Introduction
Acute myocardial infarction leads to ischemic
necrosis of the myocardium, which is greatly detrimental to the
health of the population (1). Each
year, 800,000 individuals in America are diagnosed with myocardial
infarction (2). Morbidity from
myocardial infarction in China is increasing year by year (3). Timely opening of the coronary arteries
in patients with myocardial infarction reduces the area of
myocardial infarction and significantly decreases mortality rates
(4). A clinically relevant
phenomenon that occurs is myocardial ischemia/reperfusion (I/R)
injury, which refers to damage to the myocardial structure after
regaining blood perfusion to the ischemic myocardium (5). Cardiac muscle cells may also die and
the area of myocardial necrosis may extend, which seriously affects
the prognosis of patients with myocardial infarction (6). In clinical practice, myocardial I/R
injury may result in the disorder of electrical activities in the
myocardium, arrhythmia and cardiac insufficiency (7). Pathologically, obvious bleeding and
infiltration of inflammatory cells in necrotic tissues after
reperfusion may appear (8).
Furthermore, microvascular endothelial cells are impaired and blood
vessels are blocked, thus, effective reperfusion cannot be
implemented (2).
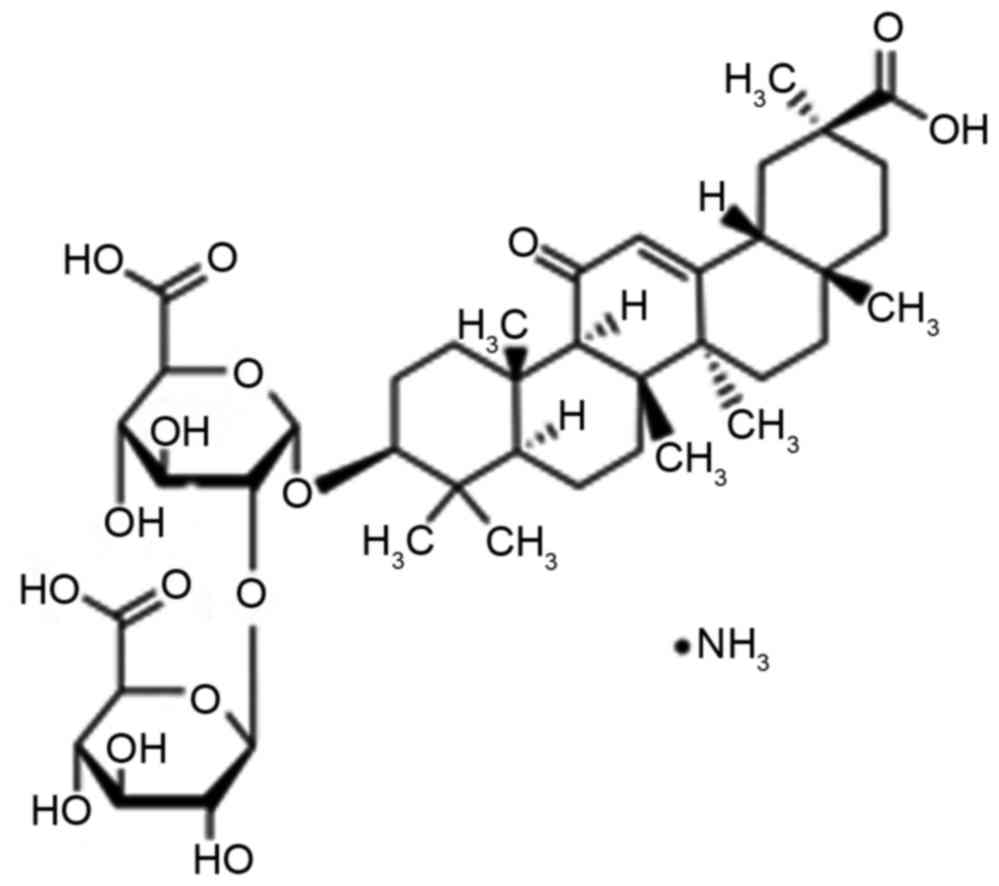
An important ingredient of root extraction from
liquorice is the water soluble acid, glycyrrhizin (Fig. 1), which consists of glucuronic acid
and glycyrrhetinic acid (9). A prior
study has indicated that glycyrrhizin is one of the most effective
ingredients of liquorice, which has anti-ulcer, anti-allergic,
anti-oxidant, immunomodulatory, anti-viral and anti-cancer effects
(10). Furthermore, it has the
functions of liver protection and membrane stabilization.
