Introduction
Advanced glycation end products (AGEs) (formed by
non-enzymatic modification of proteins, lipids and nucleic acids by
glucose), accumulated by sustained hyperglycemia, are responsible
for diabetic vascular complications (1). AGEs evoke inflammatory
response-associated macrophage migration, filtration and
inflammatory cytokine release (2,3).
Although AGEs and inflammation are critical for diabetic vascular
complications, knowledge regarding the relationship between AGEs
and inflammatory response involving macrophage migration,
filtration and inflammatory cytokine release is still unclear.
Heparanase (HPA), a unique and specific functional
endo-β-D-glucuronidase, cleaves heparan sulfate (HS) chains for
remodeling of extracellular matrix and regulation of the release of
many HS-linked molecules such as growth factors, and cytokines
(4,5). Previous findings showed that deletion
of the C-domain of HPA generated enzymatically inactive HPA
(6). Thus, the C-terminal domain may
be essential for HPA enzymatic activity. On the other hand,
syndecans are a family of HS-decorated cell-surface proteoglycans
degraded by HPA (7). Syndecan-1
(Syn-1), a member of the syndecan family, which comprises HP
proteoglycans (HSPGs), displayed the capacity to modulate cell
migration and inflammatory responses by binding chemokines and
cytokines (8,9). A study has shown that HPA has the
ability to regulate Syn-1 expression (10).
Based on the above studies, we speculated that HPA
may mediate AGEs-induced inflammatory response via Syn-1 protein
expression. Therefore, we examined the relation between C-domain of
HPA and Syn-1 in AGE-induced macrophage migration and inflammation.
Inflammatory response mediators including, tumor necrosis factor-α
(TNF-α) and interleukin-1β (IL-1β), were studied to evaluate the
role of C-domain of HPA between AGEs and inflammatory response in
macrophages.
Materials and methods
Cell culture
Macrophage cells (the Cell Bank of the Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai, China) were cultured in RPMI-1640 medium containing 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and
100 µg/ml streptomycin. Cells were cultured at 37°C in a humidified
incubator, in which the concentration of CO2 was 5%, and
were used in the exponential growth phase.
Cell viability analysis by 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Macrophages were plated at 5×104 cells/ml
and incubated with or without AGEs (Shanghai Yixin Biotechnology
Co., Ltd., Shanghai, China), and antibody against C-domain of HPA
(Wuhan Boster Bio-Engineering Co., Ltd., Wuhan, China) or anti-HPA
plus Syn-1 antibody (R&D Systems, Inc., Minneapolis, MN, USA)
was applied for various periods of time (4–24 h). After incubation,
MTT solution was added to each well for 4 h. DMSO was added to
dissolve the formazan crystals formed. Finally, the plates were
read by a microplate reader after the blue salt in each well was
dissolved.
Cell treatments
The cells were treated with AGEs (0, 25, 50 and 100
mg/l), and anti-HPA or anti-HPA plus Syn-1 antibody for 24 h,
respectively, and were analyzed by migration assay. RT-PCR and
enzyme-linked immunosorbent assay (ELISA) were performed to
evaluate the role of C-domain of HPA and Syn-1 in AGE-induced
macrophage migration and release of inflammatory cytokine IL-1β or
TNF-α. Subsequently, the cells were treated with 100 mg/l AGEs, and
anti-HPA antibody for 24 h. This was followed by analyses with the
help of heparan degrading enzyme assay in order to examine the
effects of C-domain of HPA on HPA enzyme activity in AGE-induced
macrophages. Finally, the cells were treated with 100 mg/l AGEs,
and anti-HPA antibody for 24 h, and harvested for western blot
analysis to assess of the role of the C-domain of HPA in
AGE-induced Syn-1 expression.
Migration assay
Macrophages were seeded onto the upper chamber of
6-well Transwell plates (Becton Dickinson, Franklin Lakes, NJ, USA)
with 8 µm pores. Medium containing 2% FBS was used in the lower
chamber. After 6 h, the cells were removed from the upper surface
of the filter with cotton swabs. Macrophages migrated to the
Transwell were fixed with 4% paraformaldehyde. Migrated macrophages
were then stained with hematoxylin and subsequently counted (6
random fields/slide).
