Introduction
In China, we are facing a rapidly increasing aging
population, and this contributes to a gradual increase in the
incidence of cerebral vascular diseases. Currently, the etiology
and relevant mechanism of small vessel disease (SVD) remains
unclear. In clinical practice, the treatment of small vessel is in
need of improvement, especially those that could not be correctly
diagnosed during imagology. Consequently, it often leads to a delay
in treatment and increases the risk for patients. This condition
may lead to transient ischemic attack (TIA), cerebral hemorrhage,
infarction, vascular cognitive impairment, affective and gait
disorder, or even decreased general functions (1). Therefore, early intervention and
prophylaxis of SVD are considered to be significant parameters for
the prognosis of the disease (2).
Generally, TIA refers to transient insufficiency of
blood supply occuring in the carotid artery or vertebro-basilar
arterial system, causing local cerebral ischemia, and a sudden,
transient and reversible neurological dysfunction (3). This can only last for several minutes
from onset and can disappear after approximately 30 min. However,
if the duration is over 2 h, the patient may suffer from mild
neurologic impairment, and CT and MRI imaging results show the
signs of cerebral ischemia. TIA is frequently observed in
individuals aged 34–65 years, and those above 65 years account for
25.3%, with male patients surpassing the female ones (4). The onset of TIA is usually short, and
mostly caused by altering the supine position, excessive exercise,
or sudden rotation or extension of neck (3,4).
In this study, we investigated the correlation
between the low-density lipoprotein (LDL) and TIA caused by
cerebral small vascular disease (CSVD) and analyzed the effect of
LDL on the TIA caused by CSVD.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria for patients enrolled in the CSVD
patient group were: ⅰ) patients aged 55–90 years; ⅱ) patients with
MRI or CT results showing white matter lesion (WML) and/or lacunar
infarction (LI); ⅲ) patients with no acute cortical infarction; ⅳ)
patients whose imagological results showed no non-lacunar
infarction (>15 mm) and ⅴ) patients who signed the written
informed consent.
Exclusion criteria
Exclusion criteria for the study were: ⅰ) patients
with cardiac infarction (confirmed with echocardiography); ⅱ)
patients with intracranial or extracranial macrovascular diseases;
ⅲ) patients with pulmonary infection or infection of urinary
system; ⅳ) patients with a history of severe craniocerebral trauma
or intracranial tumor; ⅴ) patients with heart, liver, kidney or
lung failure; ⅵ) patients with any type of diseases that could lead
to death; ⅶ) patients with WML caused by non-ischemic factors, such
as poison, genetic variability, infection, demyelinating disease,
metabolic diseases, or hydrocephalus and ⅷ) non-cooperative
patients and those with a history of mental illnesses.
Inclusion criteria of patients that were enrolled in
the control group were: ⅰ) patients who had risk factors associated
with cerebral vascular diseases as well as stroke-like symptoms,
such as dizziness, headache, onset of epilepsy, inarticulate speech
or weakness of limbs but with normal results of MRI examination of
head and ⅱ) patients who signed the written informed consent.
Exclusion criteria were: patients with infection or malignant
diseases.
We enrolled 139 patients into the patient group.
According to the MRI and/or CT of head, the patients were further
divided into two subgroups: the WML group (n=86) and the LI group
(n=53). We also enrolled 107 subjects into the control group. All
participants and their families voluntarily accepted the conditions
of this study. This study was approved by the Ethics Committee of
the Affiliated Hospital of Hubei University of Arts and Science.
Signed written informed consents were obtained from all
participants before the study.
Clinical data and data collection
Inpatients and outpatients who were admitted to the
hospital between September 2012 and September 2015 were enrolled.
These cases were divided into the patient and control groups to
investigate the correlation between the level of LDL and
CSVD-induced TIA. Basic clinical data are presented in Table I. An experienced physician in the
department of neurology was assigned to collect the detailed
history of the disease and physical examinations including the
patients age, sex, height, weight, BMI, smoking and drinking
history, history of hypertension, diabetes mellitus and heart
disease.
 | Table I.History of baseline diseases and
clinical data of enrolled patients (mean ± SD). |
Table I.
