Introduction
Mammalian oocyte growth and development depends on a
functional two-way communication axis between oocytes and ovarian
granulosa cells (GCs) (1–5). The cellular and molecular mechanisms
associated with this process are poorly understood, but
communication between oocytes and GCs are able to form a paracrine
signaling pathway via soluble oocyte-secreted factors (OSFs)
(6,7). This communication is essential for
oocyte developmental competence. Examples of OSFs include bone
morphogenetic protein (BMP)-15, BMP-6 and growth differentiation
factor (GDF)-9, which are produced in ovarian follicles (8).
BMPs, which are a subfamily of the transforming
growth factor-β superfamily, are produced by mammalian oocytes
(9,10). In addition to its effect on the
regulation of bone development, accumulating data suggest that the
BMP signaling pathway also serves a key role in the process of
ovarian follicle development (11–13).
Blocking the BMP signaling pathway in mouse GCs has previously been
demonstrated to impair female fertility and to induce breast cancer
progression (14–16).
BMP-6 is one of the BMP ligands that are produced by
mammalian oocytes, GCs and other cell types (9–11,17,18).
Researchers have identified various functions of BMP-6 in the
ovaries, such as inducing decreases in FSH receptor, inhibin-α, and
inhibin/activin β subunit mRNA expression in rat GCs (19) and increasing inhibin-α and activin-α
production in bovine GCs (20). The
regulation of activins and FSH receptor is crucial during
folliculogenesis (21,22); therefore, these findings provided a
rationale to investigate the roles of BMP-6 in human oocyte
development. To the best of our knowledge, whether BMP-6 is
important for oocyte quality and associated with pregnancy outcomes
following in vitro fertilization (IVF) had not been
previously assessed. Therefore, to define the reproductive function
of BMP-6 in vivo, the present study was conducted to compare
the gene and protein expression levels of BMP-6 in vivo in
follicular fluid and in GCs between pregnant and non-pregnant
patients following IVF and embryo transfer.
Materials and methods
Study design
The Ethics Committee of Shijiazhuang Obstetrics and
Gynecology Hospital (Shijiazhuang, China) approved the present
methodology and all patients gave their informed consent, prior to
participation. Furthermore, the study protocol followed the
principles of the Declaration of Helsinki. In total, 80 female
patients who were recruited to the Reproductive Medicine Center,
Shijiazhuang Obstetrics and Gynecology Hospital, between September
2012 and July 2014 for the present study and subsequently underwent
intracytoplasmic sperm injection (ICSI). A total of 44 patients
were pregnant (pregnant group; mean age, 28.9±4.7 years), and 36
patients were not pregnant (non-pregnant group; mean age, 29.4±3.8
years) based on pregnancy outcomes, assessed according to serum hCG
levels measured 14 days after embryo transfer. The inclusion
criteria for all patients included: Previous treatment for ovarian
stimulation, age ≤38 years and a basal follicle-stimulating hormone
(bFSH) level <10 IU/l. The exclusion criteria included the
following: The inability to bear pregnancy due to severe diseases;
severe psychiatric disorders; urinary system infection; sexually
transmitted infections; a previous history of drug and/or alcohol
abuse; a teratogenic level of exposure to X-ray radiation;
endometriosis; uterine cavity lesions, including endometrial polyps
and intrauterine adhesions; and other factors, including
endometrial infertility.
Treatment
Both groups underwent long-term
gonadotropin-releasing hormone (GnRH) downregulation. All patients
were administered 0.1 mg/day Triptorelin (Ipsen Pharma Biotech
S.A.S., Z.E. de Signes, France) intramuscularly, which is a GnRH
agonist, prior to the mid-luteal phase of menstruation for ≥14 days
until patients met the study criteria: Endometrium, ≤5 mm by
ultrasonic testing (23,24); serum estradiol (E2),
<50 pg/ml by chemiluminiscence method, as previously described
(25). Patients were then
administered gonadotropins [(Gn) 250–300 IU recombinant human
follitropin alfa solution for injection (Gonal-F; Merck Serono;
Merck KGaA, Darmstadt, Germany)] daily for 9–12 days via abdominal
subcutaneous injection to advance follicle growth. The duration of
Gn was associated with the different ovary responses to Gn for each
individual. During Gonal-F administration, the rate of follicular
development was monitored via ultrasound examination and the levels
of E2, progesterone (P), and luteinizing hormone (LH)
were measured using the chemiluminiscence method to regulate the
dose of Gonal-F to generate a follicular diameter ≥18 mm in both
ovaries. The dosage of gonadotropin (Gn) used was calculated by
multiplying the Gn dosage by the number of Gn days. The use of
Gonal-F and Triptorelin was stopped on the evening of the same day
and 250 µg human chorionic gonadotrophin (hCG; Merck Serono; Merck
KGaA) was administered via intramuscular injection. After 36–38 h,
fertilized eggs were harvested via ultrasound-guided transvaginal
puncture oocyte retrieval surgery (26). Following oocyte retrieval surgery, a
selected oocyte-corona-cumulus complex was placed into a
CO2 incubator at 37°C, cultured for 4–6 h.
