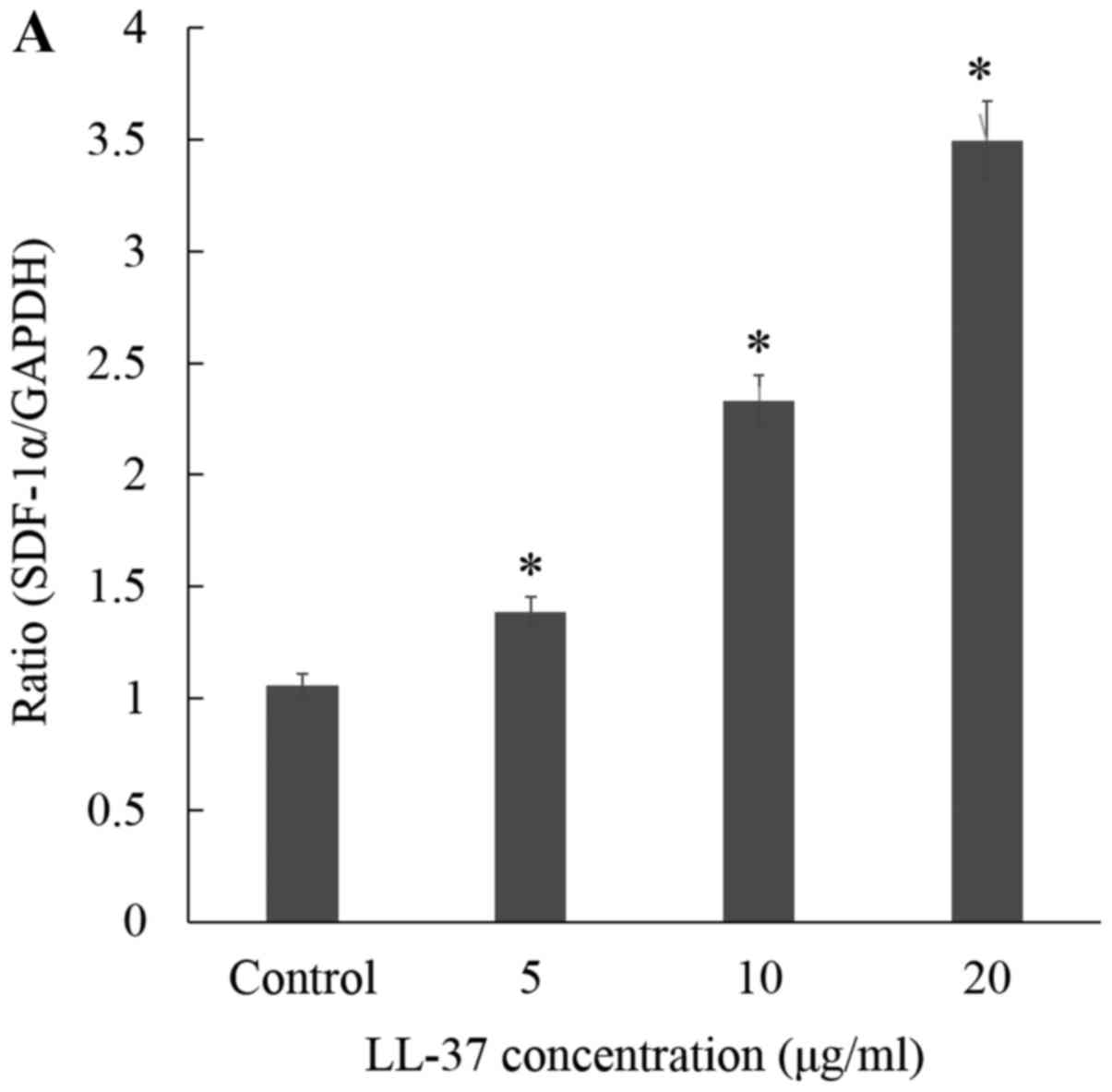
treated ASCs-induced HDF migration. As expected, the mRNA levels of
SDF-1α were significantly increased by CM from ASCs treatment with
LL-37 in a dose-dependent manner (Fig.