History of baseline diseases and
clinical data of enrolled patients (mean ± SD).
| Group | Sex
(male/female) | Age | Cases (n) | Weight (kg) | Height (cm) | BMI |
|---|
| Patient group | 76/63 | 54.3±16.4 | 139 | 70.2±21.5 | 169.7±20.4 | 30.85±14.64 |
| Control group | 61/46 | 46.5±17.3 | 107 | 68.4±19.8 | 160.2±15.3 | 28.63±16.71 |
| F-value |
| 0.82 | – | 0.79 | 0.84 | 0.63 |
| P-value |
| >0.05 | – | >0.05 | >0.05 | >0.05 |
Diagnostic criteria
After admission, patients were subjected to head MRI
examination, including T1- and T2-weighted image, T2 fluid
attenuated inversion recovery (FLAIR) and diffusion weighted
imaging (DWI). Fasting blood glucose and lipid levels, liver and
kidney function, and prothrombin chart were determined. Blood
routine, CRP and regular electrocardiographic examination were
conducted. For patients with aortic stenosis, we performed cerebral
angiography examination, and for patients with cardiac thrombus,
echocardiography was carried out.
Imagological examination
Imagological examination of SVD
After admission, patients received the MRI and/or CT
examinations. The results were independently analyzed by two
physicians in the imaging department to identify the types of
lesion, i.e., the WML and LI and corresponding imaging diagnoses
were given. Imaging diagnosis and relevant analysis was conducted
independently from the general clinical data.
WML
Uniform or slightly decreased T1WI signal, highly
enhanced T2WI signal or a low signal density in CT, unclear rim, no
significant mass effect, patch-shaped, or mutually fused, uneven
signals, border between the corpus callosum and septum pellucidum
not affected, and diameters >5 mm. Most of the lesions were
distributed in the frontal, temporal and parietal-occipital lobe,
basal ganglia and infratentorial region (Fig. 1A-F).
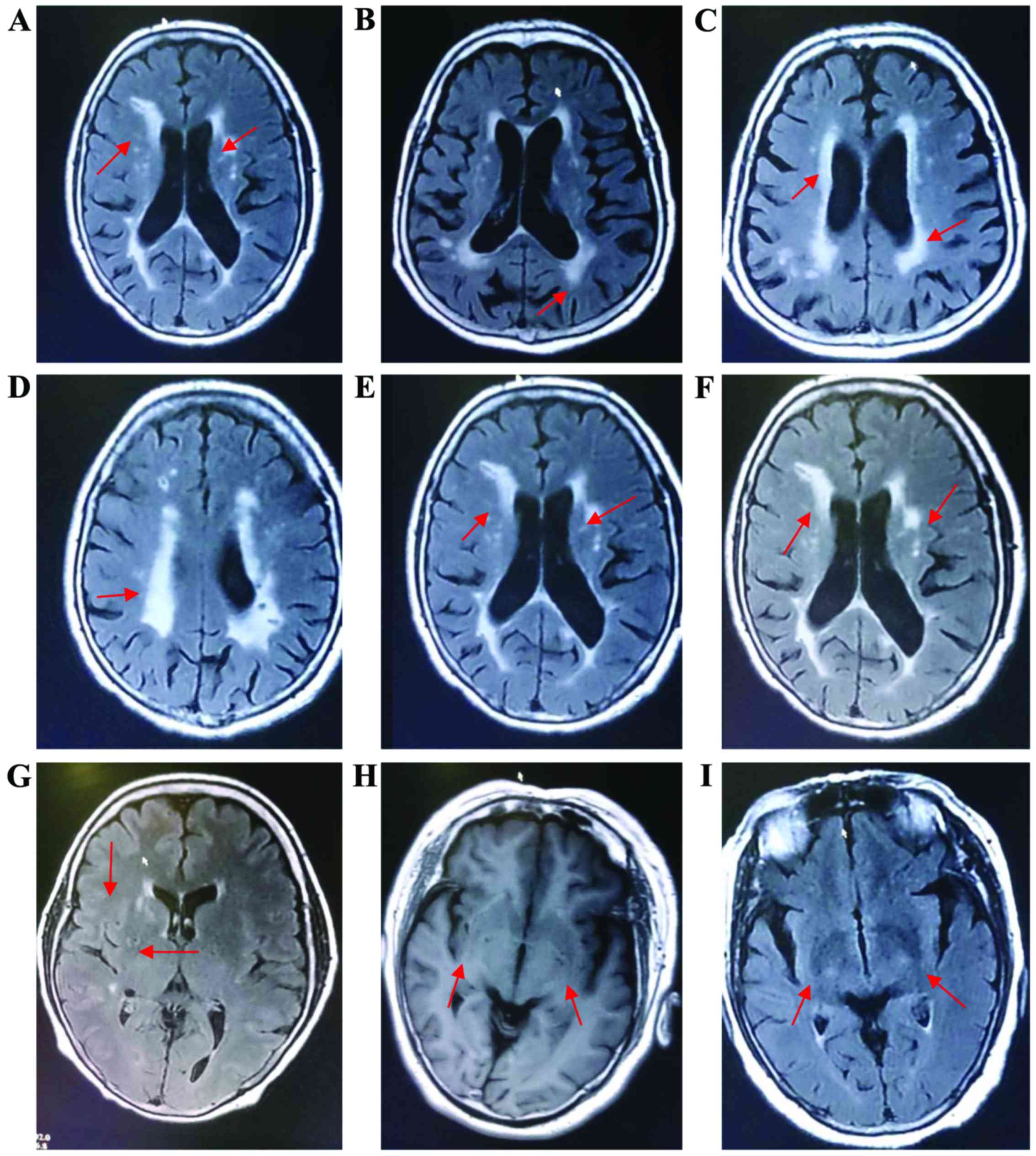 | Figure 1.MRI images for CSVD. (A) (MRI FLAIR):