Glycyrrhizin has been widely used in Europe and the Middle East
(11).
The aim of the present study was to investigate the
protective effect of glycyrrhizin, as well as the related
mechanisms, against myocardial I/R injury in model rats.
Materials and methods
Animals, groups and experimental
design
A total of 48 male Sprague-Dawley rats, weighing
280–320 g, were obtained from the Experimental Animal Centre of the
Sichuan Neurosurgical Institute (Sichuan, China) and were
maintained in standard conditions: 25°C; 50% humidity; a 12-h light
cycle (8:00-20:00); and free access to laboratory chow and water.
Rats were divided into five groups: Sham group (Sham; n=8);
myocardial I/R injury + non-treated group (NS; n=10); myocardial
I/R injury + 2 mg/kg glycyrrhizin (n=10); myocardial I/R injury + 4
mg/kg glycyrrhizin (n=10); and myocardial I/R injury + 10 mg/kg
glycyrrhizin (n=10). In the myocardial I/R injury + 2, 4 or 10
mg/kg glycyrrhizin groups, myocardial I/R injury rats were
administered glycyrrhizin at 0, 2, 4 or 10 mg/kg by intraperitoneal
injection at 30 min prior to ischemia. The sham and NS groups were
treated with an equal volume of normal saline.
In vivo myocardial I/R injury
model
Sprague-Dawley rats were anesthetized with 40 mg/kg
of sodium pentobarbital (intraperitoneally, Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China). Myocardial I/R injury was
induced by left thoracic incision to expose the heart and a 6/0
silk suture was sewn around the left anterior descending coronary.
The slipknot was released for reperfusion for 24 h, following
ischemia for 30 min.
Determination of myocardial infarct
size
Sprague-Dawley rats were sacrificed using the
beheaded method and the hearts were subsequently harvested. Hearts
were stained with 1.5% Evans blue (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), washed with saline and stored at −80°C.
Subsequently, the frozen hearts were cut into 5–7 mm slices and
incubated with 1.2% triphenyltetrazolium chloride (TTC) (Ameresco,
Inc., Framingham, MA, USA) for 15 min at 37°C. Viable non-ischemic
myocardium presented as blue (stained with Evans blue) and
ischemic, viable myocardium was red (stained with TTC). The ratio
of infarct size was calculated as red area/total area of heart, and
expressed as a percentage.
Measurement of aspartate
aminotransferase (AST), lactate dehydrogenase (LDH) alanine
aminotransferase (ALT) and creatine kinase (CK)
Blood samples were obtained from the right carotid
artery and centrifuged at 1,000 × g for 15 min at 4°C. ELISA assay
kits were used to determine serum AST (cat. no. C010-2), LDH (cat.
no. A020-2), ALT (cat. no. C009-1) and CK (cat. no. H197) levels
according to the manufacturer's instructions (all Nanjing Jianchen
Bioengineering Institute, Nanjing, Jiangsu, China).
Measurement of glutathione (GSH) and
glutathione peroxidase (GSH-PX)
Blood samples were obtained from the right carotid
artery and centrifuged at 1,000 × g for 15 min at 4°C. Assay kits
were used to determine serum GSH and GSH-PX levels (cat. nos.
A006-2 and A005) according to the manufacturer's instructions
(Nanjing Jianchen Bioengineering Institute).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from heart tissue samples
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific Inc.,
Waltham, MA, USA), and DNase I (Invitrogen; Thermo Fisher
Scientific Inc.) was used to remove genomic DNA, and RNA samples (1
µg) were used for cDNA synthesis using an RT-PCR kit (Roche
Diagnostics GmbH, Mannheim, Germany). Total RNA (200 ng) was
subjected to RT-qPCR using SYBR Green Premix (Takara Biotechnology
Co., Ltd., Dalian, China) on an ABI 7500 Real-time PCR system
(Thermo Fisher Scientific, Inc.). Primers used to amplify the
fragment of HMGB1 and inducible nitric oxide synthase (iNOS) were
designed as follows: HMGB1, forward 5′-CTGATGCAGCTTATACGAAG-3′ and
reverse 5′-TCAGGTAAGGAGCAGAACAT-3′; iNOS, forward
5′-GCATCCCAAGTACGAGTGGT-3′ and reverse 5′-GAAGGCGTAGCTGAACAAGG-3′;
and GAP DH, forward 5′-CCATCACTGCCACTCAGAAGA-3′ and reverse
5′-CATGAGGTCCACCACCCTGT-3′. The PCR consisted of an initial
denaturation step at 94°C for 5 min followed by 40 cycles at 94°C
for 30 sec, 58°C for 30 sec and 72°C for 45 sec, with a final
extension at 72°C for 10 min. The relative gene expression was
calculated using the 2−ΔΔCqmethod (12).