ELISA for TNF-α, and IL-1β
The levels of TNF-α and IL-1β in culture
supernatants were determined using ELISA kits (Wuhan Boster
Bio-Engineering Co., Ltd.) according to the manufacturer's
instructions. The levels of TNF-α and IL-1β were quantified using a
standard curve established by a serial dilution of standard
concentration.
Detection of mRNAs by RT-qPCR
Total RNA from macrophages was isolated with TRIzol
reagent. Isolated RNA was reversed transcribed into complementary
DNA by RevertAid First Strand cDNA Synthesis kit (Fermentas,
Vilnius, Lithuania). RT-qPCR was used for First Strand DNA and to
further amplify specific cDNA fragments over 35 cycles using RNA
PCR kit (Takara Biotechnology Co., Ltd., Dalian, China). The amount
of TNF-α and IL-1β was determined and normalized by the amount of
GAPDH cDNA.
Western blot analysis
Total cell lysates were obtained by dissociation
solution containing protease inhibitors and PMSF. Purified proteins
were subjected to SDS-PAGE, transferred onto a nitrocellulose
membrane and probed with anti-Syn-1 antibody (polyclonal goat IgG,
1:1,000 dilution and cat. no. AF3190). A second
peroxidase-conjugated Ab (polyclonal rabbit IgG, 1:10,000 dilution
and cat. no. BA1060) was used and the activity was detected using
enhanced chemiluminesence detection system. Anti-GAPDH antibody
(polyclonal goat IgG, 1:1,000 dilution and cat. no. sc-48167) was
used as a loading control.
Heparan degrading enzyme assay for HPA
activity
The medium was collected from each culture plate.
HPA activity was assessed using a heparan degrading enzyme assay
kit (Otsu, Shiga, Japan). A standard curve was established using
the standards; plot enzyme activity on the x-axis and the
corresponding absorbance on the y-axis. The enzymatic activity was
then calculated corresponding to each sample's measured
absorbance.
Statistical analysis
Data were expressed as mean ± SEM. Statistical