2A). In addition, expression of SDF-1α protein was markedly
increased in CM from LL-37 treated ASCs in a time-dependent manner,
with maximum expression noted 48 h after stimulation with 10 µg/ml
of LL-37 (Fig. 2B).
Chemokine SDF-1α blockade decreases
HDF migration activity
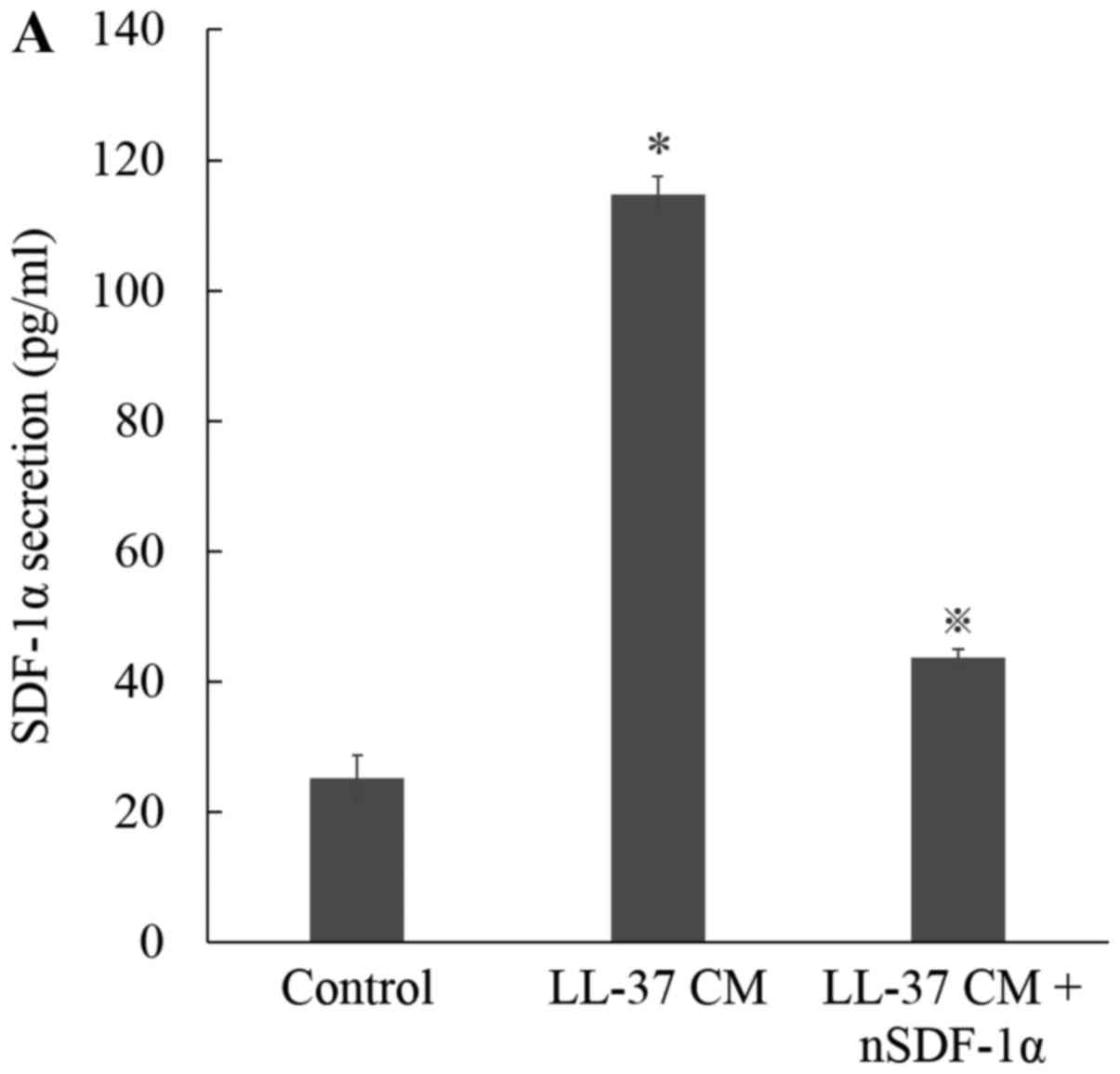
To characterize the involvement of SDF-1α in CM from
LL-37 treated ASCs-enhanced cell migration, we treated CM from
LL-37 treated ASCs with a monoclonal anti-SDF-1α neutralizing
antibody. Blocking of SDF-1α in this manner effectively decreased
LL-37-induced SDF-1α secretion from ASCs (Fig. 3A). Furthermore, photomicrographs
revealed that CM from LL-37 treated ASCs induced HDF migration,
while treatment with neutralizing SDF-1α antibody followed by LL-37
stimulation resulted in significant inhibition of HDF migration
(Fig. 3B and C).
