Introduction
Liver transplantation is an effective approach for
the treatment of end-stage liver diseases, including hepatic
carcinoma and hepatic cirrhosis (1,2), and
currently accounts for 4% of liver transplants annually (3). With the progress of medical science,
liver transplantation has been widely used in patients with
hepatitis B virus (HBV) infection, specifically patients with
chronic and severe HBV infection (4,5).
However, liver transplantation is a complicated surgery that takes
5–10 h on average. In addition, patients with end-stage liver
diseases typically have blood coagulation dysfunction, which may
lead to substantial blood loss during liver transplantation
surgery. Therefore, perioperative blood transfusion is critically
important for the success of liver transplantation surgery
(6).
Intraoperative autologous blood transfusion is a
medical procedure involving the recovery of blood lost during
surgery and re-infusing it into the patient after rinsing.
Intraoperative autologous blood transfusion may resolve the problem
of limited blood supply sources and reduce the economic burden on
patients. In addition, intraoperative autologous blood transfusion
may prevent transfusion-transmitted infections and avoid allergic,
hemolytic and graft-versus-host reactions caused by allogeneic
blood transfusion (7,8). As a major form of autotransfusion,
intraoperative autologous blood transfusion has been widely used in
clinical practice. Sankarankutty et al (9) demonstrated that autologous blood
transfusion significantly reduced blood usage in liver
transplantation surgery by recovering >90% of red blood cells
(RBCs) in the surgical field, the recovered RBCs accounted for
>50% of the total amount of RBCs used in the liver
transplantation surgery. Therefore, autologous blood transfusion
may markedly relieve the limitation of blood supply source in
clinical settings.
Autologous blood transfusion may cause morphological
and functional changes of RBCs. Different autologous blood
transfusion methods may have distinct influences on the morphology
and function of recovered RBCs (10). A study conducted by Wan et al
(11) suggests that autologous blood
transfusion had no significant adverse effects on the morphology
and deformability of RBCs during coronary artery bypass surgery.
Ling (12) reported that autologous
transfusion of rinsed RBCs in orthopaedic surgeries had no
evidently negative impacts on the overall hemorheology in the
patients. Another study also reported a limited influence of
intraoperative autologous blood transfusion on the function of RBCs
in patients with liver and kidney diseases (13).
In end-stage HBV patients, the membrane structure
and function of RBCs have been altered by the accumulation of toxic
substances due to decompensation and liver dysfunction (14). It remains unclear whether autologous
blood transfusion has an influence on the morphology and function
of RBCs in HBV patients with decompensation. The present study
examined the morphological and functional changes of RBCs and
evaluated the alternation of hemorheology caused by intraoperative
autologous blood transfusion in chronic HBV patients at the
end-stage. The results of the current study provide a theoretical
basis for the application of autologous blood transfusion in liver
transplantation surgery.
Materials and methods
Patients
All human studies have been approved by The
Institute Research Medical Ethics Committee of the Third Affiliated
Hospital, Sun Yat-Sen University (Guangdong, China). All human
studies have been performed in accordance with the ethical
standards outlined in the 1964 Declaration of Helsinki and its
later amendments. All patients provided their informed consent
prior to their inclusion in the study.
From January 2014 to June 2015, a total of 15 male
patients with HBV at the end-stage underwent liver transplantation
for HBV-related liver disease in our center, were included in the
present study. Inclusion criteria for participating in the study
were: Patients (only male) were aged 18–70 years, were hepatitis B
surface antigen-positive for at least 6 months, HBV DNA-positive,
accepted to sign the informed consent paper and were expected to
survive >6 months after surgery. Exclusion criteria for the
present study were: hepatitis B surface antigen-negative, expected
to survive <6 months after surgery, were positive for hepatitis
C virus or HIV co-infection, hepatpcellular carinoma and exhibited
drug allergies. Due to liver decompensation, the 15 patients
received orthotopic liver transplantation under general anesthesia
with midazolam (0.1 mg/kg), propofol (1.0 mg/kg), fentanyl (4
µg/kg) and vecuronium (0.1 mg/kg) (all from Yangtze River
Pharmaceutical Group, Jiangsu, China) at the Third Affiliated
Hospital, Sun Yat-Sen University. The patients (age, 18–70 years;
weight, 50–75 kg) were classified as ASA III–IV according to the
ASA physical status classification system (15). Whole blood, serum, plasma and urine
from each patient were tested, and no blood, endocrine or
autoimmune diseases were identified in the 15 patients. No pus,
bile or cancer cells were identified in the blood from the
patients. Informed consent was obtained from the patients and their
families prior to the liver transplantation surgery.
Reagents and instruments
All reagents used in the present study were provided
by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). An
Autologous Blood Recovery System (Autologous 2000) was purchased
from Wandong Medical Equipment Company (Beijing, China). Automatic
blood analyzer (Sysmex XT 2000i) was purchased from Sysmex Corp.