On day 3 after fertilization, the cleavage and
embryo quality was observed, embryo quality was classified as
follows: Grade 1, uniform cell size, 0 or <5% debris; grade 2,
the majority of cells exhibited characteristics of the stage of 6–9
cells on day 3 embryonic development, fragments of 10–25% or grade
3, the cells are different, blastomere size uneven, >25%
fragments. Grade 1 and grade 2 embryos were classed as high quality
embryos, as previously described (27).
Preparation of sperm
The corresponding husbands (aged from 22–40 years
old) all provided signed informed consent. On the oocyte retrieval
surgery day, ejaculated spermatozoa were obtained by masturbation
following 3–5 days of ejaculatory abstinence. After liquefaction of
semen at room temperature, sperm samples were prepared by
discontinuous density-gradient centrifugation. For discontinuous
density-gradients, the bottom fraction was aspirated and washed
with Quinn's sperm wash medium (ART-2040, SAGE; BioPharma,
Trumbull, CT, USA) twice at 300 × g for 20 min at room temperature
and incubated at 37°C until use.
Collection of follicular fluid and
GCs
Following oocyte retrieval, the collected follicular
fluid was transferred to a centrifuge tube for separation at 4°C
(125 × g; 5 min), and the supernatant was stored at −80°C until
required. The remaining fluid was discarded, and PBS was added to
bring the volume of the sediment to 5 ml. Subsequently, 5 ml human
lymphocyte separation medium (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) was placed in another
centrifuge tube, into which the PBS-sediment sample was added
slowly via a straw. The sample was then centrifuged at 4°C with 80
× g for 20 min. When a layer of white GCs was observed in the
middle of the tube, granular cell centrifugation at 4°C was
performed at 503 × g for 5 min. The supernatant was subsequently
discarded and the granular cells were flash frozen in liquid
nitrogen and stored at −80°C until required.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from GCs with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. cDNA was synthesized
according to the manufacturers instructions of the M-MLV First
Strand kit (Thermo Fisher Scientific, Inc.). qPCR of mRNA was
performed according to the manufacturer instructions of the
Platinum SYBR-Green qPCR Super Mix UDG kit (Invitrogen; Thermo
Fisher Scientific, Inc.) on an ABI 7300 system (Thermo Fisher
Scientific, Inc.). A GAPDH endogenous control was used for
normalization, and the relative amount of mRNA was calculated using
the 2−ΔΔCq method (28).
The primers used were as follows: BMP-6, forward,
5′-GCAATCTGTGGGTTGTGACT-3′ and reverse, 5′-AAGGGCTGCTTGTCGTAAG-3′;
and GAPDH forward, 5′-TGAACGGGAAGCTCACTGG-3′ and reverse,
5′-GCTTCACCACCTTCTTGATGTC-3′. The PCRs were performed in 20 µl
reaction volumes with 10X PCR buffer, 25 mM MgCl2, 25 mM
dNTPs, 1 µl primers and 0.5 U AmpliTaq Gold (4311816; Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed as
follows: 95°C for 10 min followed by 40 cycles of 95°C for 5 sec
and 60°C for 1 min. All samples were assessed in triplicate and the
value of the cycle threshold (CT) was determined using ABI 7500
software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Western blot analysis
Lysates from follicular fluid and GCs were prepared
with 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% Nonidet P-40, 0.5%
sodium deoxycholic acid, and complete protease inhibitor mixture
tablets (Roche Applied Science, Penzberg, Germany), and protein was
then isolated as previously described (29,30). A
total of 10 ng protein from each sample were separated by 10%
SDS-PAGE and electrotransfered to a PVDF membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% milk for 2 h at
room temperature and incubated overnight at 4°C using the following
primary antibodies: Rabbit anti-rat BMP-6 (1:1,000; cat no.
ab155963; Abcam, Cambridge, UK) and mouse anti-rat β-actin
(1:1,000; cat no. sc-130656; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Membranes were subsequently incubated with
horseradish peroxidase-conjugated secondary antibody (1:10,000, cat
no. 611-1302; Rockland) for 2 h at room temperature and then
evaluated with an ECL (enhanced chemiluminescence) Fusion Fx
(Vilber Lourmat, Marne-la-Vallée, France). Images were captured and
processed using Quantity One software, version 4.62 (Bio-Rad
Laboratories, Inc.). All experiments were replicated three
times.
Immunofluorescence staining
GCs were performed on coverslips fixed at room
temperature for 15 min in 50:50 methanol:acetone. Sections were
deparaffinized with xylene and rehydrated in a graded ethanol
series (from 70, 80, 90 to and 100% absolute ethyl alcohol), the
slides were pre-incubated at room temperature for 30 min with 10%
normal goat serum (cat no. 710027, KPL; SeraCare Life Sciences
Inc., Milford, MA, USA) and then incubated with primary antibodies
BMP-6 (1:50; cat no. ab155963; Abcam) at 4°C overnight. The
secondary antibodies were fluorescein-labeled rabbit IgG antibody
(1:200; cat no. 021516; KPL; SeraCare Life Sciences Inc.) at room
temperature for 30 min. In each experiment, DAPI (cat no. 157574;
MB Biomedicals, LLC., Santa Ana, CA, USA) was used for nuclear
counter staining. Images were acquired using a Leica microscope
(DM6000B) and digitized using LAS version 4.4 software (both from
Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Data analysis was conducted using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). All data are presented as the mean
± standard deviation. Differences between groups were assessed
using Student's t-test or one-way analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
General conditions and treatment
A total of 80 female patients who underwent ICSI
were recruited for the present study. The inclusion criteria for
all patients included a long protocol for ovarian stimulation, age
≤38 years, and a bFSH level <10 IU/l. Age, duration of
infertility, and bFSH levels did not differ significantly between
the pregnant and non-pregnant groups (Table I).
 | Table I.Patient infertility characteristics
according to pregnancy status. |
Table I.