Measurement of nuclear factor
kappa-light-chain-enhancer of activated B cells-p65 (NF-κB-p65),
tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and
IL-6
Blood samples were obtained from the right carotid
artery and centrifuged at 1,000 × g for 15 min at 4°C. ELISA
assay kits were used to detect serum NF-κB-p65 (cat. no. H202),
TNF-α (cat. no. R019), IL-1β (cat. no. H002) and IL-6 (cat. no.
R016) levels according to the manufacturer's instructions (Nanjing
Jianchen Bioengineering Institute).
Western blot assays
Total protein was extracted from heart tissue
samples and homogenized in 0.5 ml of radio-immunoprecipitation
assay buffer. The supernatant was collected for protein
concentration, using the BCA protein assay reagent kit (Beijing
Boaosen Biotechnology Co., Ltd., Beijing, China). Equal quantities
of protein (50 µg) samples were separated using 6–12% SDS-PAGE and
transferred onto polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skimmed milk
for 2 h and incubated with rabbit anti-phosphorylated (p)-p38
(1:4,000; cat. no. 4511), rabbit anti p-JNK (1:2,000; cat. no.
4668) and p-extracellular signal-regulated kinase (ERK; 1:3,000;
4376; all Cell Signaling Technology, Inc., Danvers, MA, USA), at
4°C overnight. Following washing with Tris-buffered saline solution
with Tween-10, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
bs-0295G; Beijing Boaosen Biotechnology Co., Ltd.) at 37°C for 1 h.
Blots were visualized with a Low Background Luminescence ECL
Detection kit (Nanjing Jianchen Bioengineering Institute) and
quantified using the Image J 3.0 system (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Experimental data were expressed as mean ± standard
deviation. SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) software was
used for statistical analysis of data, with significant
within-group and between-group differences analyzed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Protective effect of glycyrrhizin on