Conditioned medium from LL-37 treated
ASCs increases the expression of CXCR4 in HDFs
Because CXCR4 is a CXC chemokine receptor that is
known to the recruitment of HDFs, we next examined the expression
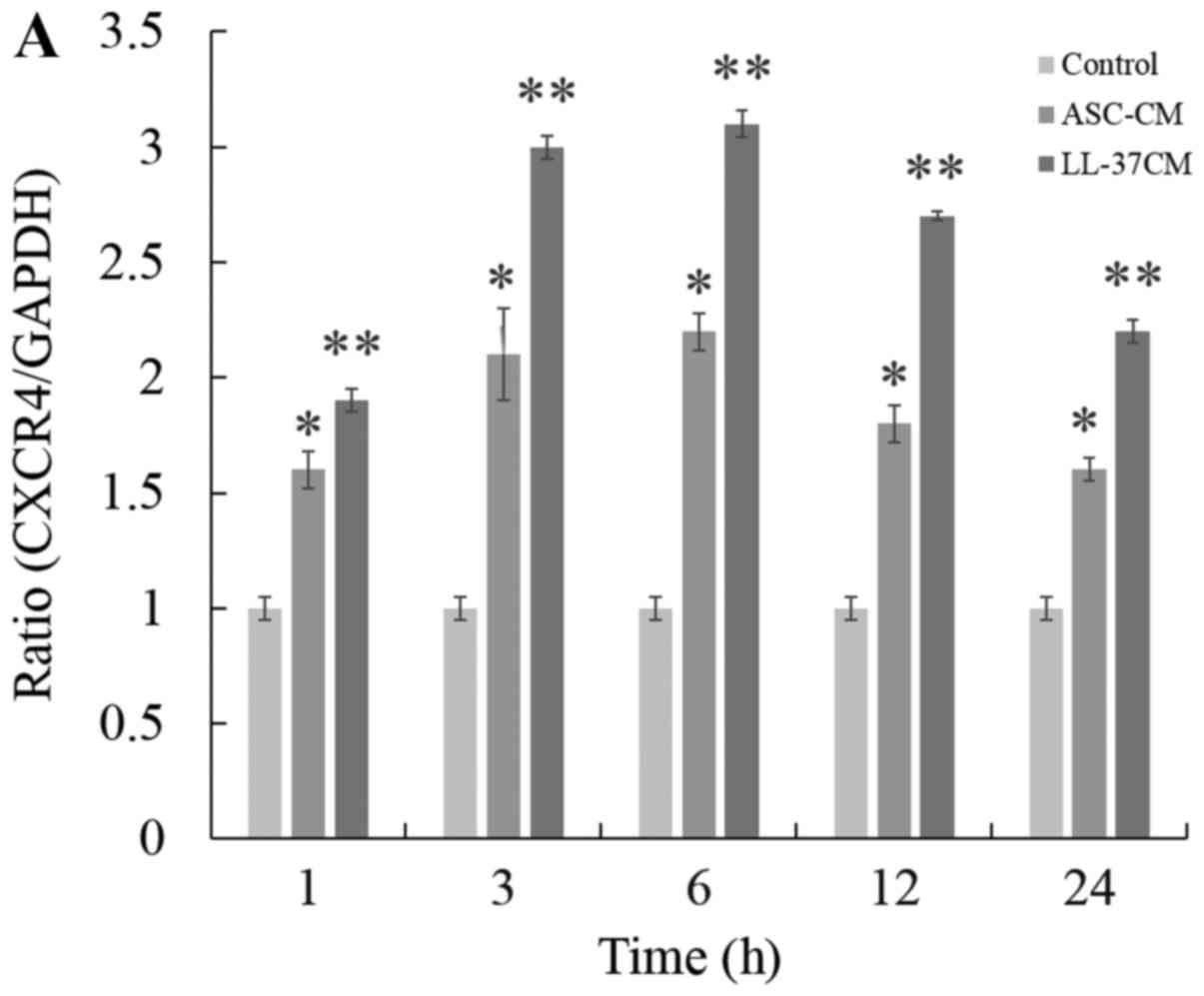
of CXCR4 in HDFs. Fig. 4A shows that
CM from untreated ASCs produced a notable increase in the
expression of CXCR4 compared to media control. Furthermore,
expression of CXCR4 was further markedly increased by CM from LL-37
treated ASCs. The transcription of CXCR4 mRNA on HDFs reached a
maximum at 6 h with CM from LL-37 treated ASCs. To investigate the
effect of LL-37-inhibition in ASCs on CXCR4 expression in HDFs, CM
from LL-37 treated ASCs were treated with a specific αLL-37
antibody, and the changes in CXCR4 expression were monitored by
Real-Time PCR. As expected, the enhanced expression of CXCR4
observed in CM from LL-37-treated ASCs was significantly reduced in
the presence of a neutralizing antibody (αLL-37) (Fig. 4B). To further confirm the requirement
of LL-37 in enhanced CXCR4 expression, ASCs were treated with