Patient infertility characteristics
according to pregnancy status.
| Group | Patients, n | Age, years | Infertility duration,
years | bFSH, IU/l |
|---|
| Non-pregnant | 36 | 29.4±3.8 | 5.2±3.7 | 4.6±2.7 |
| Pregnant | 44 | 28.9±4.7 | 4.6±2.1 | 5.8±4.0 |
| P-values |
| 0.608 | 0.364 | 0.129 |
The dosage of Gn used, and the serum E2,
LH and P levels on the day of hCG administration were compared, and
no significant differences were observed between the two groups
(Table II).
 | Table II.Patients' controlled ovarian
hyperstimulation parameters according to pregnancy status. |
Table II.
Patients' controlled ovarian
hyperstimulation parameters according to pregnancy status.
| Group | Patients, n | Total dose of Gn,
IU | Serum level on day
of hCG administration |
|---|
|
|
|
| E2,
pg/ml | LH, mIU/ml | P, ng/ml |
|
|
|
|
|
|
|
| Non-pregnant | 36 | 2868±958 | 4757±1745 | 0.81±0.43 | 0.91±0.58 |
| Pregnant | 44 | 3038±832 | 4529±2005 | 1.00±0.72 | 0.89±0.71 |
| P-values |
| 0.389 | 0.594 | 0.168 | 0.892 |
The number of oocytes was also compared, and no
significant differences were observed between groups (Table III); however, the fertilization
rate and the high quality embryo rate were significantly higher in
the pregnant group compared with the non-pregnant group (P<0.01;
Table III).
 | Table III.Patient indexes of clinical treatment
according to pregnancy status. |
Table III.