infarct size in rats with myocardial I/R injury
Infarct size of rats was measured by Evans blue/TTC
staining in each of the five groups (Fig. 2). Glycyrrhizin significantly
inhibited infarct size in myocardial I/R injury rats, inhibition
was demonstrated to correspond with the increasing concentrations
of glycyrrhizin administered when compared with the NS group
(P<0.01; Fig. 2).
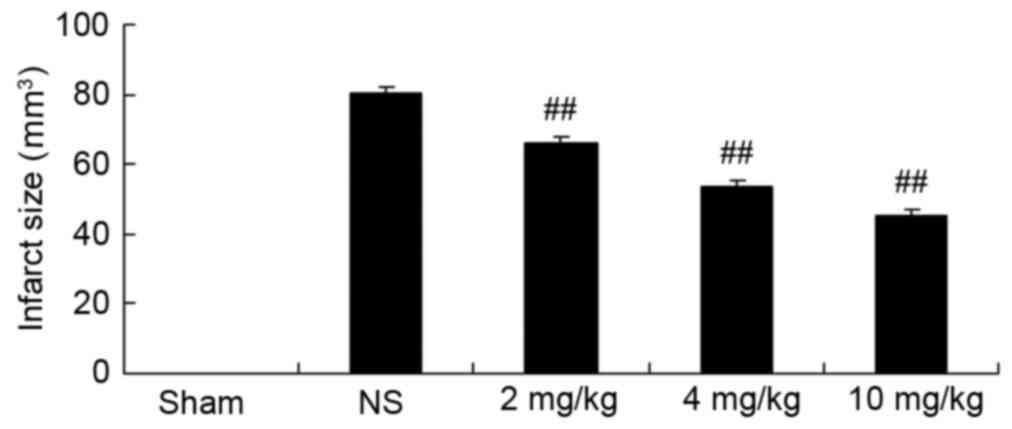 | Figure 2.Protective effect of glycyrrhizin on
infarct size in rats with myocardial I/R injury. Sprague-Dawley
rats were divided into five groups: Sham, NS and myocardial I/R
injury + pre-treatment with 2, 4 and 10 mg/kg glycyrrhizin groups,
respectively, to investigate the effect of glycyrrhizin on infarct
size in myocardial I/R injury. Data are presented as the mean ±
standard deviation. ##P<0.01 vs. NS. I/R,
ischemia/reperfusion; Sham, sham-treated group; NS, myocardial I/R
injury + non-treated group; 2 mg/kg, myocardial I/R injury +
pre-treatment with 2 mg/kg glycyrrhizin group; 4 mg/kg, myocardial
I/R injury + pre-treatment with 4 mg/kg glycyrrhizin group; 10
mg/kg, myocardial I/R injury + pre-treatment with 10 mg/kg
glycyrrhizin group. |
Protective effect of glycyrrhizin on
AST, LDH, ALT and CK in rats with myocardial I/R injury
Plasma AST, LDH, ALT and CK levels were measured,
which are important indicators of the extent of myocardial injury
(13). Compared with the sham group,
plasma AST, LDH and ALT levels were significantly increased as a
result of myocardial I/R injury in rats, as was also depicted in
the NS group compared with the sham-operated group (P<0.01);
although, no significant increase was observed for CK (Fig. 3A-D, respectively). Increased levels
of the indicators for myocardial injury were significantly hindered
by treatment with 2–10 mg/kg glycyrrhizin (P<0.01); however,