right cerebral hemisphere (i.e., left part of the figure):
leukoaraiosis (arrow), and acute cerebral infarction in left
cerebral hemisphere (i.e., right part of the figure). Corresponding
patient with acute cerebral infarction. (B) (MRI FLAIR):
leukoaraiosis (arrow). Corresponding patient with TIA. (C) (MRI
FLAIR): leukoaraiosis (arrow). Corresponding patient with TIA. (D)
(MRI FLAIR): leukoaraiosis (arrow). Corresponding patient with TIA.
(E) (MRI FLAIR): right cerebral hemisphere (i.e., left part of the
figure): leukoaraiosis (arrow), and acute cerebral infarction in
left cerebral hemisphere (i.e., right part of the figure).
Corresponding patient with acute cerebral infarction. (F) (MRI
FLAIR): right cerebral hemisphere (i.e., left part of the figure):
leukoaraiosis (arrow), and acute cerebral infarction in left
cerebral hemisphere (i.e., right part of the figure). (E and F) for
the same corresponding patient with acute cerebral infarction. (G)
(MRI FLAIR): LI (arrow). Corresponding patient with TIA. (H) (MRI
FLAIR): bilateral LI (arrow). Corresponding patient with TIA. (I)
(MRI FLAIR): bilateral LI (arrow). Corresponding patient with TIA.
CSVD, cerebral small vascular disease; FLAIR, fluid attenuated
inversion recovery; TIA, transient ischemic attack; LI, lacunar
infarction. |
LI
Imagological manifestation: clear rim, diameters of
3–15 mm, and regions with the characteristics of cerebrospinal
fluid (CSF) on MRI. In CT and T1WI, low intensity/signal was
identified, and was similar to the features of CSF in previous LI.
Thus, enhanced signal presented in diffusion-weighted images was
used for diagnosis in case of the newly developed LI. LI was mainly
distributed in the midline, such as deep cerebral hemispheres and
brainstem (bilateral thalamus, basal ganglia, periventricular white
matter and subcortical white matter) (Fig. 1G-I).