Patient indexes of clinical treatment
according to pregnancy status.
| Group | Patients, n | Oocytes, n | Fertilization rate,
% (fertilized/unfertilized) | Rate of good
quality embryos (%) |
|---|
| Non-pregnant | 36 | 14.6±6.2 | 58.6 (269/461) | 34.4 (106/308) |
| Pregnant | 44 | 15.3±4.8 | 82.1
(345/420)a | 55.3
(166/300)(166/300)a |
| P-values |
| 0.571 |
<0.0001a | 0.0002a |
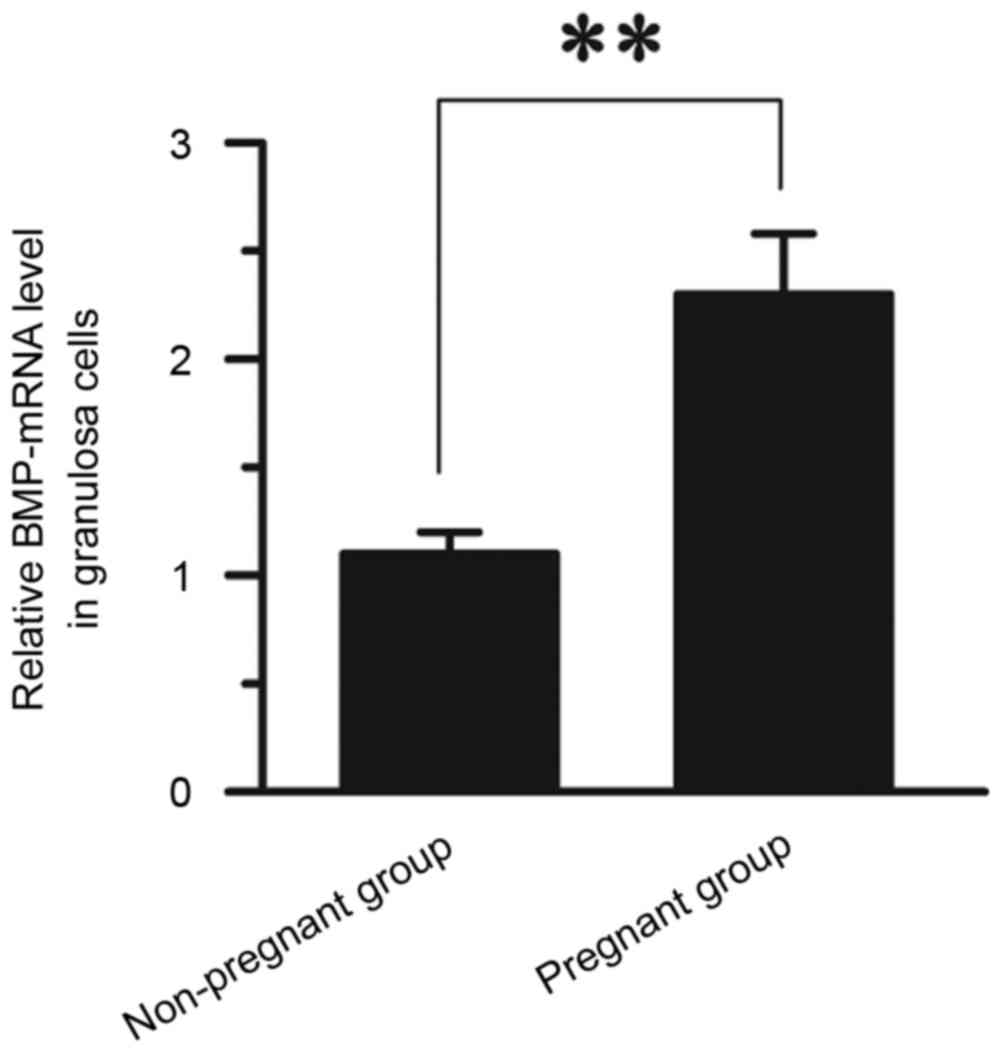
Comparison of BMP-6 mRNA expression in
GCs between pregnant and non-pregnant patients
RT-qPCR was used to compare the mRNA levels of BMP-6
in GCs between pregnant and non-pregnant patients. The expression
of BMP-6 mRNA in the pregnant group (2.30±0.28) was significantly
higher than that observed in the non-pregnant group (1.10±0.10;
P<0.01; Fig. 1).
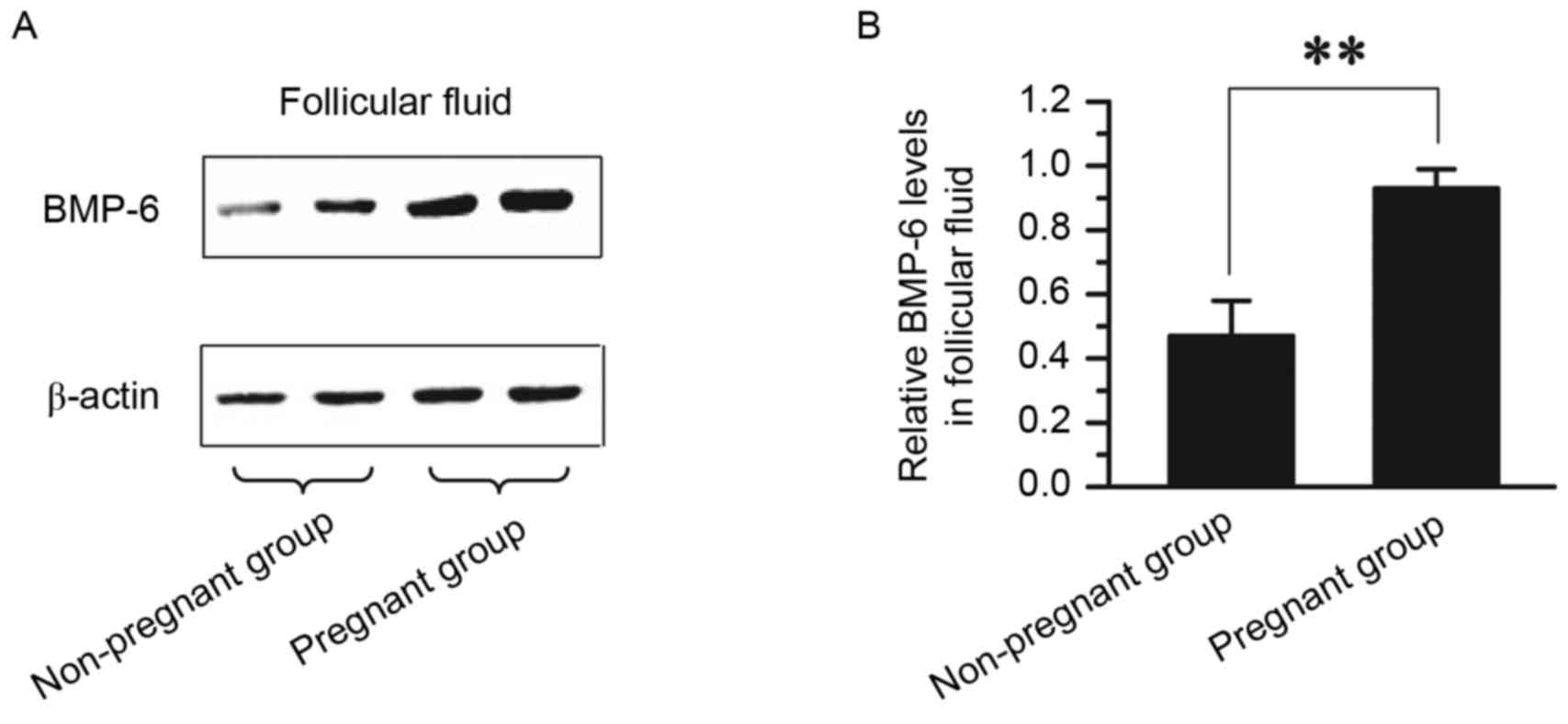
Comparison of BMP-6 protein expression
in follicular fluid between pregnant and non-pregnant patients
The level of BMP-6 protein in follicular fluid
between pregnant and non-pregnant patients was compared. Follicular
fluid was collected from 36 patients in the non-pregnant group and
44 patients in the pregnant group. The protein expression level of
BMP-6, as determined by western blot, are presented in Fig. 2. The protein expression level of
BMP-6 was significantly higher in follicular fluid from the
pregnant group (0.93±0.06) than in follicular fluid from the
non-pregnant group (0.47±0.11; P<0.01; Fig. 1).