pertussis toxin (Ptx), a Gαi inhibitor, before activation with
LL-37 to prevent SDF-1/CXCR4 signaling. Ptx treatment had a similar
effect on CXCR4 expression in HDFs as the LL-37 antibody. Ptx
treatment followed by LL-37 stimulation resulted in a significant
inhibition of CXCR4 expression (Fig.
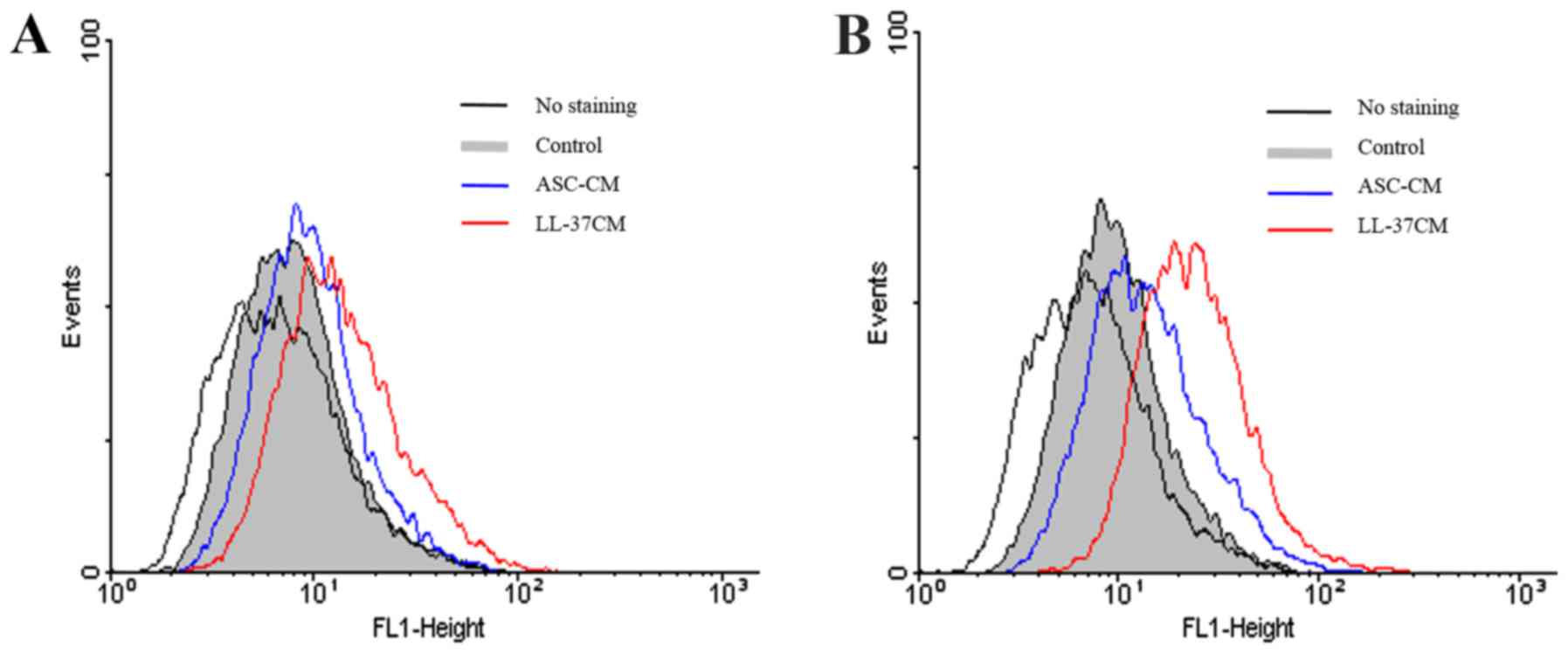
4B). In addition, we used flow cytometry to confirm that CM
from LL-37 treated ASCs augments CXCR4 expression in HDF donor
pools compared with CM from ASCs alone at 18 h (Fig. 5A and B). Taken together, our results
indicated that CM from LL-37 treated ASCs enhanced the expression
of CXCR4, which participates in HDF migration, at the mRNA and
protein levels. These results suggest that CM from LL-37-treated
ASCs may be a novel trophic factor for stimulating human HDF
migration.
Discussion
Adipose stem cell-conditioned media (ASC-CM)
contains a number of active peptides and has been successfully used
to treat multiple types of tissue defects, including skin wounds
both in vivo and in vitro (1,3,6,10,11). A
paracrine mechanism might be the most effective way for ASCs to
promote wound healing (9). We
hypothesized that ASC stimulated with LL-37 would increase
expression of soluble factors promoting human dermal fibroblast
migration, which is a key step in the wound healing process.
Therefore, we set out to determine how ASCs would react to an
LL-37-rich microenvironment to address whether the chemokine axis,
which is well known to control cell migration properties, is
influenced by CM from LL-37-treated ASCs, and whether it can
regulate the migration of HDFs.
LL-37 is involved in wound healing from the first
encounter with microbes to recovery of the tissue damaged during
infection (12). LL-37 is stored at
high concentrations (40 µM or 630 µg/109 cells),
predominantly in human neutrophil granules, and is up regulated in
response to infections. The concentrations of LL-37 vary within
different tissues and cells, and the physiological significance of
the different activities of LL-37 has been actively debated.
Ultimately, the functions of LL-37 in vivo are dependent on
the peptide concentration and tissue composition at specific sites.