Mini-mental status examination (MMSE)
The scale was 1 point for each correct answer, and 0
for wrong answers, 9 points for inappropriate answer, and 8 points
for patients who refused to answer or did not understand the
question. In adding up the total scores, 8 and 9 points were all
recorded as 0. The maximum score was 30 points. During the
examination, the education levels and dementia were also
considered. Therefore, elderly patients were judged as dementia if
they met the following conditions: illiterate, score <17 points;
primary school, score <20 points and high school or above, score
<24 points. The mental status of patient was judged by the
following criteria: 27–30 points, normal; 21–26 points, mild; 10–20
points, moderate and 0–9 points, severe.
Montreal cognitive assessment (MoCA)
Detection items included: visuospatial, executive
and naming ability, attention, language, abstraction, delayed
recall, and orientation, with a total of 30 points; 1 point was
added for an education duration <12 years for calibration.
Higher scores represented stronger cognitive ability, and patients
with scores <26 points were considered as normal.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(SPSS, Chicago, IL, USA). Measurement data were presented as (means
± standard deviation), and ANOVA or t-test was used for intergroup
comparison. Count data were presented as percentage, and Chi-square
test was used for intergroup comparison. For each risk factor,
multi-factor logistic regression analysis and correlation analysis
of paired material were carried out. P<0.05 indicated that the
difference was statistically significant.
Results
Statistics of categorized test for
risk factors
We summarized clinically collected results of
physical and laboratory examinations, including blood pressure and
glucose, total cholesterol (TC), low-density lipoprotein
cholesterol (LDL-C), HDL-C, creatinine (Cr), total triglyceride
(TG), smoking and drinking capacity and duration, and performed the
statistical analysis. Results indicated that LDL-C, TG and TC
levels in the LI group were significantly higher than those in the
other two groups, but ages in the control group were significantly
lower than those in the other two groups with statistically
significant differences (p<0.05) (Table II).
 | Table II.Statistics of categorized test for
risk factors of patients in each group (mean ± SD). |
Table II.
Statistics of categorized test for
risk factors of patients in each group (mean ± SD).
| Indexes | Group | Cases | Detection
results |
|---|
| Fasting blood glucose
(mmol/l) | Control group | 107 | 5.68±7.51 |
|
| WML group | 86 | 6.32±3.3 |
|
| LI group | 53 | 5.87±4.6 |
|
| F-value | – | 0.62 |
|
| P-value | – | >0.05 |
| Systolic pressure
(mmHg) | Control group | 107 | 119.3±25.3 |
|
| WML group | 86 | 136.7±13.5 |
|
| LI group | 53 | 128.2±22.8 |
|
| F-value | – | 0.94 |
|
| P-value | – | >0.05 |
| Diastolic pressure
(mmHg) | Control group | 107 | 92.12±9.6 |
|
| WML group | 86 | 87.81±10.2 |
|
| LI group | 53 | 95.63±15.7 |
|
| F-value | – | 0.72 |
|
| P-value | – | >0.05 |
| LDL-C (mmol/l) | Control group | 107 | 1.58±2.67 |
|
| WML group | 86 | 3.15±1.21 |
|
| LI group | 53 | 4.65±0.86 |
|
| F-value | – | 1.95 |
|
| P-value | – | <0.05 |
| HDL-C (mmol/l) | Control group | 107 | 1.45±1.22 |
|
| WML group | 86 | 0.97±0.63 |
|
| LI group | 53 | 1.25±0.57 |
|
| F-value | – | 0.83 |
|
| P-value | – | >0.05 |
| Cr (µmol/l) | Control group | 107 | 88.52±16.82 |
|
| WML group | 86 | 79.42±27.21 |
|
| LI group | 53 | 90.16±25.33 |
|
| F-value | – | 0.37 |
|
| P-value | – | >0.05 |
| TG (mmol/l) | Control group | 107 | 1.32±0.81 |
|
| WML group | 86 | 2.52±1.53 |