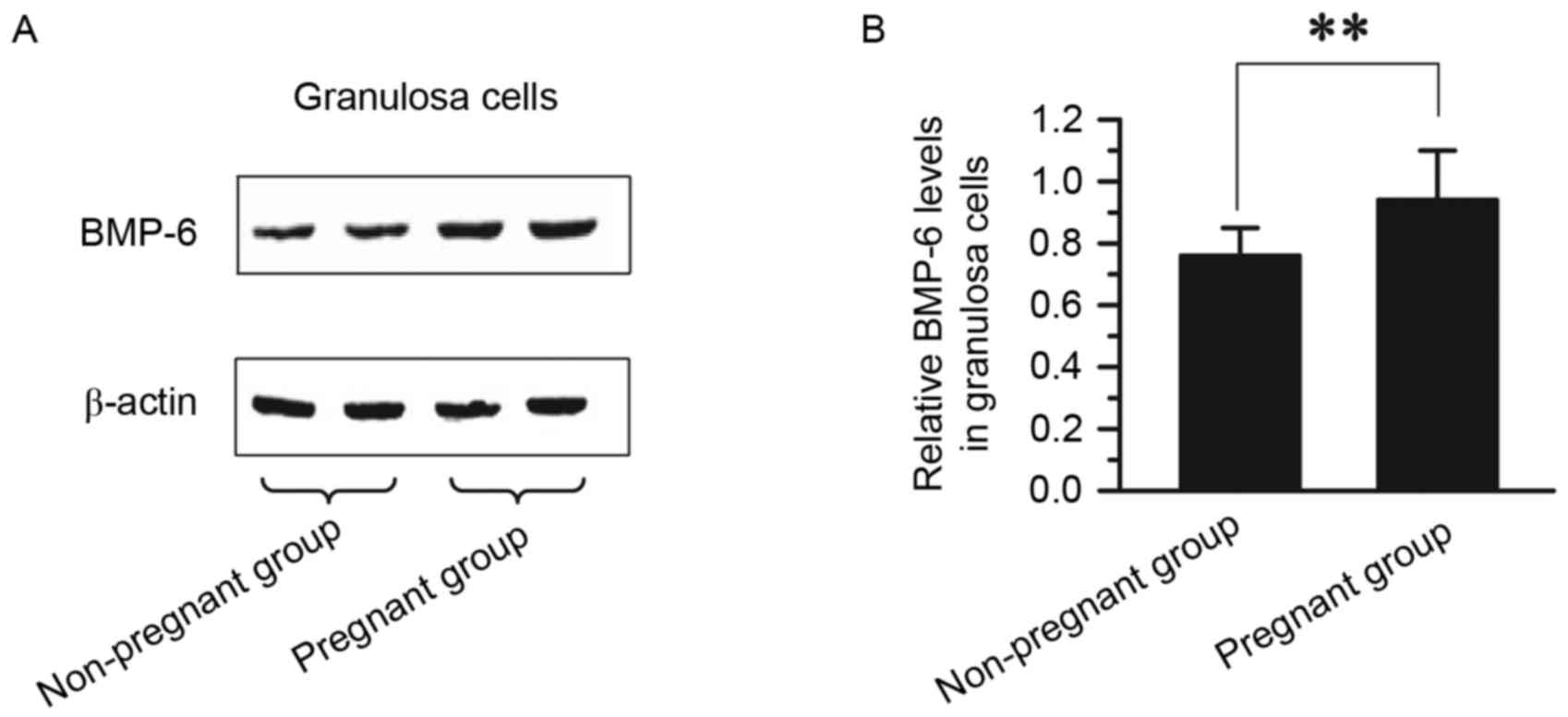
Comparison of BMP-6 protein expression
in GCs between pregnant and non-pregnant patients
The expression of BMP-6 protein in GCs was compared
between pregnant and non-pregnant patients. GCs were collected from
36 patients in the non-pregnant group and 44 patients in the
pregnant group. The protein expression of BMP-6, as determined via
western blotting, is presented in Fig.