analysis was performed by SPSS statistical software (SPSS, Inc.,
Chicago, IL, USA) using one-way analysis of variance (ANOVA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell viability
Cell viability was decreased significantly following
exposure to AGE concentrations of 200 µM and higher for 24 h. No
significant difference of the cell viability was observed in 25–100
mg/l AGE-treated macrophage following 4–24 h treatments (Fig. 1A). Therefore, we chose the
concentration of AGEs ≤100 mg/l, as these concentrations did not
change the cell viability significantly in the 4–24 h
incubation.
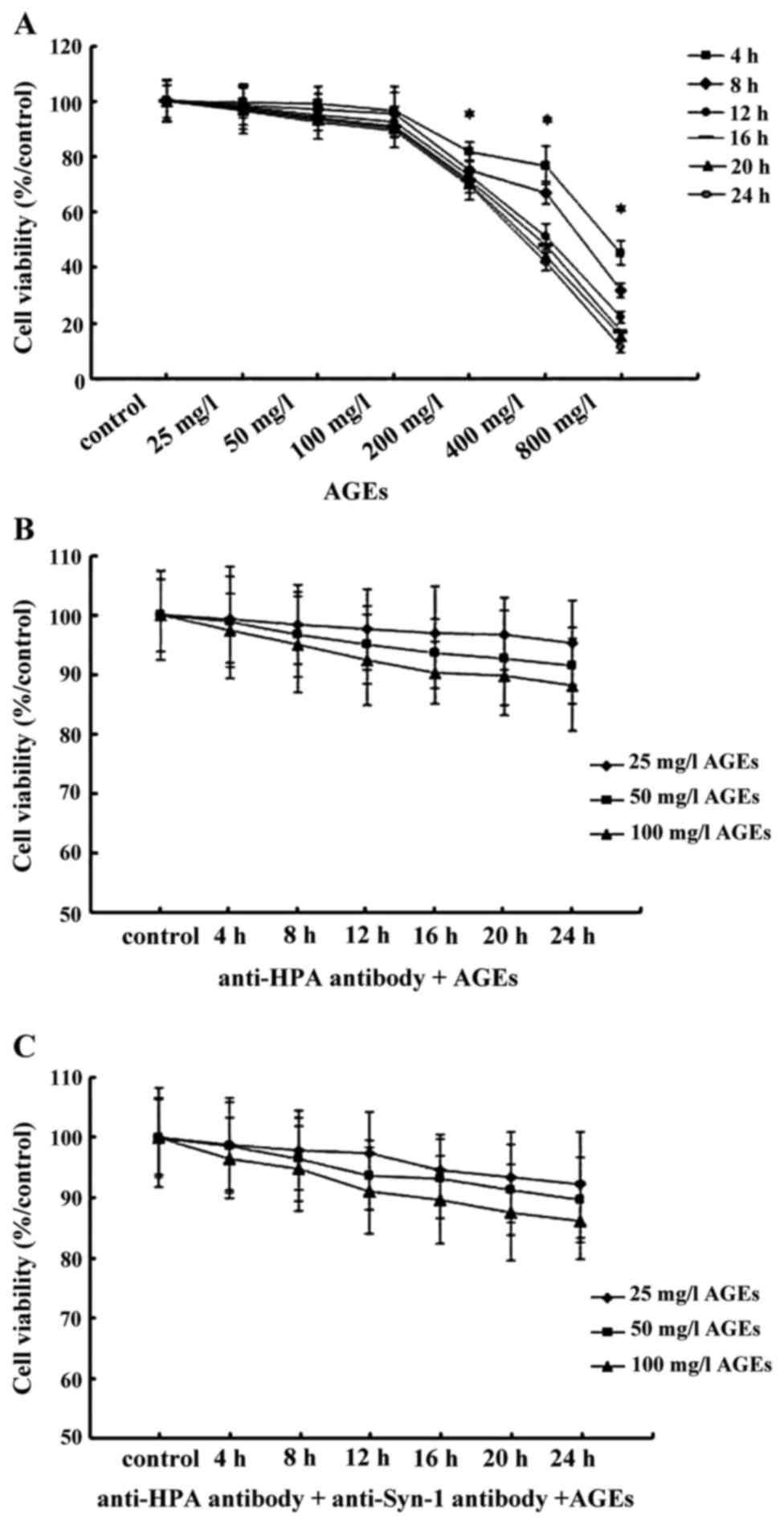 | Figure 1.The viability of macrophages treated
with AGEs, anti-HPA and Syn-1 antibody. The viability of
macrophages was measured using MTT. (A) Macrophages were treated
with AGEs (0, 25, 50, 100, 200, 400 and 800 mg/l) for 4, 8, 12, 16,
20 and 24 h. (B) Macrophages were pretreated with anti-HPA before
culture with 25, 50 and 100 mg/l AGEs for 4, 8, 12, 16, 20 and 24
h. (C) Macrophages were pretreated with anti-HPA plus anti-Syn-1
antibody before culture with 25, 50 and 100 mg/l AGEs for 4, 8, 12,
16, 20 and 24 h. The results are the mean of 6 culture wells (mean
± SEM). *P<0.05, as compared to the control group. All of the
experiments were performed independently in triplicate. AGEs,
advanced glycation end products; HPA, heparanase; Syn-1,
syndecan-1. |
Subsequently, macrophages were pre-treated with 10
µg/ml antibody that recognized the C-domain of HPA, and 10 µg/ml
anti-Syn-1 antibody, before culture with 25–100 mg/l AGEs for 4 to
24 h except control group. No significant change in macrophage
viability was observed in the studied concentrations for 24 h
(Fig. 1B and C). Therefore, 25–100
mg/l AGE incubation with macrophages for 24 h after pretreatment
with 10 µg/ml anti-HPA antibody, and 10 µg/ml anti-Syn-1 antibody
was selected for further experiments.