(Kobe, Japan). A blood rheometer (LVDV-III+) was purchased from the
Brookfield Co. (San Francisco, CA, USA).
Vital signs of the patients were monitored during
surgery under anesthesia induced with midazolam (0.1 mg/kg),
propofol (1 mg/kg), fentanyl (4 µg/kg) and vecuronium (0.1 mg/kg),
and maintained with sufentanil (0.5 mg/kg), propofol (0.5 mg/kg),
heptafluoro ether (1 mg/kg), and vecuronium (0.08 mg/kg) (all from
Yangtze River Pharmaceutical Group, Jiangsu, China). Each patient
underwent invasive arterial pressure measurement, deep vein
puncture and floating catheter manometry. Anticoagulation of
pre-operative blood (15 ml) collected from the deep veins of 15
patients (n=15) was conducted using EDTA. Blood collected during
the surgery using the autologous blood recovery machine was mixed
with anticoagulants and filtrated (filter diameter, 20–40 µm) in
the blood storage. Subsequently, the filtrated blood was
centrifuged at 6,000 × g for 15 min at 4°C and rinsed twice with
PBS (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing,
China) to remove cell debris, free hemoglobin (FHb) and
anticoagulants. The hematocrit of recovered RBCs was between 30 and
50%. Following this, 15 ml of the recovered blood was used in
subsequent experiments to compare with the pre-operative blood
samples (n=15).
Morphological and structural
evaluation of RBCs
To evaluate the morphology of RBCs,
EDTA-anticoagulated blood samples (2 ml) were analyzed in an
automatic blood analyzer to determine the mean corpuscular volume
(MCV), mean corpuscular hemoglobin (MCH), mean corpuscular
hemoglobin concentration (MCHC) and red cell distribution width
(RDW). A drop of blood (~50 µl) was spread on a glass slide and
left for 2 min at room temperature. Following Wright staining, the
slide was examined under an inverted optical microscope (IX80;
Olympus Corporation of the Americas, Center Valley PA, USA) with an
oil lens at a magnification of ×100 to evaluate the morphology of
100 RBCs according to the morphological scoring system (16). In this scoring system, 1 point was
assigned to RBCs of biconcave-discoid shape. Points 2, 3, 4 and 5
were assigned to spiny RBCs of disc shape, spiny RBCs, spherical
and spiny RBCs and spherical RBCs, respectively. Imaging was
conducted under an inverted microscope.
To evaluate the structure of RBCs using a scanning
electron microscope (SEM), blood samples were washed using 10X
normal saline and centrifuged twice at 2,000 × g at 4°C for 5 min.
Subsequently, the precipitate was fixed in 2.5% glutaraldehyde for
1 h at 4°C and dehydrated in phosphate-buffered saline (0.1 M),
graded ethanol solution (50, 70, 90 and 100%), and 100% iso-amyl
acetate. Following critical point drying using a Hitachi HCP-2 type
critical point dryer (Tokyo, Japan) and metal coating using E-1045
ion-beam sputtering apparatus (Techcomp Ltd., Beijing, China)
(17), the samples were examined
under a SEM to evaluate the ultrastructure of the surface of
RBCs.
Analysis of RBC membrane proteins
Following centrifugation of the blood samples in a
heparinized tube at 2,000 × g for 10 min at 4°C, the supernatant
and upper white fluffy layer (primarily leukocytes) were removed
using a pipette. Packed RBCs were obtained by washing the
precipitate with normal saline for 5 min and centrifuged at 6,000 ×
g for 10 min at 4°C three times. Packed RBCs were suspended in
hypotonic buffer and incubated at 4°C for 20 min. Following
centrifugation at 20,000 × g for 30 min at 4°C, a pink precipitate
was observed in the bottom. The precipitate was washed with
hypotonic buffer three times for 10 min to collect the ‘spectrin
layer’ that primarily contains the RBC membrane proteins. A total
of 2 µg of RBC membrane proteins were stained with Coomassie
brilliant blue to measure the protein content. Other RBC membrane
proteins were stored at 30°C for subsequent experiments.
RBC membrane proteins were appropriately homogenized
using an ultrasonic homogenizer and mixed with 5X loading buffer.
Following boiling for 5 min, the proteins (15 µl) were separated by
10% SDS-PAGE. Following electrophoresis, the discolored gel was
scanned and imaged on a LiCor Odyssey scanner (Li-Cor Biosciences,
Lincoln, NE, USA). The electrophoresis pattern of RBC membrane
proteins was analyzed using Quantity One software version 4.5.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Evaluation of RBCs physiological
functions
Levels of 2,3-diphosphoglycerate (2,3-DPG), FHb,
erythrocyte membrane ATPase and malondialdehyde (MDA) in
pre-operative and recovered blood samples were measured using
different kits, including the 2,3 DPG kit (HY-60073; eBioscience;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), FHB kit
(HY-60068; eBioscience; Thermo Fisher Scientific, Inc.), ATPase
assay kit (A016-1; Nanjing Jiancheng Bioengineering Institute) and
MDA assay kit (MAK085; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to the manufacturer's protocol.