3. The protein expression level of BMP-6 was significantly
higher in GCs from the pregnant group (0.94±0.16) than in GCs from
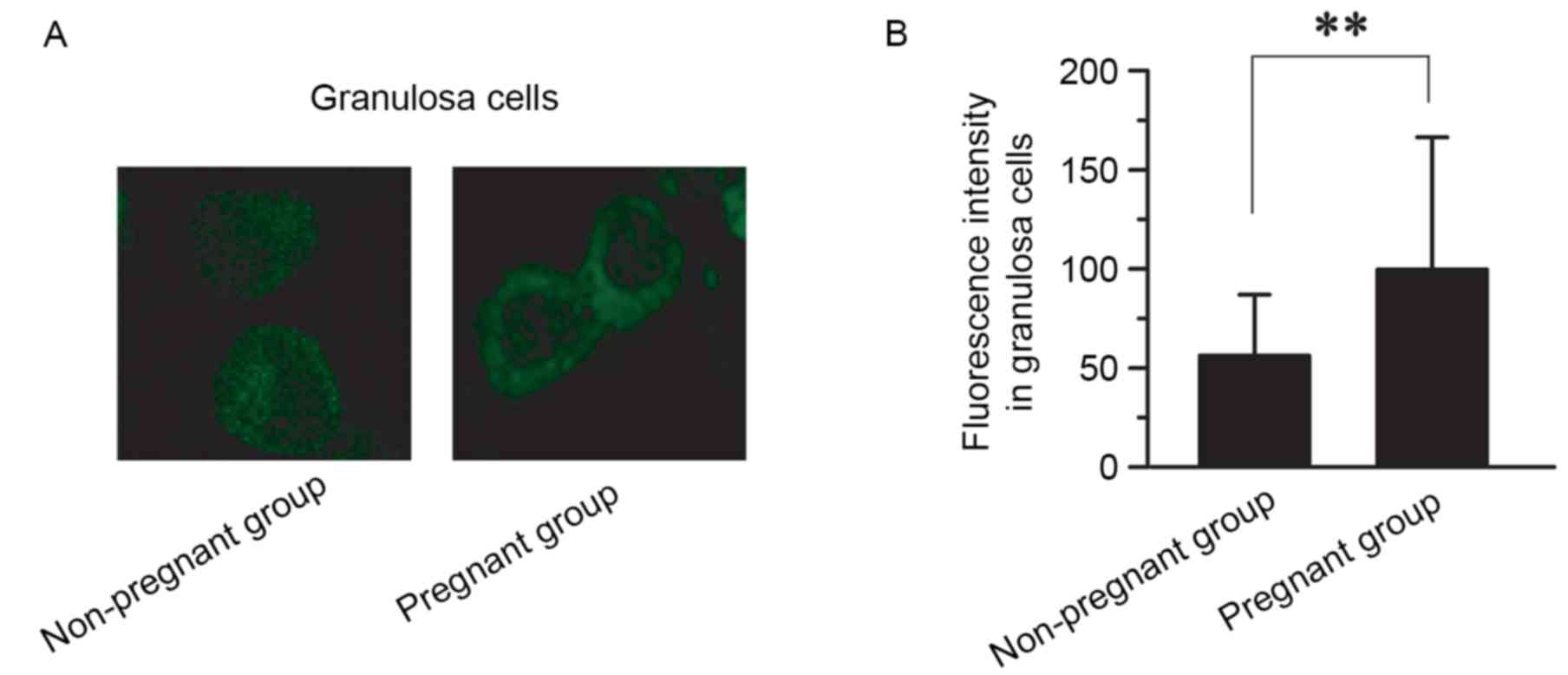
the non-pregnant group (0.76±0.01; P<0.01; Fig. 3). The expression and distribution of
BMP-6 protein in GCs between the pregnant and non-pregnant groups
was also compared using immunofluorescence staining (Fig. 4). The level of BMP-6 was
significantly higher in the pregnant group (99.6±66.8) than in the
non-pregnant group (56.2±30.8; P<0.01; n=3; Fig. 4).
Discussion
In the present study, intrafollicular
granulosa-lutein cell BMP-6 mRNA and protein expression was
detected in patients undergoing IVF-embryo transfer treatment,
which indicated that BMP-6 may serve a role in folliculogenesis
during ovarian hyperstimulation. Based on the BMP-6 expression
results obtained for follicular fluid and granular cells, it was
demonstrated that BMP-6 expression was significantly higher in the
pregnant group compared with the non-pregnant. However, no
additional statistically significant correlations were identified
between BMP-6 expression and the number of oocytes, the number of
transferred embryos, the fertilization rate or the clinical
pregnancy rate. The obtained results suggest that BMP-6 expression
in the human ovary may serve a role in oocyte maturation.
Appropriate expression of BMP-6 may be associated with good oocyte
quality in IVF cycles.
Folliculogenesis is the process by which primordial
follicles grow and develop to the ovulatory follicle stage.
Previous studies have investigated BMP-6, BMP-15 and GDF-9
expression during folliculogenesis. Species differ widely in the
initiation of follicle growth. Mice with null mutations in the
BMP-6 and BMP-15 genes undergo normal follicle development and have
normal fertility (31). Conversely,
mutations in the BMP-15 gene are associated with infertility in
sheep and premature ovarian failure in humans (32,33).
Other studies have demonstrated that BMP-6 is associated with
follicle growth in the pre-antral and antral stages (34,35). In
addition, some studies have demonstrated that BMP-15 and GDF-9 are
able to stimulate granulosa cell mitosis in pre-antral follicles
(12,31,36).