|
| LI group | 53 | 2.87±1.36 |
|
| F-value | – | 2.76 |
|
| P-value | – | <0.05 |
| Smoking duration
(year) | Control group | 107 | 7.62±3.8 |
|
| WML group | 86 | 6.51±1.5 |
|
| LI group | 53 | 8.42±1.6 |
|
| F-value | – | 0.82 |
|
| P-value | – | >0.05 |
| Smoking amount
(/day) | Control group | 107 | 15.27±10.36 |
|
| WML group | 86 | 18.48±9.68 |
|
| LI group | 53 | 10.25±7.83 |
|
| F-value | – | 0.98 |
|
| P-value | – | >0.05 |
| Drinking duration
(year) | Control group | 107 | 5.87±4.81 |
|
| WML group | 86 | 5.77±6.74 |
|
| LI group | 53 | 7.46±2.21 |
|
| F-value | – | 0.34 |
|
| P-value | – | >0.05 |
| Drinking amount
(ml/day) | Control group | 107 | 108.26±22.64 |
|
| WML group | 86 | 125.47±12.35 |
|
| LI group | 53 | 116.35±23.56 |
|
| F-value | – | 0.94 |
|
| P-value | – | >0.05 |
| Age (years) | Control group | 107 | 38.7±11.2 |
|
| WML group | 86 | 53.6±5.3 |
|
| LI group | 53 | 58.2±7.7 |
|
| F-value | – | 5.84 |
|
| P-value | – | <0.05 |
| TC (mmol/l) | Control group | 107 | 4.8±3.65 |
|
| WML group | 86 | 8.7±2.24 |
|
| LI group | 53 | 6.8±1.24 |
|
| F-value | – | 1.68 |
|
| P-value | – | <0.05 |
MMSE and MoCA examination
We conducted MMSE and MoCA examinations on all
patients and the results revealed that scores in WML and LI groups
were significantly lower than those in the control group and
differences were statistically significant (p<0.05). No
significant difference was detected when the LI group was compared
with the WML group (p>0.05) (Table
III).
 | Table III.MMSE and MoCA examination. |
Table III.
MMSE and MoCA examination.
| Group | Cases | MMSE score | MoCA score |
|---|
| Control group | 107 | 28.38±0.82 | 27.32±1.24 |
| WML group | 86 | 21.27±1.78 | 22.47±1.35 |
| LI group | 53 | 21.25±2.18 | 20.35±1.56 |
| F-value | – | 19.38 | 20.03 |
| P-value | – | 0.017 | 0.012 |
Logistic regression analysis between
CSVD and risk factors
We collected and summarized all possible risk
factors for CSVD-induced TIA, including fasting glucose, lipid
level, Cr level, smoking and drinking amount and duration. The
logistic regression analysis and correlation analysis of paired
material revealed that the serum level of LDL-C was correlated to
the CSVD-induced TIA (OR=1,321) and the difference was
statistically significant despite the enrollment of TG into the
multivariable analysis (p<0.05) (Table IV).
 | Table IV.Analysis of correlation of
CSVD-induced TIA with LDL-C and TG. |
Table IV.
Analysis of correlation of
CSVD-induced TIA with LDL-C and TG.
|
| LDL-C | TG |
|
|
|---|
|
|
|
|
|
|
|---|
| CSVD |
Positivea | Negative |
Positiveb | Negative | r | P-value |
|---|
| Positive | 139 | 0 | 125 | 11 | 0.108 | <0.05 |
| Negative | 23 | 86 | 17 | 90 | 0.024 | <0.05 |
Discussion
Small cerebral vessels refer to the small perforator
arteries, small arteries, capillary and small veins with diameters
of 40–200 µm that are distributed inside the brain. Small cerebral
vessels constitute the basic units that supply blood for cerebral
tissues, and play a significant role in maintaining the cerebral
function. The small cerebral vessels, consisting of endothelial
cells and a few smooth muscle cells, are characterized by poor
vascular elasticity and are usually susceptible to injuries due to
lack of outer layer in histological structure (1–3). Small
thrombus could be easily developed due to the increase in blood
viscosity caused by augmentation of red blood cells or blood fat,
causing endothelial injuries, and secondarily leading to the TIA
and/or cerebral infarction. The main pathological manifestations
include fibrinoid necrosis and amyloidosis, which may lead to
cerebral hemorrhage and infarction (4–7).