The concentration-dependent effects of LL-37 range from
anti-biofilm activity and an ability to block neutrophil apoptosis
at low nM levels to antimicrobial and chemotactic effects at 0.1–10
µM levels, and cytotoxic effects at levels above 10 µM. Thus,
defining the optimal efficacious concentration of a peptide within
the wound environment is a challenge to the development of
antimicrobial peptides for the treatment of wounds (9,13). The
optimal concentration of LL-37 that we found to stimulate ASC-CM to
induce HDFs was (10 µg/ml) can be estimated as 2.2 µM. According to
reported activities and the effects of LL-37 at particular
concentrations, the concentration of LL-37 in this study may be
considered not enough to develop chemotactic effects in
vitro but sufficient to achieve chemotactic effects in
vitro. Further investigation will be required to define the
clinically effective doses of LL-37 needed to achieve functional
augmentation of ASC-CM for treatment of wounds.
Phamacological preconditioning of stem cells to
boost the release of cytoactive factors may represent the effective
way to enhance their functional efficacy (14). Our experiments indicated that the
paracrine effect of ASCs was significantly augmented by stimulation
of media with LL-37. Regarding the results, we found that LL-37
stimulated SDF-1α m RNA and secretion from ASCs, which play an
important role in the HDFs migration since the neutralization of
SDF-1α block the stimulatory effects of CM from LL-37 treated ASCs.
Evidence from our experiment supports the existence of secreting
protein from ASC treated with LL-37 and the participant to regulate
migration of HDFs. Identification of both active proteins from
ASC-CM and their mechanism, as well as drug development using these
peptides, will contribute to the establishment of effective ASC-CM
therapeutic strategies for skin repair (4). Furthermore, a number of recent data
suggest that the regulatory proteins involved in the regulation of
self-renewal, growth, survival, and differentiation of premalignant
neoplastic stem cells. Targeting of cancer stem cell using drugs
that kill of permanently suppress these cells may be a prerequisite
for the development of new curative treatment. Therefore, current
research is seeking novel markers and targets that are
preferentially expressed on cancer stem cells (15,16).
We previously showed that CXC chemokine receptor 4
is essential for migration of HDFs (17). As expected, HDFs migration and
expression of CXCR4/SDF-1α on HDFs was significantly increased by
CM from LL-37-treated ASCs followed by CM from untreated ASCs and
control media, respectively. CXCR4 expression was enhanced by CM
and the neutralizing experiments and Ptx treated data demonstrated
that this effects were mediated by LL-37-G coupled protein receptor
axis. However, we also found that cell migration was not decreased
to the level of control media in the presence of neutralizing
SDF-1α (Fig. 3C). This observation
contrasts with the dose-dependent relationship between LL-37
concentration and the secretion of active peptides such as SDF-1α
by human ASCs, suggesting that in addition to SDF-1α, other soluble
factors and mechanisms may mediate the effects of LL-37 with
respect to stimulation of HDF migration. For example, LL-37 also
leads to a considerable increase in secretion of monocyte
chemoattractant protein-1 (MCP-1) in human ASCs (3). Thus, MCP-1 may affect not only multiple
behaviors of human ASCs, including proliferation and migration, but
also HDF migration. There still remains the possibility to have
additional unknown factors in CM from ASCs to regulate CXCR4 in
HDFs. Further investigations will be necessary to clarify the
additional potential mechanisms and factors involved in CM from
LL-37-treated ASCs induced migration of HDFs.
CXCR4 is a G-protein coupled receptor belonging to
the CXC family of chemokine receptors. CXCR4 expression is
dynamically regulated by external cues like hypoxia and can be
upregulated following in vitro priming with a mixture of
cytokine, as shown to enhance migration in vitro toward an
SDF-1α gradient (18,19). Interaction of CXCR4 with its ligand,
SDF-1α directs the movements of cells in hematopoietic stem cell
homing, leukocyte trafficking, tumor metastasis (2,20–22).