During selection of the dominant follicle, the growth of small
pre-antral follicles is impaired by restricting androgen output
from theca cells. Granulosa-derived activin and BMP-6 and
oocyte-derived GDF-9, BMP-15 and BMP-6 are associated with this
process. These factors may attenuate the output of LH-dependent
androgen by theca cells in small pre-antral follicles (13).
In addition, BMP-6 protein was strongly expressed in
the GCs of healthy tertiary follicles and weakly expressed in the
GCs of atretic follicles. BMP-6 increased the expression of
anti-mullerian hormone, preserving the ovarian reserve. This
research indicated that BMP-6 serves an important role during this
period (11).
In addition to promoting ovulation and inhibiting
premature luteinization, BMP-6, BMP-15 and GDF-9 also inhibit
premature luteinization and limit progesterone biosynthesis by
suppressing progesterone synthesis (37). Following ovulation, these
oocyte-derived luteinization inhibitors are lost. In addition,
BMP-6 may increase the accumulation of neutrophils in the ovulatory
follicle and suppress the effect of protease inhibitors (35). Accordingly, it is essential to
investigate the molecular aspects of folliculogenesis in future
clinical applications. The findings of the present study suggested
that BMP-6 may be associated with fertilization and embryo quality
in humans. Therefore, BMP-6 may become a useful marker in IVF
procedures; however, further research must be done on this
topic.
Taken together, these findings suggest important
paracrine and autocrine roles for BMP-6 in the regulation of
ovarian functions. To the best of our knowledge, the present study
provides the first evidence of an autocrine role for BMP-6 in the
regulation of oocyte quality.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81173294).
Glossary
Abbreviations
Abbreviations:
|
GCs
|
granulosa cells
|
|
BMP-6
|
bone morphogenetic protein-6
|
References
|
1
|
Buccione R, Vanderhyden BC, Caron PJ and
Eppig JJ: FSH-induced expansion of the mouse cumulus oophorus in
vitro is dependent upon a specific factor(s) secreted by the
oocyte. Dev Biol. 138:16–25. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eppig JJ, Wigglesworth K, Pendola F and
Hirao Y: Murine oocytes suppress expression of luteinizing hormone
receptor messenger ribonucleic acid by granulosa cells. Biol
Reprod. 56:976–984. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilchrist RB, Ritter LJ and Armstrong DT:
Mouse oocyte mitogenic activity is developmentally coordinated
throughout folliculogenesis and meiotic maturation. Dev Biol.
240:289–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salustri A, Yanagishita M and Hascall VC:
Mouse oocytes regulate hyaluronic acid synthesis and mucification
by FSH-stimulated cumulus cells. Dev Biol. 138:26–32. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanderhyden BC, Telfer EE and Eppig JJ:
Mouse oocytes promote proliferation of granulosa cells from
preantral and antral follicles in vitro. Biol Reprod. 46:1196–1204.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li R, Norman RJ, Armstrong DT and
Gilchrist RB: Oocyte-secreted factor(s) determine functional
differences between bovine mural granulosa cells and cumulus cells.
Biol Reprod. 63:839–845. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eppig JJ, Wigglesworth K and Pendola FL:
The mammalian oocyte orchestrates the rate of ovarian follicular
development. Proc Natl Acad Sci USA. 99:pp. 2890–2894. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McNatty KP, Moore LG, Hudson NL, Quirke
LD, Lawrence SB, Reader K, Hanrahan JP, Smith P, Groome NP,
Laitinen M, et al: The oocyte and its role in regulating ovulation
rate: A new paradigm in reproductive biology. Reproduction.
128:379–386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elvin JA, Yan C and Matzuk MM:
Oocyte-expressed TGF-beta superfamily members in female fertility.
Mol Cell Endocrinol. 159:1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paradis F, Novak S, Murdoch GK, Dyck MK,
Dixon WT and Foxcroft GR: Temporal regulation of BMP2, BMP6, BMP15,
GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the
oocyte, granulosa and theca cells of developing preovulatory
follicles in the pig. Reproduction. 138:115–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi J, Yoshino O, Osuga Y, Koga K, Hirota
Y, Hirata T, Yano T, Nishii O and Taketani Y: Bone morphogenetic
protein-6 stimulates gene expression of follicle-stimulating
hormone receptor, inhibin/activin beta subunits, and anti-Mullerian
hormone in human granulosa cells. Fertil Steril. 92:1794–1798.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimasaki S, Moore RK, Otsuka F and
Erickson GF: The bone morphogenetic protein system in mammalian
reproduction. Endocr Rev. 25:72–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knight PG and Glister C: TGF-beta
superfamily members and ovarian follicle development. Reproduction.
132:191–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK
and Lyons KM: The type I BMP receptor BmprIB is essential for
female reproductive function. Proc Natl Acad Sci USA. 98:pp.