C-domain of HPA mediates AGE-induced
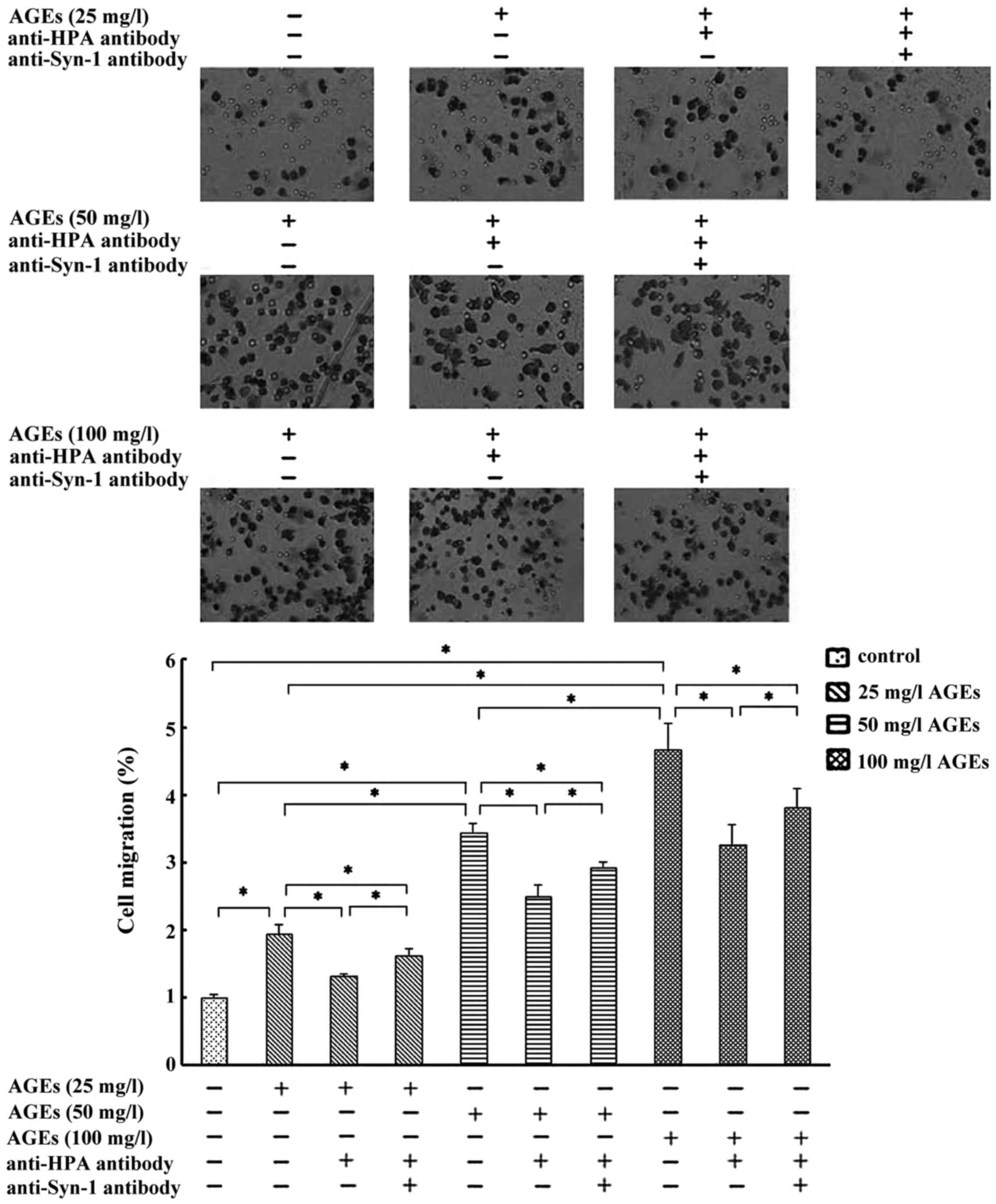
macrophage migration via Syn-1
AGEs (25–100 mg/l) induced a non-linear
concentration-dependent significant increase for 24 h in macrophage
migration. Pretreatment with the antibody recognized the C-domain
of HPA, thereby decreasing the AGE-induced macrophages migration
significantly (Fig. 2). These
results indicated that C-domain of HPA mediates AGEs-induced
macrophage migration. Compared with anti-HPA antibody pretreatment,
the co-pretreatment with anti-HPA antibody and anti-Syn-1 antibody
markedly promoted cell migration in 25–100 mg/l AGE-induced
macrophage but not to the levels close to the control (Fig. 2). These results suggested that
AGE-induced macrophage migration may be mediated by C-domain of HPA
via Syn-1.