Pre-operative or recovered blood samples (2 ml) were
diluted with normal saline in a 10:1 ratio and centrifuged at 2,000
× g for 10 min at 4°C to prepare packed RBCs. Graded NaCl solution
(1–8.5 g/l) was added to the packed RBCs (0.05 ml) following
incubation at 37°C for 5 min. To compare the osmotic fragility
between pre-operative and recovered RBCs, the RBC hemolysis rate at
different concentrations of NaCl was calculated according to a
previous study (18).
Hemorheology
Pre-operative or recovered blood samples (3 ml) were
treated with lithium heparin anticoagulant. Anticoagulated blood
samples were analyzed in a blood rheometer to evaluate low-shear
blood viscosity, high-shear blood viscosity, plasma viscosity,
blood relative viscosity, erythrocyte aggregation index, Casson
viscosity and yield stress.
Statistical analysis
Measurement data were presented as the mean ±
standard deviation. Statistical analyses were performed using SPSS
statistical software version 13.0 (SPSS, Inc., Chicago, IL, USA).
Comparison of values between the pre-operative and recovered blood
samples was conducted using paired t-tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological and structural changes
in RBCs recovered during liver transplantation surgery
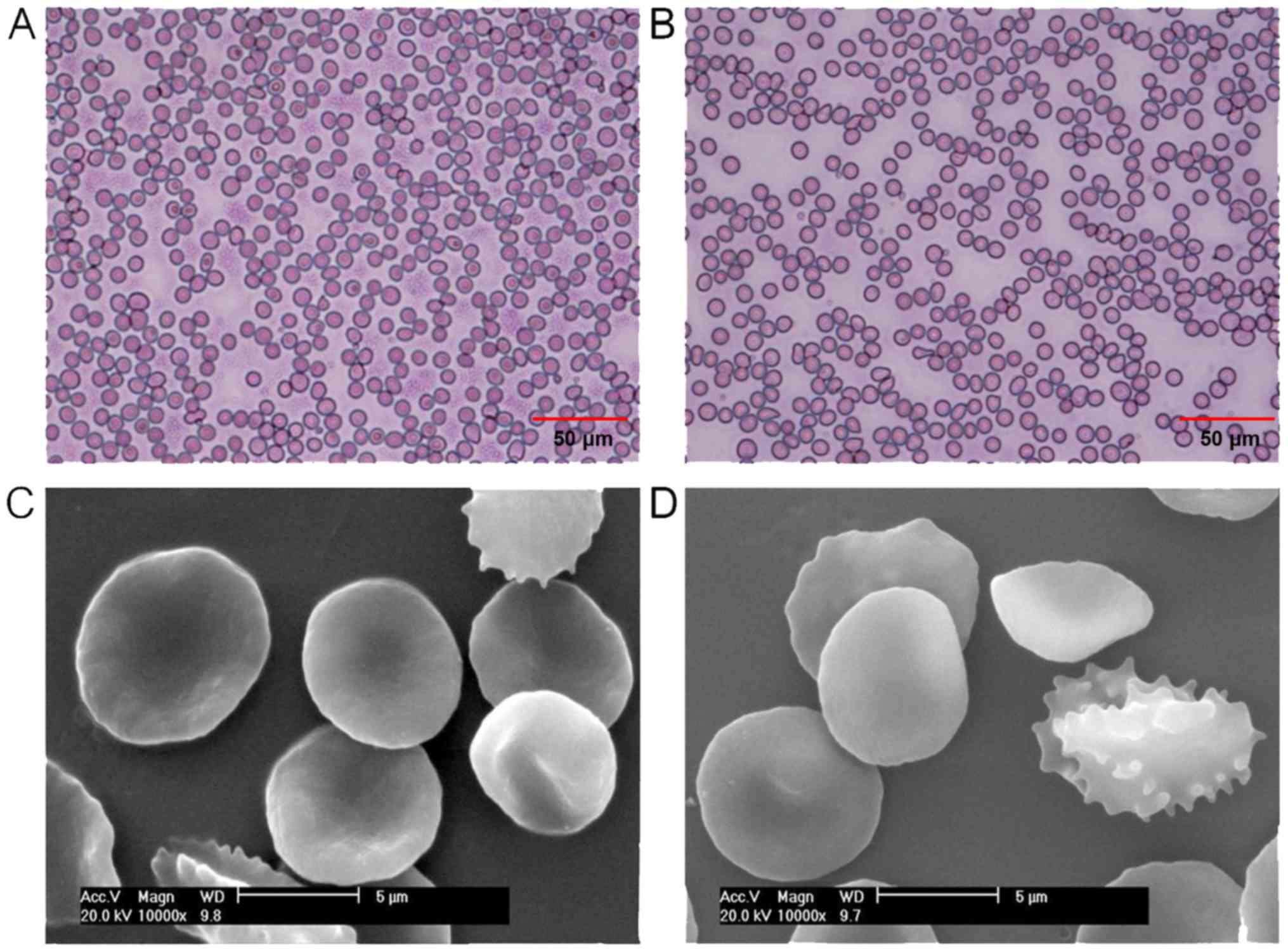
As presented in Fig.