decreased levels of LDH in the 4 and 10 mg/kg groups were not
statistically significant (Fig.
3A-D).
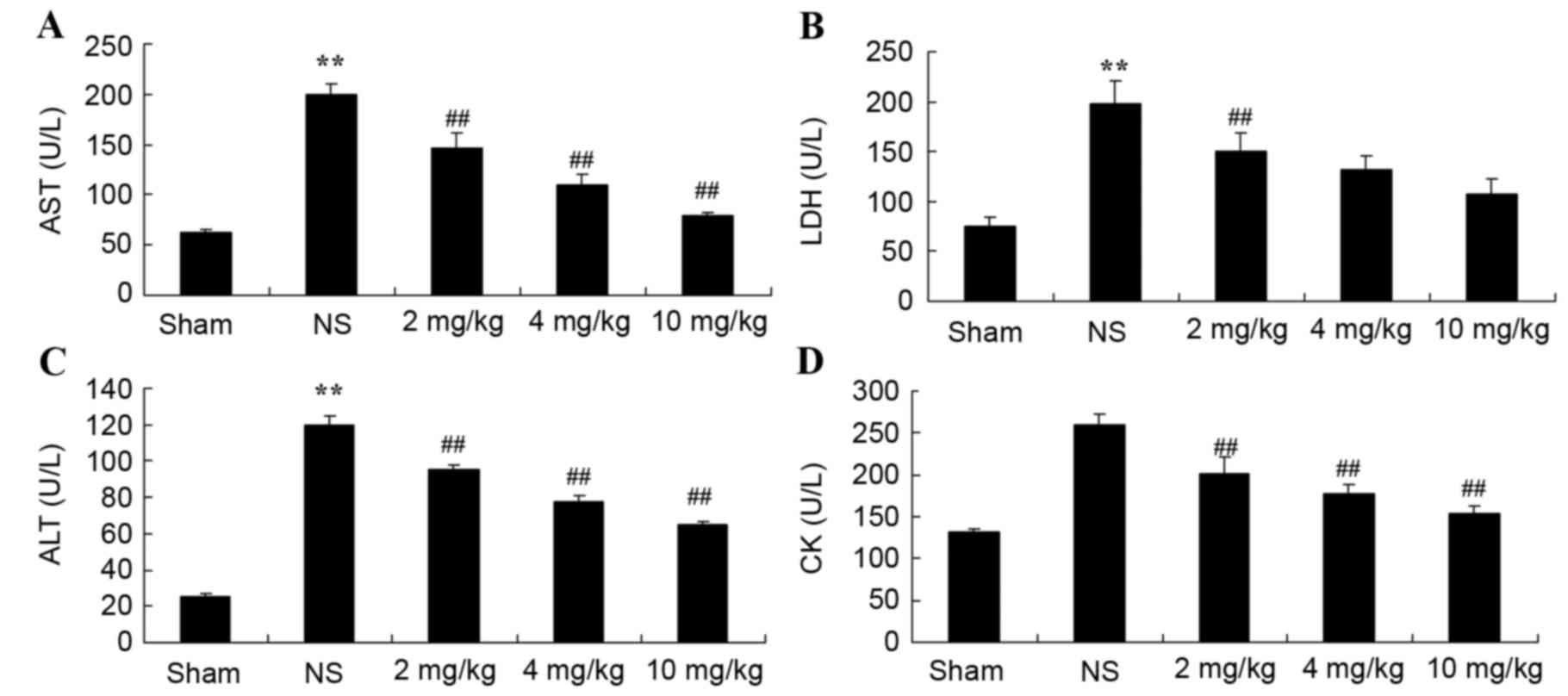 | Figure 3.Protective effect of glycyrrhizin on
AST, LDH, ALT and CK in rats with myocardial I/R injury. The
protective effect of glycyrrhizin was investigated by determining
the levels of (A) AST, (B) LDH, (C) ALT and (D) CK in rats with
myocardial I/R injury. Rats were divided into five groups: Sham, NS
and myocardial I/R injury + pre-treatment with 2, 4 and 10 mg/kg
glycyrrhizin groups, respectively, to investigate the effect of
glycyrrhizin on infarct size in myocardial I/R injury. Data are
presented as the mean ± standard deviation. **P<0.01 vs. Sham;
##P<0.01 vs. NS. I/R, ischemia/reperfusion; AST,
aspartate aminotransferase; LDH, lactate dehydrogenase; ALT,
alanine aminotransferase; CK, creatine kinase; Sham, sham-treated
group; NS, myocardial I/R injury + non-treated group; 2 mg/kg,
myocardial I/R injury + pre-treatment with 2 mg/kg glycyrrhizin
group; 4 mg/kg, myocardial I/R injury + pre-treatment with 4 mg/kg
glycyrrhizin group; 10 mg/kg, myocardial I/R injury + pre-treatment
with 10 mg/kg glycyrrhizin group. |
Protective effect of glycyrrhizin on
oxidative stress in rats with myocardial I/R injury
Plasma GSH and GSH-PX levels significantly decreased
after myocardial I/R injury, compared with the sham-operated group
(P<0.01; Fig. 4A and B,
respectively). When rats were pre-treated with 2–10 mg/kg
glycyrrhizin, plasma GSH and GSH-PX levels were significantly
elevated when compared with the levels observed in the NS group in
myocardial I/R injury rats (P<0.01; Fig. 4A and B, respectively).
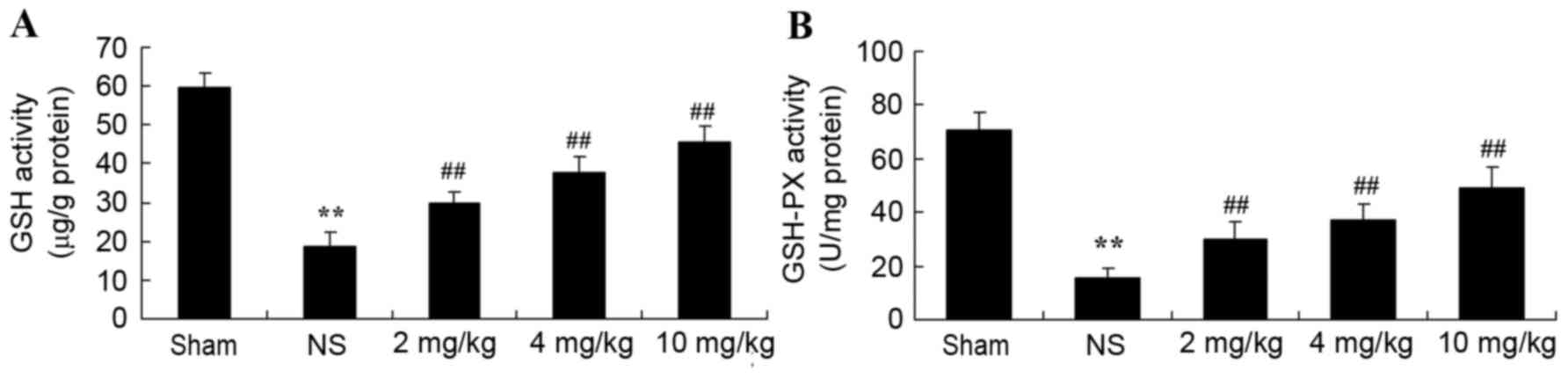 | Figure 4.Protective effect of glycyrrhizin on
oxidative stress in myocardial I/R injury rats. Protective effect
of glycyrrhizin on the levels of plasma (A) GSH and (B) GSH-PX in
rats with myocardial I/R injury. Data are presented as the mean ±