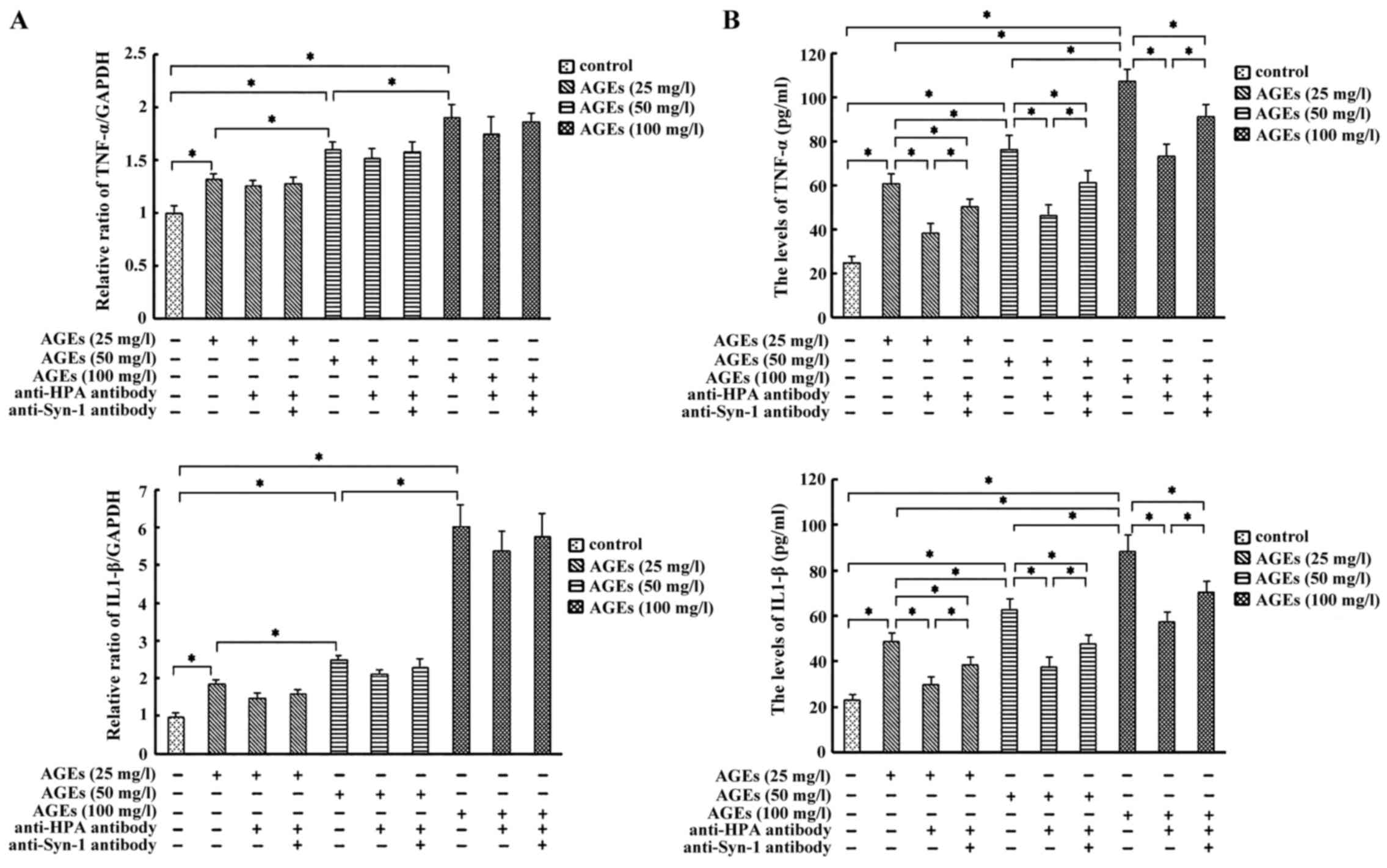
C-domain of HPA mediates the release
of TNF-α and IL-1β via Syn-1 in AGE-induced macrophages
We observed that treatment of Ana-1 macrophages with
AGEs promoted a non-linear concentration-dependent increase in the
secretion of IL-1β and TNF-α significantly. Moreover, pretreatment
with anti-HPA antibody significantly inhibited the secretion of
IL-1β and TNF-α, whereas the inhibition was markedly attenuated by
co-pretreatment with anti-HPA and Syn-1 antibody, while
pretreatment of anti-HPA or anti-HPA plus Syn-1 antibody did not
significantly affect the mRNA levels of IL-1β and TNF-α in the
identical concentration of AGE-induced macrophages, respectively
(Fig. 3A and B). These results
suggested that C-domain of HPA mediated the release of inflammation
cytokine IL-1β and TNF-α in AGE-induced macrophage via Syn-1
independently of IL-1β and TNF-α mRNA expression.