1A, the majority of RBCs in the pre-operative blood samples
were a normal biconcave-discoid shape. However, the number of RBCs
exhibiting a normal biconcave-discoid shape decreased in the
recovered blood samples. Furthermore, spherical and spiny RBCs were
observed in the recovered blood samples (Fig. 1B). As presented in Fig. 1C, RBCs in the pre-operative blood
samples were observed to have a smooth surface without abnormal
protrusions, based on SEM examination. Protrusions were observed on
the surface of RBCs in the recovered blood samples (Fig. 1D).
While the morphological scores of RBCs in the
recovered blood samples were higher than that of RBCs in the
pre-operative blood samples, the difference was not statistically
significant. In addition, no significant differences in the MCV,
MCH, MCHC, red cell distribution width-coefficient of variation
(RDW-CV) and red cell distribution standard deviation (RDW-SD)
values were identified between RBCs in the pre-operative blood
samples and recovered blood samples (Table I).
 | Table I.Comparison between the morphological
scores of MCV, MCH, MCHC and RWD between red blood cells in the
pre-operative blood samples and recovered blood samples. |
Table I.
Comparison between the morphological
scores of MCV, MCH, MCHC and RWD between red blood cells in the
pre-operative blood samples and recovered blood samples.
| Variable | Pre-operative blood
samples (n=15) | Recovered blood
samples (n=15) | P-value |
|---|
| Morphological
score | 121±16 | 130±19 | P>0.05 |
| MCV | 94±11 | 95±10 | P>0.05 |
| MCH | 33±4 | 31±5 | P>0.05 |
| MCHC | 350±13 | 333±38 | P>0.05 |
| RDW-SD | 47±9 | 48±9 | P>0.05 |
| RDW-CV | 0.14±0.02 | 0.14±0.03 | P>0.05 |
Membrane protein changes in RBCs
recovered during liver transplantation surgery
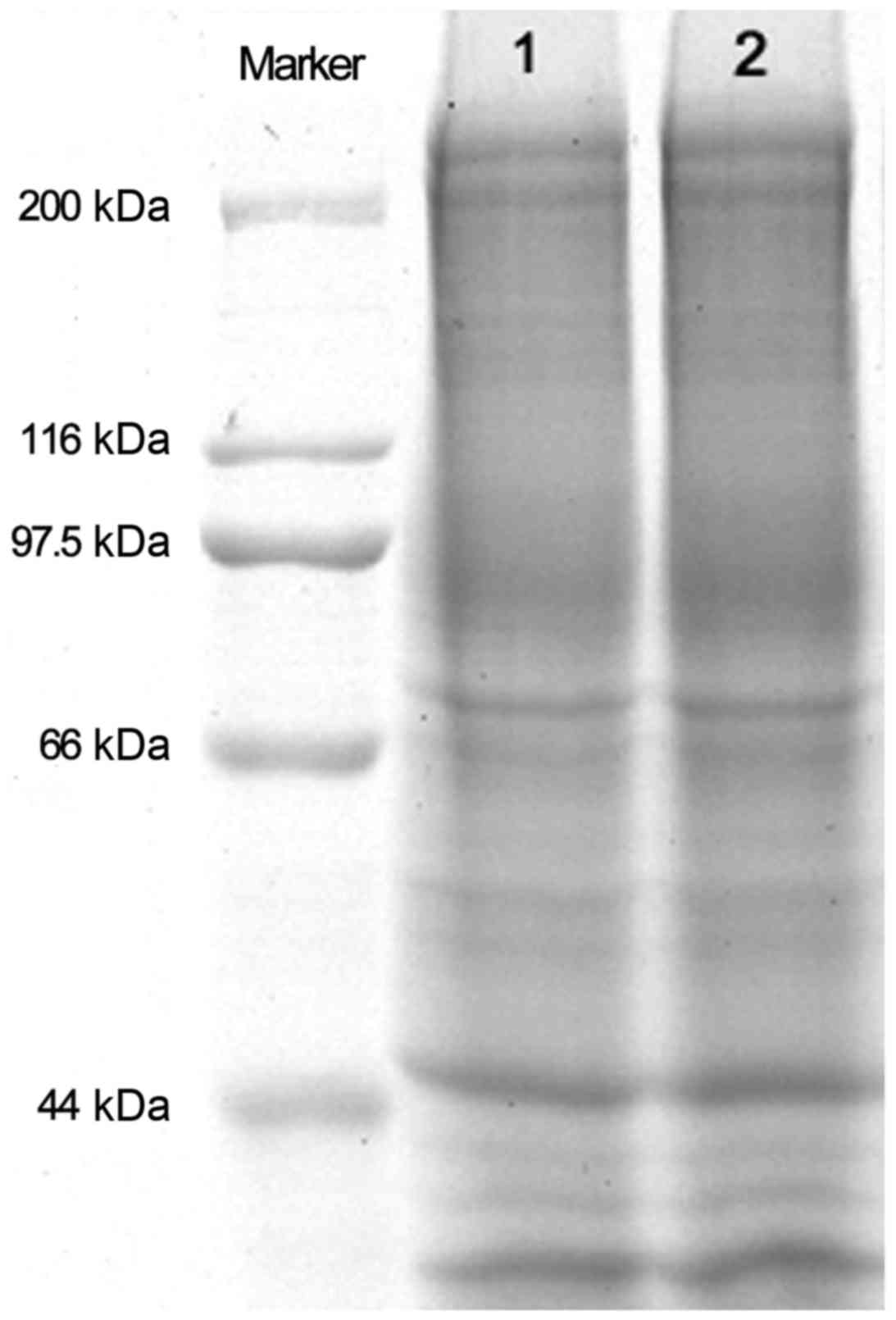
As presented in Fig.