Generally, CSVD refers to the syndrome consisting of the clinical,
imagological and pathological manifestations caused by various
lesions. CSVD can be divided into two categories: ⅰ) acute
neurological impairment, which is manifested by stroke, deep
cerebral infarction and cerebral hemorrhage with acute onset, rapid
progression, poor prognosis and a high mortality and disability
rate and ⅱ) chronic and progressive neuropsychic dysfunction,
including vascular cognitive impairment, affective disorder,
decreased comprehension ability, memory impairment, gait disorder
and general function reduction (8–12).
Imagological features are typically manifested by LI,
leukoaraiosis, microbleeding, which can be easily missed or
misdiagnosed (13–15). In the International Stroke Conference
and European Stroke Conference held in 2006, experts introduced a
document titled ‘Little strokes, big trouble’ (1), which was repeated in the International
Stroke Conference and European Stroke Conference held in 2008,
suggesting that CSVD has gradually gained the attention of
international medical communities.
Currently, the consensus is that CSVD-induced TIA is
caused by various factors, and lipid metabolism disorders is one of
the risk factors of CSVD. However, the harm posed by the increase
of LDL level is the most serious compared to all other factors.
Therefore, CSVD can be prevented by controlling the level of LDL in
clinical practice (7). Prior studies
often investigated CSVD and the abnormal level of blood fat
independently, and partially neglected the effect of other risk
factors on this disease. Therefore, it is imperative to clarify the
exact relationship between the CSVD and other related risk factors,
in order to perform early intervention in this disease.
Studies conducted on small-sample groups showed that
CSVD is mainly correlated with the age, hypertension,
hyperlipidemia and atherosclerosis, but the specific mechanism
remained unknown (2). Results
obtained from prior studies suggested that the treatment with
statins could effectively inhibit the development of SVD (3–6). These
results suggested that there may be a certain association between
the abnormal lipid metabolism and SVD. In the present study we
evaluated a larger group, and discovered a positive correlation
between LDL-C level and the incidence rate of CSVD. Currently,
lipid infiltration theory has been confirmed in experimental and
clinical practice, suggesting that the increase in LDL can promote
cholesterol to pass through the arterial wall and localized
aggregation can induce the macrophages and smooth muscle cells to
transform into the foam cells by consuming the lipid (16). This is also considered an important
mechanism of atherosclerotic plaque formation. LDL-C plays an
important role in the formation of atherosclerosis by the following
mechanism: after LDL is transported into the lower layer of
arterial endothelium, it is transformed into ox-LDL through
oxidative modification, which is transported into the macrophages
by the scavenger receptor on the cell membrane. Macrophages that
devoured and digested the lipid are transformed into the foam
cells, constituting the fatty streak through aggregation.
Cholesterol can be released after the rupture of fatty streak,
forming the lipid core of atherosclerotic plaque. Previous findings
revealed that the major component of LDL is LDL-3, i.e., small but
intensive LDL shows the strongest effect in inducing
atherosclerosis. This is mainly manifested as follows: the
clearance rate of LDL is very slow through the pathway mediated by
its receptor and LDL-3 adheres to the vascular wall and is
transported into the endothelial cells of a vessel (16,17). LDL
can easily pass through the arterial wall to be devoured by the
macrophages. LDL is oxidized and ox-LDL is formed. Shen et
al confirmed that there was a correlation between LDL-C in
low-density and cardiovascular events in patients with chronic
renal diseases (16). Currently,
ox-LDL is considered a relatively sensitive index for predicting
the small vascular lesions. Thus, we consider that LDL-C may be an
important risk factor in the occurrence of CSVD-induced TIA, among
which the LDL in a low density shows the strongest effect.
We studied a relatively large sample, and combined
the investigations of the various factors. We found that LDL is the
cause for CSVD and explained the correlation between the risk
factors and CSVD.
We concluded that LDL is a major risk factor
affecting the onset of CSVD. We believe that CSVD should be studied
in patients with hyperlipidemia. Patients should be examined by
head MRI or CT in order to eliminate the probability of CSVD. In
order to reduce the occurrence of adverse events in clinical
practice, early intervention in SVD through decreasing the level of
LDL, improving the endothelial function of small vessels and
applying the anti-inflammation and nerve-protection methods should
be considered.