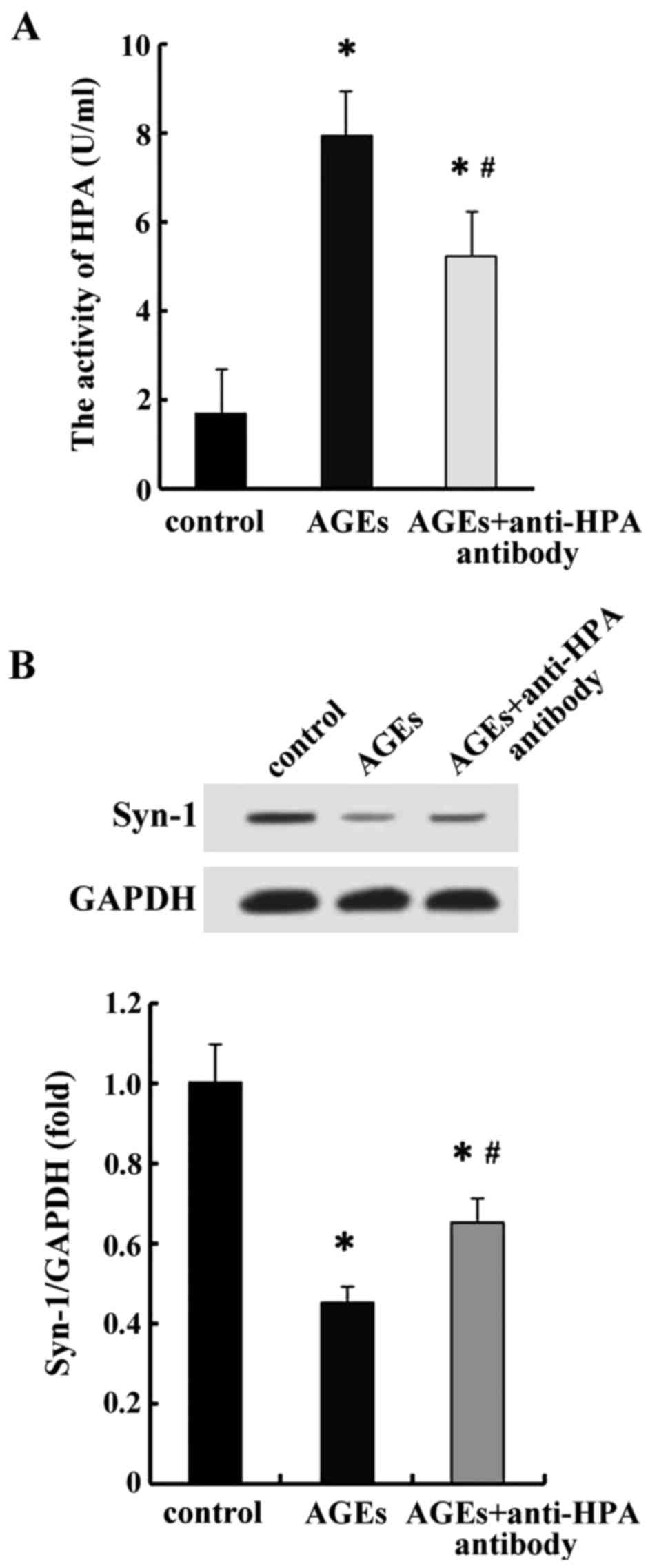
AGE stimulation increased HPA enzyme
activity and decreased Syn-1 protein C-domain
dependence-domain
Data from earlier studies confirmed that HPA enzyme
activity and Syn-1 were crucial for inflammation. HPA enzymatic
activity was observed to cause degradation of Syn-1 in order to
release Syn-1 binding cytokines. To address the effect of C-domain
of HPA on HPA enzyme activity and Syn-1 protein, we selected the
maximum concentration of AGEs (100 mg/l) that induced the maximum
cell migration and secretion of IL-1β and TNF-α. We observed that
AGEs promoted HPA activity and inhibited the protein expression of
Syn-1 significantly in macrophages. Moreover, pretreatment with the
antibody that recognized C-domain of HPA significantly inhibited
HPA activity and elevated the protein expression of Syn-1 in
AGE-induced macrophages (Fig. 4).