2, no marked changes in the molecular weight of membrane
proteins were observed between pre-operative and recovered
RBCs.
Comparison of physiological functions
between RBCs in the pre-operative and recovered blood samples
Tables II and
III demonstrate no significant
differences for 2,3-DPG, Na+ K+-ATPase,
Ca2+-ATPase, Mg2+-ATPase, MDA and osmotic
fragility were identified between RBCs in the pre-operative blood
samples and recovered blood samples (P>0.05). However, FHb in
recovered RBCs (50.7±12.6 mg/l) was significantly higher than that
in pre-operative RBCs (37.1±5.7 mg/l; P<0.05; Table II).
 | Table II.Comparison of physiological functions
between red blood cells in the pre-operative blood samples and
recovered blood samples. |
Table II.
Comparison of physiological functions
between red blood cells in the pre-operative blood samples and
recovered blood samples.
| Variable | Pre-operative blood
samples (n=15) | Recovered blood
samples (n=15) | P-value |
|---|
| 2, 3-DPG,
µmol/gHb | 15.9±4.5 | 19.4±4.0 | P>0.05 |
| Na+
K+-ATPase, U/mg protein | 5.91±1.93 | 5.29±1.88 | P>0.05 |
|
Ca2+-ATPase, U/mg protein | 8.23±2.46 | 7.98±2.01 | P>0.05 |
|
Mg2+-ATPase, U/mg protein | 4.99±1.32 | 4.78±1.01 | P>0.05 |
| MDA, nmol/ml | 4.3±0.1 | 5.7±0.5 | P>0.05 |
| FHb, mg/l | 37.1±5.7 |
50.7±12.6a | P<0.05 |
 | Table III.Comparison of the hemolysis rates
between red blood cells in the pre-operative blood samples and
recovered blood samples in all 15 patients. |
Table III.
Comparison of the hemolysis rates
between red blood cells in the pre-operative blood samples and
recovered blood samples in all 15 patients.
| NaCl concentration,
g/l | Pre-operative blood
samples, % | Recovered blood
samples, % |
|---|
| 8.5 | 100 | 100 |
| 7.5 | 85±9 | 80±13 |
| 7.0 | 77±6 | 65±13 |
| 6.5 | 61±22 | 56±21 |
| 6.0 | 47±33 | 45±27 |
| 5.5 | 30±34 | 41±21 |
| 5.0 | 34±46 | 20±11 |
| 4.5 | 36±46 | 20±13 |
| 4.0 | 32±12 | 22±14 |
| 3.5 | 14±26 | 10±3 |
| 3.0 | 14±20 | 9±2 |
| 2.0 | 13±22 | 8±2 |
| 1.0 | 10±11 | 7±10 |
Influence of autologous blood
transfusion in liver transplantation surgery on hemorheology
Medium- (3.56±1.35 mpa.s) and high-shear (2.33±0.7
mpa.s) blood viscosities in the recovered blood samples were
significantly lower than those in the pre-operative blood samples
(5.64±2.35 and 3.64±1.06 mpa.s, respectively; P<0.05). Casson
viscosity (1.7±0.62 mpa.s) in the recovered blood samples was
significantly higher compared with the pre-operative blood samples
(0.9±0.03 mpa.s; P<0.05). However, no significant differences in
the low-shear blood viscosity, plasma viscosity, relative blood
viscosity, erythrocyte aggregation index and Casson yield stress
were identified between recovered and pre-operative blood samples
(Table IV).
 | Table IV.Comparison of the hemorheology
between recovered and pre-operative blood samples. |
Table IV.