standard deviation. **P<0.01 vs. Sham; ##P<0.01
vs. NS. GSH, glutathione; GSH-PX, glutathione peroxidase; I/R,
ischemia/reperfusion; AST, aspartate aminotransferase; LDH, lactate
dehydrogenase; ALT, alanine aminotransferase; CK, creatine kinase;
Sham, sham-treated group; NS, myocardial I/R injury + non-treated
group; 2 mg/kg, myocardial I/R injury + pre-treatment with 2 mg/kg
glycyrrhizin group; 4 mg/kg, myocardial I/R injury + pre-treatment
with 4 mg/kg glycyrrhizin group; 10 mg/kg, myocardial I/R injury +
pre-treatment with 10 mg/kg glycyrrhizin group. |
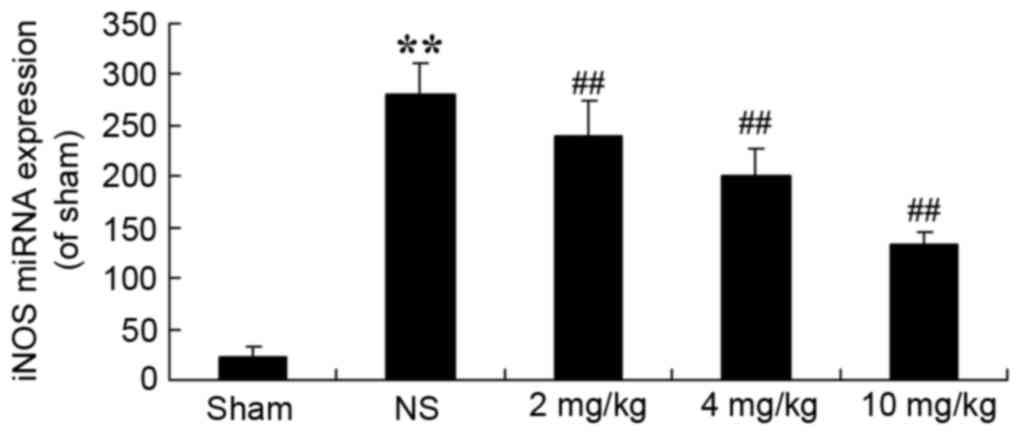
Protective effect of glycyrrhizin on
iNOS in rats with myocardial I/R injury
To demonstrate that the protective effect of
glycyrrhizin on myocardial I/R injury is associated with iNOS
production, iNOS miRNA expression levels of myocardial I/R injury
rats were determined. In the sham group, iNOS miRNA expression
levels were significantly decreased when compared with the
expression exhibited in myocardial I/R injury rats (P<0.01;
Fig. 5). Pre-treatment with
glycyrrhizin resulted in a significant decrease in the iNOS miRNA
expression of myocardial I/R injury groups when compared with the
NS group (P<0.01; Fig. 5).
Protective effect of glycyrrhizin on
inflammatory reactions in rats with myocardial I/R injury
The protective effect of glycyrrhizin on
inflammatory reactions in myocardial I/R injury rats was
investigated. Compared with the sham-operated group,
NF-κB-p65TNF-α, IL-1β and IL-6 levels were significantly increased
in all myocardial I/R injury groups when compared with the sham
group (Fig. 6A-D, respectively).
Increased levels of plasma NF-κB-p65, TNF-α, IL-1β and IL-6 were
significantly hindered by treatment with glycyrrhizin, with
significantly decreased levels exhibited with increased
concentrations of glycyrrhizin administered when compared with the
NS group (P<0.01; Fig. 6).