Manipulation of the CXCR4/SDF-1α provides also clinical benefit to
recipients of hematopoietic stem cell transplantation (23). It is postulated that CXCR4 axis
control may also have a potential effect in HDF migration and skin
repair. Recently, SDF-1α exposure was shown to up regulate low
basal CXCR4 surface expression in dermal fibroblast, which
increased chemotaxis (17). Our
results in the present study revealed that the expression of CXCR4
at mRNA levels was augmented by LL-37 stimulated ASC-CM followed by
untreated ASC-CM and control media respectively, as shown to
enhance migration toward an SDF-1α gradient (Fig. 4). Nevertheless, present study showed
discrepancy between mRNA and protein expression of CXCR4 in dermal
fibroblast induced by LL-37 stimulated ASC-CM. Although CXCR4 mRNA
showed the enhanced expression, flow cytometry data indicated no
significant expression level of CXCR4 protein (Fig. 5A). Instead of surface expression,
intracellular localization of CXCR4 protein in dermal fibroblast
was considerably expressed by stimulated media (Fig. 5B). The prominent intracellular
localization of CXCR4 suggests that dynamic equilibrium between the
cytoplasm and plasma membrane may modulate CXCR4 availability at
the cell surface, and thus cell responsiveness to SDF-1α gradient
(24). Although CXCR4 undergoes
internalization after interaction with ligand, like other G-protein
coupled receptor, the extent of intracellular expression and
endocytosis kinetics differs from one cell type to the other
(24,25).
In conclusion, this study demonstrated that
conditioned medium from LL-37-treated ASCs accelerated migration of
HDFs by upregulating CXCR4 expression in vitro. This is the
first report to demonstrate the ameliorative effect of conditioned
medium from LL-37-treated ASCs on HDFs migration. Blocking LL-37
with pertussis toxin and a neutralizing LL-37 antibody markedly
reduced HDF cell migration. Together, these data suggested that
conditioned medium from LL-37-treated ASCs enhances CXCR4
expression in HDFs, which may possibly stimulate cutaneous wound
healing. These results also suggest that conditioned medium from
LL-37-treated ASCs may be an effective wound healing therapeutic
candidate. Our data also clarified that SDF-1 is one of the active
soluble factors responsible for mediating the effects of
conditioned medium from LL-37-treated ASCs in wound healing. In
addition, the effect of mechanism of LL-37 treated ASC-CM on HDFs
in mouse model will be investigated in future studies.
References
|
1
|
Kim WS, Park BS, Sung JH, Yang JM, Park
SB, Kwak SJ and Park JS: Wound healing effect of adipose-derived
stem cells: A critical role of secretory factors on human dermal
fibroblasts. J Dermatol Sci. 48:15–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qu Y, Mao M, Li X, Zhang L, Huang X, Yang
C, Zhao F, Xiong Y and Mu D: Enhanced migration and CXCR4
over-expression in fibroblasts with telomerase reconstitution. Mol
Cell Biochem. 313:45–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS One.
3:e18862008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nie C, Yang D, Xu J, Si Z, Jin X and Zhang
J: Locally administered adipose-derived stem cells accelerate wound
healing through differentiation and vasculogenesis. Cell
Transplant. 20:205–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WS, Park BS and Sung JH: The
wound-healing and antioxidant effects of adipose-derived stem
cells. Expert Opin Biol Ther. 9:879–887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heilborn JD, Nilsson MF, Kratz G, Weber G,
Sørensen O, Borregaard N and Ståhle-Bäckdahl M: The cathelicidin
anti-microbial peptide LL-37 is involved in re-epithelialization of
human skin wounds and is lacking in chronic ulcer epithelium. J
Invest Dermatol. 120:379–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coffelt SB, Marini FC, Watson K, Zwezdaryk
KJ, Dembinski JL, LaMarca HL, Tomchuck SL, zu Bentrup K Honer,
Danka ES, Henkle SL and Scandurro AB: The pro-inflammatory peptide
LL-37 promotes ovarian tumor progression through recruitment of
multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA.
106:pp. 3806–3811. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami M, Ohtake T, Dorschner RA,
Schittek B, Garbe C and Gallo RL: Cathelicidin anti-microbial
peptide expression in sweat, an innate defense system for the skin.