These results indicated that AGEs promoted HPA enzyme activity and
inhibited Syn-1 protein expression that in turn was observed to be
mediated by C-domain of HPA. Thus, C-domain of HPA could regulate
inflammatory response via HPA enzyme activity associated with Syn-1
protein.
Discussion
AGEs and inflammation induced by macrophage have
emerged as the important mechanistic components responsible for
diabetic vascular complications (1).
Previous studies have shown that AGEs evoke inflammation (11). However, the key molecule responsible
for these complications remains unclear. The present study revealed
that C-domain of HPA is crucial for AGE-induced macrophage
inflammatory response and migration via Syn-1 expression.
Our initial experiment was aimed to select the
appropriate AGE concentration and time for further experimentation
by MTT assay. Exposure of macrophage cultures to increasing
concentrations of AGEs for 4, 8, 12, 16, 20 and 24 h resulted in
dose- and time-dependent inhibition, but nonlinear dose and time
response to cell viability. Marked inhibition was observed
following treatments with AGE concentrations of 200 mg/l or higher
for 4, 8, 12, 16, 20 and 24 h. The results did not reveal any
differences in the range of 25–100 mg/l for 24-h incubation period.
Thus, we chose the concentration of AGEs of not more than 100 mg/l
for 24 h culture for further experimentation in the present study.
Subsequently, pretreatment with 10 µg/ml anti-HPA antibody or 10
µg/ml anti-HPA plus 10 µg/ml anti-Syn-1 antibody revealed a
decreased tendency but not marked difference in the viability of
macrophages incubated at 25, 50 and 100 mg/l AGEs, respectively. We
utilized preincubation of 10 µg/ml anti-HPA antibody, and 10 µg/ml
anti-Syn-1 plus 10 µg/ml anti-HPA antibody along with tested AGE
concentration range for 24 h, in further experiments on
macrophages.
HPA is the unique and specific functional
endoglycosidase. Extracellular HPA exerted its enzymatic activity
to cleave HS chains from HSPGs. It resulted in remodeling of the
extracellular matrix to enhance cell migration and release many
HS-linked molecules such as cytokines involved in inflammation
(12). Recent data demonstrated that
the COOH-terminal domain (C-domain) of HPA was critical for HPA
enzymatic activity (6).
We studied the role of C-domain of HPA in
AGE-induced macrophage migration and release of inflammatory
cytokines. These results confirmed that the C-domain of HPA
mediated the AGE-induced macrophage inflammatory response. Recent
data from other studies also supported the fact that Syn-1, a
member of HS proteoglycan, regulates various inflammatory processes
involving cell migration and binding cytokines (8,13,14). In
the lung, Syn-1 attenuated allergic lung inflammation by
suppressing Th2 cell recruitment (15). In acute myocardial infarction and
remodeling, Syn-1 protected against exaggerated inflammation and
adverse infarct healing, thereby reducing cardiac dilatation and
dysfunction (16). Furthermore, in
endothelial cells, loss of Syn-1 resulted in a pro-inflammatory
phenotype (17). In the present
study, the treatment of similar AGE-induced macrophages, with
anti-HPA or anti-HPA plus anti-Syn-1 antibody, did not
significantly affect the mRNA expression of TNF-α and IL-1β. This
result revealed that C-domain of HPA and Syn-1 may regulate the
release of TNF-α and IL-1β through post-transcription
Moreover, in the present study, AGEs led to a
significant increase in extracellular HPA activity and this
response is diminished by the pretreatment of antibody recognized
C-domain of HPA. The above observation clearly defined the role of
C-domain in AGE-induced HPA enzymatic activity in macrophage. On
the basis of HPA enzyme activity associated with C-domain of HPA
and Syn-1 protein expression (HPA degrades HSPG include Syn-1), our
results further supported that AGEs could decrease Syn-1 protein
expression via AGE-induced HPA activity associated with C-domain of
HPA.
In conclusion, the present findings show that
C-domain of HPA mediates AGE-induced inflammation-associated
macrophages involving HPA enzymatic activity and Syn-1 protein
expression. The study provided novel insights into the role of
C-domain of HPA in AGE-induced macrophage inflammatory response
associated with vascular complication of diabetes.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Shanghai Municipality (13ZR1432600), Key
Disease Project of Integrated Traditional and Western Medicine of
Shanghai Municipality (zxbz2012-19), Key Basic Research Project of
the Science and Technology Commission of Shanghai Municipality
(2010-10JC1413000), and the National Natural Science Foundation of
China (2009-30871175).