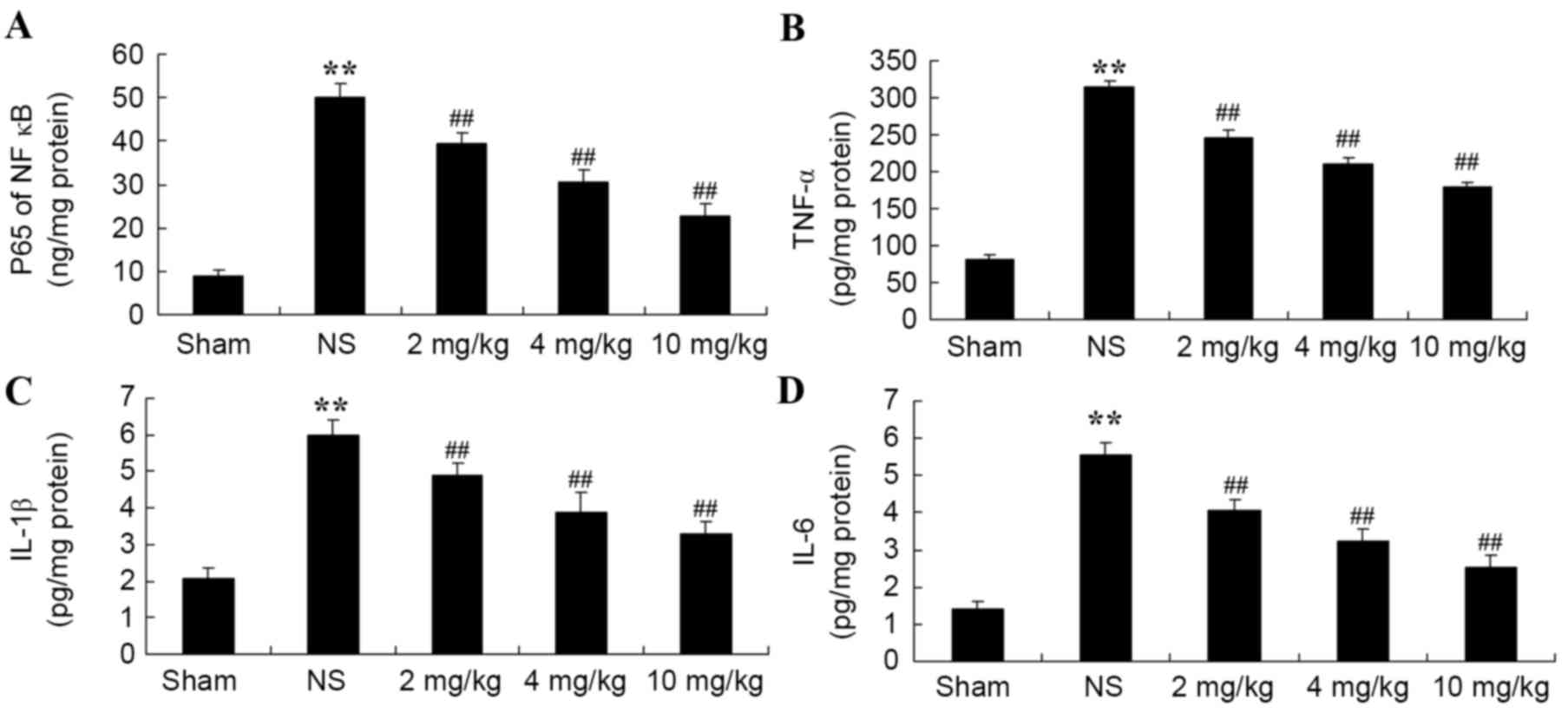 | Figure 6.Protective effect of glycyrrhizin on
inflammatory reactions in myocardial I/R injury rats. Protective
effect of glycyrrhizin on plasma (A) NF-κB-p65, (B) TNF-α, (C)
IL-1β and (D) IL-6 levels in rats with myocardial I/R injury. Data
are presented as the mean ± standard deviation. **P<0.01 vs.