7994–7999. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edson MA, Nalam RL, Clementi C, Franco HL,
Demayo FJ, Lyons KM, Pangas SA and Matzuk MM: Granulosa
cell-expressed BMPR1A and BMPR1B have unique functions in
regulating fertility but act redundantly to suppress ovarian tumor
development. Mol Endocrinol. 24:1251–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Yang S, An D, Hu F, Yuan W, Zhai C
and Zhu T: BMP-6 inhibits microRNA-21 expression in breast cancer
through repressing deltaEF1 and AP-1. Cell Res. 19:487–496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solloway MJ, Dudley AT, Bikoff EK, Lyons
KM, Hogan BL and Robertson EJ: Mice lacking Bmp6 function. Dev
Genet. 22:321–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lyons KM, Pelton RW and Hogan BL: Patterns
of expression of murine Vgr-1 and BMP-2a RNA suggest that
transforming growth factor-beta-like genes coordinately regulate
aspects of embryonic development. Genes Dev. 3:1657–1668. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Otsuka F, Moore RK and Shimasaki S:
Biological function and cellular mechanism of bone morphogenetic
protein-6 in the ovary. J Biol Chem. 276:32889–32895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glister C, Kemp CF and Knight PG: Bone
morphogenetic protein (BMP) ligands and receptors in bovine ovarian
follicle cells: Actions of BMP-4, −6 and −7 on granulosa cells and
differential modulation of Smad-1 phosphorylation by follistatin.
Reproduction. 127:239–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamoto M, Minami S, Nakano R and Kobayashi
M: Immunohistochemical localization of inhibin/activin subunits in
human ovarian follicles during the menstrual cycle. J Clin
Endocrinol Metab. 74:989–993. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minegishi T, Tano M, Igarashi M, Rokukawa
S, Abe Y, Ibuki Y and Miyamoto K: Expression of
follicle-stimulating hormone receptor in human ovary. Eur J Clin
Invest. 27:469–474. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ata B and Tulandi T: Ultrasound automated
volume calculation in reproduction and in pregnancy. Fertil Steril.
95:2163–2170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corbacioğlu A and Baysal B: Effects of
endometrial thickness and echogenic pattern on assisted
reproductive treatment outcome. Clin Exp Obstet Gynecol.
36:145–147. 2009.PubMed/NCBI
|
|
25
|
Zhang F, Liu XL, Rong N and Huang XW:
Clinical value of serum anti-mullerian hormone and inhibin B in
prediction of ovarian response in patients with polycystic ovary
syndrome. J Huazhong Univ Sci Technolog Med Sci. 37:70–73.
2017.PubMed/NCBI
|
|
26
|
Wu YT, Wang TT, Chen XJ, Zhu XM, Dong MY,
Sheng JZ, Xu CM and Huang HF: Bone morphogenetic protein-15 in
follicle fluid combined with age may differentiate between
successful and unsuccessful poor ovarian responders. Reprod Biol
Endocrinol. 10:1162012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alpha Scientists in Reproductive Medicine
and ESHRE Special Interest Group of Embryology: The Istanbul
consensus workshop on embryo assessment: Proceedings of an expert
meeting. Hum Reprod. 26:1270–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM,
Luo Q and Huang HF: High bone morphogenetic protein-15 level in
follicular fluid is associated with high quality oocyte and
subsequent embryonic development. Hum Reprod. 22:1526–1531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Otsuka F, Yao Z, Lee T, Yamamoto S,
Erickson GF and Shimasaki S: Bone morphogenetic protein-15.
Identification of target cells and biological functions. J Biol
Chem. 275:39523–39528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin
JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, et al:
Synergistic roles of bone morphogenetic protein 15 and growth
differentiation factor 9 in ovarian function. Mol Endocrinol.
15:854–866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galloway SM, McNatty KP, Cambridge LM,
Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds
KG, Montgomery GW, et al: Mutations in an oocyte-derived growth
factor gene (BMP15) cause increased ovulation rate and infertility
in a dosage-sensitive manner. Nat Genet. 25:279–283. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Pasquale E, Beck-Peccoz P and Persani
L: Hypergonadotropic ovarian failure associated with an inherited
mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J
Hum Genet. 75:106–111. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Araújo VR, Silva GM, Duarte AB,
Magalhães-Padilha DM, Almeida AP, Lunardi FO, Serafim MK, Moura AA,
Campello CC, Rodrigues AP and Figueiredo JR: Bone morphogenetic
protein-6 (BMP-6) stimulates the antrum formation by the regulation
of its signalling pathway in caprine pre-antral follicles cultured
in vitro. Reprod Domest Anim. 51:59–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akiyama I, Yoshino O, Osuga Y, Shi J,
Takamura M, Harada M, Koga K, Hirota Y, Hirata T, Fujii T, et al:
The role of bone morphogenetic protein 6 in accumulation and
regulation of neutrophils in the human ovary. Reprod Sci.
21:772–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paulini F and Melo EO: The role of
oocyte-secreted factors GDF9 and BMP15 in follicular development
and oogenesis. Reprod Domest Anim. 46:354–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otsuka F, Moore RK, Iemura S, Ueno N and
Shimasaki S: Follistatin inhibits the function of the
oocyte-derived factor BMP-15. Biochem Biophys Res Commun.
289:961–966. 2001. View Article : Google Scholar : PubMed/NCBI
|