Comparison of the hemorheology
between recovered and pre-operative blood samples.
| Variable | Pre-operative blood
samples (n=15) | Recovered blood
samples (n=15) | P-value |
|---|
| Low-shear blood
viscosity | 15.5±10.5 | 15.3±8.73 | P>0.05 |
| Medium-shear blood
viscosity | 5.64±2.35 |
3.56±1.35a | P<0.05 |
| High-shear blood
viscosity | 3.64±1.06 |
2.33±0.70a | P<0.05 |
| Plasma
viscosity | 2.1±1.5 | 1.3±0.6 | P>0.05 |
| Relative blood
viscosity | 13.5±8.19 | 12.2±6.27 | P>0.05 |
| Erythrocyte
aggregation index | 4.75±0.89 | 7.00±3.21 | P>0.05 |
| Casson
viscosity | 0.90±0.03 |
1.7±0.62a | P<0.05 |
| Yield stress | 6.2±6.5 | 7.8±6.1 | P>0.05 |
Discussion
A biconcave disc shape is an important feature of
RBCs, essential for the deformability, suspension stability and
osmotic fragility of RBCs (19). In
the present study, the majority of RBCs in the pre-operative blood
samples are of the normal biconcave-discoid shape. However, the
numbers of spiny RBCs of disc shape and spiny red blood cells in
the recovered blood samples increased. In addition, spherical and
spiny RBCs and spherical RBCs in the recovered blood samples
observed. While the scores of RBCs in the recovered blood samples
were higher than that of RBCs in the pre-operative blood samples,
the difference was not statistically significant. In addition, no
significant differences in the levels of MCV, MCH, MCHC and RDW-CV
were identified between RBCs from pre-operative blood samples and
RBCs in the recovered blood samples in the liver transplantation
surgery. These results suggest that autologous blood transfusion
during liver transplantation in patients with hepatitis B with
decompensation had a limited influence on the morphology of RBCs.
Deformability and membrane fluidity are necessary for the normal
physiological function of RBCs. The composition and structure of
membrane proteins in RBCs are of importance in the function of
RBCs. The majority (~60%) of membrane proteins in RBCs form a
continuous network in the cytoplasm to support and control cell
shapes (20). Any changes in the
quality and quantity of membrane cytoskeletal proteins in RBCs may
cause abnormal morphology and function of RBCs. In the present
study, SDS-PAGE analysis demonstrated no significant difference in
the molecular weight of membrane proteins in RBCs between
pre-operative blood samples and recovered blood samples. This
suggests that autologous blood transfusion in liver transplantation
in patients with hepatitis B and decompensation had no significant
influence on the structure of RBCs.
Membrane-binding adenosine triphosphate (ATP)
enzymes have important roles in the maintenance of RBC morphology,
structure and functions. As an intermediate product in the
metabolism of RBCs, 2,3-DPG is an important indicator of the
oxygen-carrying capacity (21). In
the human body, the oxygen supply in different tissues is regulated
by the concentration of 2,3-DPG in RBCs. Under the same conditions,
an increased concentration of 2,3-DPG in RBCs promotes
O2 release from oxyhemoglobin. It has been reported that
the levels of 2,3-DPG and ATP in recovered RBCs was higher than
that in stored RBCs (22). In
addition, it has been demonstrated that recovered blood cells
exhibited an improved oxygen carrying capacity and stronger
anti-infiltration capacity than stored blood cells, which reduce
the incidence of metabolic acidosis and electrolyte imbalance
caused by the infusion of a large number of stored blood cells
(23). Other studies that compared
intraoperative cell salvage with allogeneic blood transfusion have
demonstrated an increased mean erythrocyte viability, increased
2,3-DPG (24,25) and increased ATP levels (26,27) in
salvaged blood. In the present study, no significant difference in
the levels of Na+K+-ATPase,
Ca2+-ATPase, Mg2+-ATPase and 2,3-DPG were
observed between pre-operative and recovered RBCs, suggesting that
autologous blood transfusion in liver transplantation in patients
with hepatitis B and decompensation had no significant influence on
the functions of RBCs.
FHb is plasma hemoglobin associated with the damage
of RBCs. In the present study, the concentration of FHb in the
recovered blood samples from liver transplantation in patients with
hepatitis B and decompensation was significantly higher than that
in the pre-operative blood samples in the same patients, suggesting
hemolysis in the process of blood recovery. The use of a high-speed
centrifugal machine, tubing extrusion, suction, mechanical damage
and other factors in the blood recovery process may cause hemolysis
(28). A number of measures may be
considered to reduce the chance of hemolysis. For example, the
negative suction pressure used in blood recovery may cause major
damage to RBCs, which has a large influence on the quality of
recovered RBCs. To minimize damage to RBCs, the recommended suction
pressure is typically <0.02 MPa (150 mmHg) (29). In addition, dilution of recovered
blood using saline may also markedly inhibit the damage on RBCs
(30). Osmotic fragility, which is
measured by the tensile capacity of the cell membrane, is an
important mechanical property of RBCs. Resistance to the hypotonic
solution of RBCs is associated with it membrane thickness. The
thicker membrane is associated with a higher osmotic fragility due
to a lower ratio of the membrane area to volume (18). Spain et al (31) revealed that recovered and rinsed RBCs
still have a normal volume, hemoglobin content, hemoglobin
concentration and osmotic fragility. In the present study, no
significant difference in the NaCl concentration between recovered
blood samples and pre-operative blood samples was identified. This
suggests that autologous blood transfusion during liver
transplantation in patients of hepatitis B with decompensation had
no significant influence on the osmotic fragility of RBCs.