References
|
1
|
Hachinski V: World Stroke Day 2008:
‘Little strokes, big trouble’. Stroke. 39:2407–2420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantoni L: Cerebral small vessel disease:
From pathogenesis and clinical characteristics to therapeutic
challenges. Lancet Neurol. 9:689–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amarenco P, Benavente O, Goldstein LB,
Callahan A, Sillesen H, Hennerici MG, Gilbert S, Rudolph AE,
Simunovic L, Zivin JA, et al: Stroke Prevention by Aggressive
Reduction in Cholesterol Levels Investigators: Results of the
stroke prevention by aggressive reduction in cholesterol levels
(SPARCL) trial by stroke subtypes. Stroke. 40:1405–1409. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
ten Dam VH, Van den Heuvel DM, van Buchem
MA, Westendorp RG, Bollen EL, Ford I, de Craen AJ and Blauw GJ;
PROSPER Study Group, : Effect of pravastatin on cerebral infarcts
and white matter lesions. Neurology. 64:1807–1809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok VC, Lam WW, Fan YH, Wong A, Ng PW,
Tsoi TH, Yeung V and Wong KS: Effects of statins on the progression
of cerebral white matter lesion: Post hoc analysis of the ROCAS
(Regression of Cerebral Artery Stenosis) study. J Neurol.
256:750–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu JH, Mok V, Lam W, Wong A, Chu W, Xiong
Y, Ng PW, Tsoi TH, Yeung V and Wong KS: Effects of statins on
progression of subclinical brain infarct. Cerebrovasc Dis.
30:51–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y and
Wong KS: Extent of white matter lesions is related to acute
subcortical infarcts and predicts further stroke risk in patients
with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry.
76:793–796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu JH, Wong K, Mok V, Hu X, Xiong Y, Chen
Y, Tang WK, Chen X, Wong A, Chu W, et al: Neuroimaging predictors
for depressive symptoms in cerebral small vessel disease. Int J
Geriatr Psychiatry. 25:1039–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jokinen H, Gouw AA, Madureira S, Ylikoski
R, van Straaten EC, van der Flier WM, Barkhof F, Scheltens P,
Fazekas F, Schmidt R, et al LADIS Study Group, : Incident lacunes
influence cognitive decline: The LADIS study. Neurology.
76:1872–1878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Dijk EJ, Prins ND, Vrooman HA, Hofman
A, Koudstaal PJ and Breteler MM: Progression of cerebral small
vessel disease in relation to risk factors and cognitive
consequences: Rotterdam Scan study. Stroke. 39:2712–2719. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenberg SM: Small vessels, big problems.
N Engl J Med. 354:1451–1453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pantoni L, Basile AM, Pracucci G, Asplund
K, Bogousslavsky J, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM,
Hennerici M, et al: Impact of age-related cerebral white matter
changes on the transition to disability - The LADIS study:
Rationale, design and methodology. Neuroepidemiology. 24:51–62.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pantoni L: Cerebral small vessel disease:
from pathogenesis and clinical characteristics to therapeutic
challenges. Lancet Neurol. 9:689–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel B and Markus HS: Magnetic resonance
imaging in cerebral small vessel disease and its use as a surrogate
disease marker. Int J Stroke. 6:47–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fazekas F, Chawluk JB, Alavi A, Hurtig HI
and Zimmerman RA: MR signal abnormalities at 1.5 T in Alzheimers
dementia and normal aging. AJR Am J Roentgenol. 149:351–356. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen H, Xu Y, Lu J, Ma C, Zhou Y, Li Q,
Chen X, Zhu A and Shen G: Small dense low-density lipoprotein
cholesterol was associated with future cardiovascular events in
chronic kidney disease patients. BMC Nephrol. 17:1432016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Annema W and von Eckardstein A:
Dysfunctional high-density lipoproteins in coronary heart disease:
Implications for diagnostics and therapy. Transl Res. 173:30–57.
2016. View Article : Google Scholar : PubMed/NCBI
|