J Invest Dermatol. 119:1090–1095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duplantier AJ and van Hoek ML: The human
cathelicidin antimicrobial peptide LL-37 as a potential treatment
for polymicrobial infected wounds. Front Immunol. 4:1432013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu L, Zhao J, Liu J, Gong N and Chen L:
Effects of adipose stem cell-conditioned medium on the migration of
vascular endothelial cells, fibroblasts and keratinocytes. Exp Ther
Med. 5:701–706. 2013.PubMed/NCBI
|
|
11
|
Cho JW, Kang MC and Lee KS: TGF-β1-treated
ADSCs-CM promotes expression of type I collagen and MMP-1,
migration of human skin fibroblasts, and wound healing in vitro and
in vivo. Int J Mol Med. 26:901–906. 2010.PubMed/NCBI
|
|
12
|
Vandamme D, Landuyt B, Luyten W and
Schoofs L: A comprehensive summary of LL-37, the factotum human
cathelicidin peptide. Cell Immunol. 280:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nijnik A and Hancock RE: The roles of
cathelicidin LL-37 in immune defences and novel clinical
applications. Curr Opin Hematol. 16:41–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu GS, Peshavariya HM, Higuchi M, Chan
EC, Dusting GJ and Jiang F: Pharmacological priming of
adipose-derived stem cells for paracrine VEGF production with
deferoxamine. J Tissue Eng Regen Med. 10:E167–E176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schulenburg A, Blatt K, Cerny-Reiterer S,
Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC and Valent
P: Cancer stem cells in basic science and in translational
oncology: Can we translate into clinical application? J Hematol
Oncol. 8:162015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang C, Zhang X, Hua R, Gan L,
Huang M, Zhao L, Ni S and Guo W: Bmi-1 regulates stem cell-like
properties of gastric cancer cells via modulating miRNAs. J Hematol
Oncol. 9:902016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Shim SK, Kim HA, Seon M, Yang E,
Cho D and Bang SI: CXC chemokine receptor 4 is essential for
Lipo-PGE1-enhanced migration of human dermal fibroblasts. Exp
Dermatol. 21:75–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schioppa T, Uranchimeg B, Saccani A,
Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni
M, Vago L, et al: Regulation of the chemokine receptor CXCR4 by
hypoxia. J Exp Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kucia M, Jankowski K, Reca R, Wysoczynski
M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J and Ratajczak MZ:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herzig DS, Driver BR, Fang G,
Toliver-Kinsky TE, Shute EN and Sherwood ER: Regulation of
lymphocyte trafficking by CXC chemokine receptor 3 during septic
shock. Am J Respir Crit Care Med. 185:291–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suratt BT, Petty JM, Young SK, Malcolm KC,
Lieber JG, Nick JA, Gonzalo JA, Henson PM and Worthen GS: Role of
the CXCR4/SDF-1 chemokine axis in circulating neutrophil
homeostasis. Blood. 104:565–571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koczulla R, von Degenfeld G, Kupatt C,
Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M,
Lebherz C, et al: An angiogenic role for the human peptide
antibiotic LL-37/hCAP-18. J Clin Invest. 111:1665–1672. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Green MM, Chao N, Chhabra S, Corbet K,
Gasparetto C, Horwitz A, Li Z, Venkata JK, Long G, Mims A, et al:
Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic
hematopoietic stem cell transplantation enhances hematopoietic
recovery. J Hematol Oncol. 9:712016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pelekanos RA, Ting MJ, Sardesai VS, Ryan
JM, Lim YC, Chan JK and Fisk NM: Intracellular trafficking and
endocytosis of CXCR4 in fetal mesenchymal stem/stromal cells. BMC
Cell Biol. 15:152014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding Z, Issekutz TB, Downey GP and Waddell
TK: L-selectin stimulation enhances functional expression of
surface CXCR4 in lymphocytes: Implications for cellular activation
during adhesion and migration. Blood. 101:4245–4252. 2003.
View Article : Google Scholar : PubMed/NCBI
|