Rheological properties of RBCs, primarily determined
by the aggregation and deformation of RBCs, are critically
important for normal blood circulation in blood vessels (5). Casson viscosity is associated with RBC
deformability. Low-shear and high-shear whole blood viscosities
reflect the aggregation and deformation of RBCs. RBCs of low-shear
viscosity tend to aggregate and RBCs of high-shear viscosity have
poor deformability. In the present study, Casson viscosity and low-
and high-shear whole blood viscosities in the recovered blood were
significantly higher than those in the pre-operative blood samples.
This suggests an increased deformation ability of recovered RBCs.
Suction and high-speed centrifugation may damage aging RBCs and
RBCs of poor deformability, which are removed by hemolysis to cause
increased deformation ability of recovered RBCs. In the present
study, no significant difference in the low-shear whole blood
viscosity and aggregation index was identified between recovered
blood and pre-operative blood samples. Therefore suggesting that
the autologous blood transfusion in liver transplantation of
patients with hepatitis B and decompensation had no significant
influence on the aggregation of RBCs.
In conclusion, the results of the current study
demonstrated that autologous blood transfusion in liver
transplantation in patients with hepatitis B and decompensation had
no significant influence on the morphology, structure, function and
hemorheology of recovered RBCs. Therefore, autologous blood
transfusion in liver transplantation may be widely applied.
However, the process of blood recovery and transfusion remains to
be further investigated and improved to keep the morphology,
function and hemorheology of recovered RBCs.
Glossary
Abbreviations
Abbreviations:
|
RBC
|
red blood cells
|
|
HBV
|
hepatitis B virus
|
|
MCV
|
mean corpuscular volume
|
|
MCH
|
mean corpuscular hemoglobin
|
|
MCHC
|
mean corpuscular hemoglobin
concentration
|
|
RDW
|
red cell distribution width
|
|
SEM
|
scanning electron microscope
|
|
2,3-DPG
|
2,3-diphosphoglycerate
|
|
FHb
|
free hemoglobin
|
|
MDA
|
malondialdehyde
|
|
ATP
|
adenosine triphosphate
|
References
|
1
|
Haug CE, Jenkins RL, Rohrer RJ,
Auchincloss H, Delmonico FL, Freeman RB, Lewis WD and Cosimi AB:
Liver transplantation for primary hepatic cancer. Transplantation.
53:376–382. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noble-Jamieson G, Valente J, Barnes ND,
Friend PJ, Jamieson NV, Rasmussen A and Calne RY: Liver
transplantation for hepatic cirrhosis in cystic fibrosis. Arch Dis
Child. 71:349–352. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delemos AS and Vagefi PA: Expanding the
donor pool in liver transplantation: Extended criteria donors.
Clinical Liver Disease. 2:156–159. 2013. View Article : Google Scholar
|
|
4
|
Williamson KR, Taswell HF, Rettke SR and
Krom RA: Intraoperative autologous transfusion: Its role in
orthotopic liver transplantation. Mayo Clin Proc. 64:pp. 340–345.
1989; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pratschke J, Mittler J and Neuhaus P:
Expanding the liver donor pool through extended-criteria donation.
Chirurg. 79:130–134. 2008.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuchs RJ, Levin J, Tadel M and Merritt W:
Perioperative coagulation management in a patient with
afibrinogenemia undergoing liver transplantation. Liver Transpl.
13:752–756. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waters JR, Meier HH and Waters JH: An
economic analysis of costs associated with development of a cell
salvage program. Anesth Analg. 104:869–875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carless PA, Henry DA, Moxey AJ, O'connell
DL, Brown T and Fergusson DA: Cell salvage for minimising
perioperative allogeneic blood transfusion. Cochrane Database Syst
Rev. 14:CD0018882010.
|
|
9
|
Sankarankutty AK, Teixeira AC, Souza FF,
Mente ED, Oliveira GR, Almeida RC, Andrade CM, Origuella EA and Ode
C Silva: Impact of blood salvage during liver transplantation on
reduction in transfusion requirements. Acta Cir Bras. 21 Suppl
1:S44–S47. 2006. View Article : Google Scholar
|
|
10
|
Yazer MH, Waters JH, Elkin KR, Rohrbaugh
ME and Kameneva MV: A comparison of hemolysis and red cell
mechanical fragility in blood collected with different cell salvage
suction devices. Transfusion. 48:1188–1191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan CH, Dong PQ, Yang Jing and Mei-Ling
HE: Effect of Cell-Saver on morphology and function of
erythrocytes. Chinese J Extracorpor Circul. 6:136–138. 2008.
|
|
12
|
Ling DA: Effect of autotransfusion on red
cell deformability during orthopedic operation. J Chong Med Univ.