References
|
1
|
Stinghen AE, Massy ZA, Vlassara H, Striker
GE and Boullier A: Uremic toxicity of advanced glycation end
products in CKD. J Am Soc Nephrol. 27:354–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodgson K, Morris J, Bridson T, Govan B,
Rush C and Ketheesan N: Immunological mechanisms contributing to
the double burden of diabetes and intracellular bacterial
infections. Immunology. 144:171–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen Y, Gu J, Li SL, Reddy MA, Natarajan R
and Nadler JL: Elevated glucose and diabetes promote interleukin-12
cytokine gene expression in mouse macrophages. Endocrinology.
147:2518–2525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masola V, Zaza G, Onisto M and Gambaro G:
Heparanase: another renal player controlled by vitamin D. J Pathol.
238:7–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldberg R, Rubinstein AM, Gil N, Hermano
E, Li JP, van der Vlag J, Atzmon R, Meirovitz A and Elkin M: Role
of heparanase-driven inflammatory cascade in pathogenesis of
diabetic nephropathy. Diabetes. 63:4302–4313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fux L, Feibish N, Cohen-Kaplan V,
Gingis-Velitski S, Feld S, Geffen C, Vlodavsky I and Ilan N:
Structure-function approach identifies a COOH-terminal domain that
mediates heparanase signaling. Cancer Res. 69:1758–1767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ridgway LD, Wetzel MD and Marchetti D:
Modulation of GEF-H1 induced signaling by heparanase in brain
metastatic melanoma cells. J Cell Biochem. 111:1299–1309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang C, Tseng T, Jhang Y, Tseng J, Hsieh
C, Wu WG and Lee S: Loss of cell invasiveness through PKC-mediated
syndecan-1 downregulation in melanoma cells under anchorage
independency. Exp Dermatol. 23:843–849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hassan H, Greve B, Pavao MS, Kiesel L,
Ibrahim SA and Götte M: Syndecan-1 modulates β-integrin-dependent
and interleukin-6-dependent functions in breast cancer cell
adhesion, migration, and resistance to irradiation. FEBS J.
280:2216–2227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orecchia P, Conte R, Balza E, Petretto A,
Mauri P, Mingari MC and Carnemolla B: A novel human anti-syndecan-1
antibody inhibits vascular maturation and tumour growth in
melanoma. Eur J Cancer. 49:2022–2033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moran C, Münch G, Forbes JM, Beare R,
Blizzard L, Venn AJ, Phan TG, Chen J and Srikanth V: Type 2
diabetes, skin autofluorescence, and brain atrophy. Diabetes.
64:279–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodall KJ, Poon IK, Phipps S and Hulett
MD: Soluble heparan sulfate fragments generated by heparanase
trigger the release of pro-inflammatory cytokines through TLR-4.
PLoS One. 9:e1095962014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Li R, Tan J, Peng L, Wang P, Liu
J, Xiong H, Jiang B and Chen Y: Syndecan-1 acts in synergy with
tight junction through Stat3 signaling to maintain intestinal
mucosal barrier and prevent bacterial translocation. Inflamm Bowel
Dis. 21:1894–1907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masouleh B Kharabi, Ten Dam GB, Wild MK,
Seelige R, van der Vlag J, Rops AL, Echtermeyer FG, Vestweber D,
van Kuppevelt TH, Kiesel L, et al: Role of the heparan sulfate
proteoglycan syndecan-1 (CD138) in delayed-type hypersensitivity. J
Immunol. 182:4985–4993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Park PW, Kheradmand F and Corry DB:
Endogenous attenuation of allergic lung inflammation by syndecan-1.
J Immunol. 174:5758–5765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei J, Xue S, Wu W, Zhou S, Zhang Y, Yuan
G and Wang J: Sdc1 overexpression inhibits the p38 MAPK pathway and
lessens fibrotic ventricular remodeling in MI rats. Inflammation.
36:603–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Voyvodic PL, Min D, Liu R, Williams E,
Chitalia V, Dunn AK and Baker AB: Loss of syndecan-1 induces a
pro-inflammatory phenotype in endothelial cells with a dysregulated
response to atheroprotective flow. J Biol Chem. 289:9547–9559.
2014. View Article : Google Scholar : PubMed/NCBI
|