7:282007.
|
|
13
|
Bauermann E, Shin M, Möhlmann ML, Kadar JG
and Linde I: Quality of washed autologous erythrocytes from
drainage-suction pumps. Anaesthesiol Reanim. 24:101–108. 1999.(In
German). PubMed/NCBI
|
|
14
|
Lesesve JF, Garçon L and Lecompte T:
Transient red blood cells morphological anomalies after acute liver
dysfunction. Eur J Haematol. 84:92–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringdal KG, Skaga NO, Steen PA, Hestnes M,
Laake P, Jones JM and Lossius HM: Classification of comorbidity in
trauma: The reliability of pre-injury ASA physical status
classification. Injury. 44:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohkuma S and Uda M: Effectiveness of a
morphology score as an index of the storage condition of red blood
cells. Japanese J Transfusion Cell Therapy. 32:523–527. 1986.
|
|
17
|
Yang CY, Huang LY, Shen TL and Yeh JA:
Cell adhesion, morphology and biochemistry on nano-topographic
oxidized silicon surfaces. Eur Cell Mater. 20:415–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khoshbin AR, Mohamadabadi F, Vafaeian F,
Babania A, Akbarian S, Khandozi R, Sadrebazaz MA, Hatami E and
Joshaghani HR: The effect of radiotherapy and chemotherapy on
osmotic fragility of red blood cells and plasma levels of
malondialdehyde in patients with breast cancer. Rep Pract Oncol
Radiother. 20:305–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uzoigwe C: The human erythrocyte has
developed the biconcave disc shape to optimise the flow properties
of the blood in the large vessels. Med Hypotheses. 67:1159–1163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi F, Xuan D and Xu S: Study on the
erythrocyte membrane skeletin in hemoglobinuria Cows. Scientia
Agricultura Sinica. 2000.
|
|
21
|
Che J, Tian M, Ding G, Huai Q, Dong P, Li
Y and Li S: Effects of cell salvage on erythrocyte
2,3-disphosphoglycerate and G-6-PD levels and phosphatidylserine
expression. Int J Lab Hematol. 35:385–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt H, Følsgaard S, Mortensen PE and
Jensen E: Impact of autotransfusion after coronary artery bypass
grafting on oxygen transport. Acta Anaesthesiol Scand. 41:995–1001.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phillips SD, Maguire D, Deshpande R,
Muiesan P, Bowles MJ, Rela M and Heaton ND: A prospective study
investigating the cost effectiveness of intraoperative blood
salvage during liver transplantation. Transplantation. 81:536–540.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muñoz Gómez M, Sánchez Arrieta Y, García
Vallejo JJ, Mérida de la Torre FJ, Ruíz Romero de la Cruz MD and
Eloy-García JM: Pre and post-operative autotransfusion. A
comparative study of hematology, biochemistry and red cell
metabolism in pre-donated blood and blood from post-operative
surgical drainage. Sangre (Barc). 44:443–450. 1999.(In Spanish).
PubMed/NCBI
|
|
25
|
Schmidt H, Kongsgaard U, Kofstad J, Geiran
O and Refsum HE: Autotransfusion after open heart surgery: The
oxygen delivery capacity of shed mediastinal blood is maintained.
Acta Anaesthesiol Scand. 39:754–758. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allam J, Cox M and Yentis SM: Cell salvage
in obstetrics. Int J Obstet Anesth. 17:37–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amand T, Pincemail J, Blaffart F,
Larbuisson R, Limet R and Defraigne JO: Levels of inflammatory
markers in the blood processed by autotransfusion devices during
cardiac surgery associated with cardiopulmonary bypass circuit.
Perfusion. 17:117–123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pei L and Wang J: Protective effect of
tetramethylpyrazine on red blood cells during autotransfusion.
Zhonghua Yi Xue Za Zhi. 82:322–324. 2002.(In Chinese). PubMed/NCBI
|
|
29
|
Gregoretti S: Suction-induced hemolysis at
various vacuum pressures: Implications for intraoperative blood
salvage. Transfusion. 36:57–60. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waters JH, Williams B, Yazer MH and
Kameneva MV: Modification of suction-induced hemolysis during cell
salvage. Anesth Analg. 104:684–687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spain DA, Miller FB, Bergamini TM,
Montgomery RC and Richardson JD: Quality assessment of
intraoperative blood salvage and autotransfusion. Am Surg.
63:1059–1064. 1997.PubMed/NCBI
|