Sham; ##P<0.01 vs. NS. I/R, ischemia/reperfusion;
NF-κB-p65, nuclear factor kappa-light-chain-enhancer of activated B
cells-p65; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β;
IL-6, interleukin-6; Sham, sham-treated group; NS, myocardial I/R
injury + non-treated group; 2 mg/kg, myocardial I/R injury +
pre-treatment with 2 mg/kg glycyrrhizin group; 4 mg/kg, myocardial
I/R injury + pre-treatment with 4 mg/kg glycyrrhizin group; 10
mg/kg, myocardial I/R injury + pre-treatment with 10 mg/kg
glycyrrhizin group. |
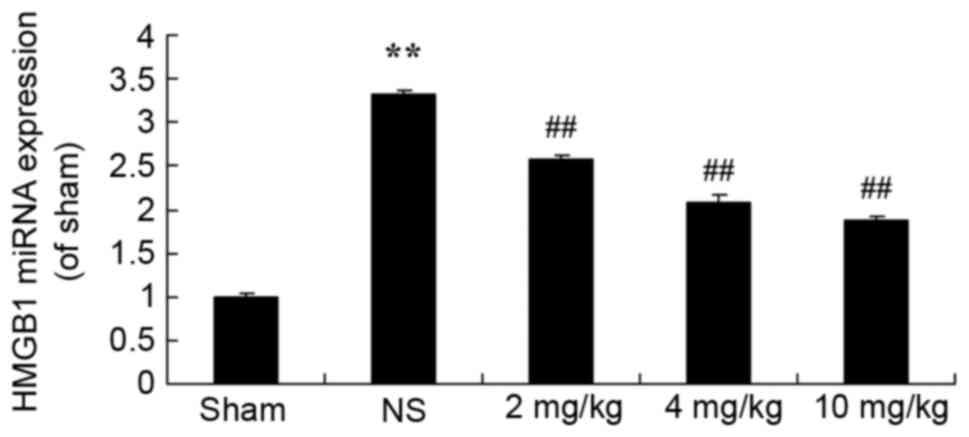
Protective effect of glycyrrhizin on
HMGB1 expression in rats with myocardial I/R injury
To determine the protective effect of glycyrrhizin
on HMGB1 expression in myocardial I/R injury rats, HMGB1 miRNA
expression was explored in the present study. Fig. 7 demonstrates thatHMGB1 miRNA
expression of the sham group was significantly lower than all
myocardial I/R injury groups (P<0.01). Treatment with
glycyrrhizin significantly decreased HMGB1 miRNA expression levels
in the myocardial I/R injury groups, when compared with the NS
group, which exhibited significantly higher miRNA expression levels
of HMGB1 in comparison with the sham group (P<0.01; Fig. 7).
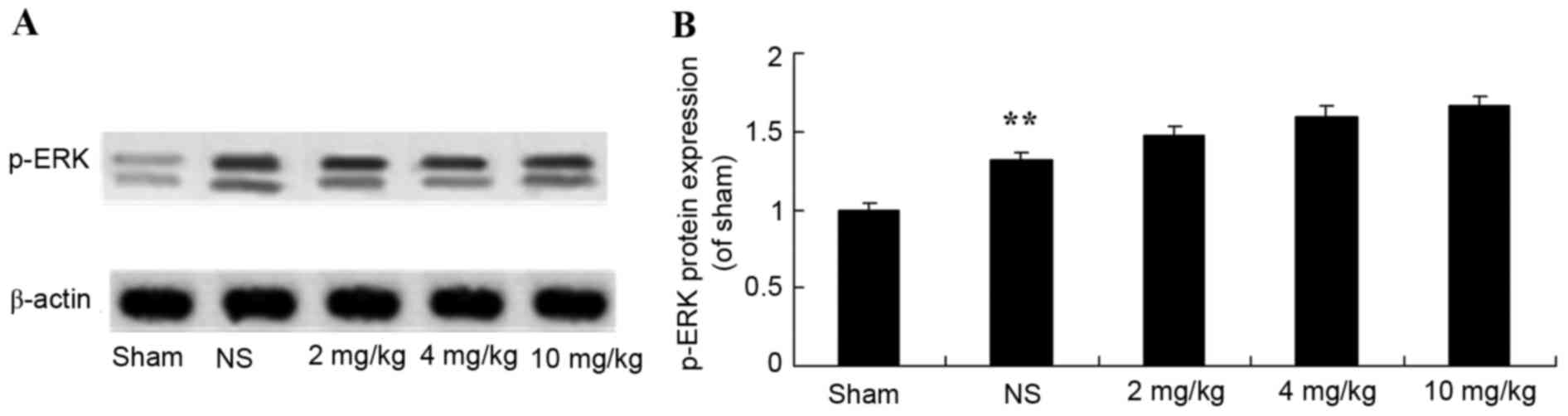
Protective effect of glycyrrhizin on
ERK protein expression in rats with myocardial I/R injury
The protective effect of glycyrrhizin on ERK protein
expression in myocardial I/R injury rats was investigated. The
protein expression levels of p-ERK were significantly elevated
following myocardial I/R when compared with the sham group
(P<0.01; Fig. 8). There were no
significant differences exhibited in the p-ERK protein expression
levels of the 2–10 mg/kg glycyrrhizin-pre-treatment groups;
however, levels were elevated in these groups when compared with
the sham and NS groups (Fig. 8).
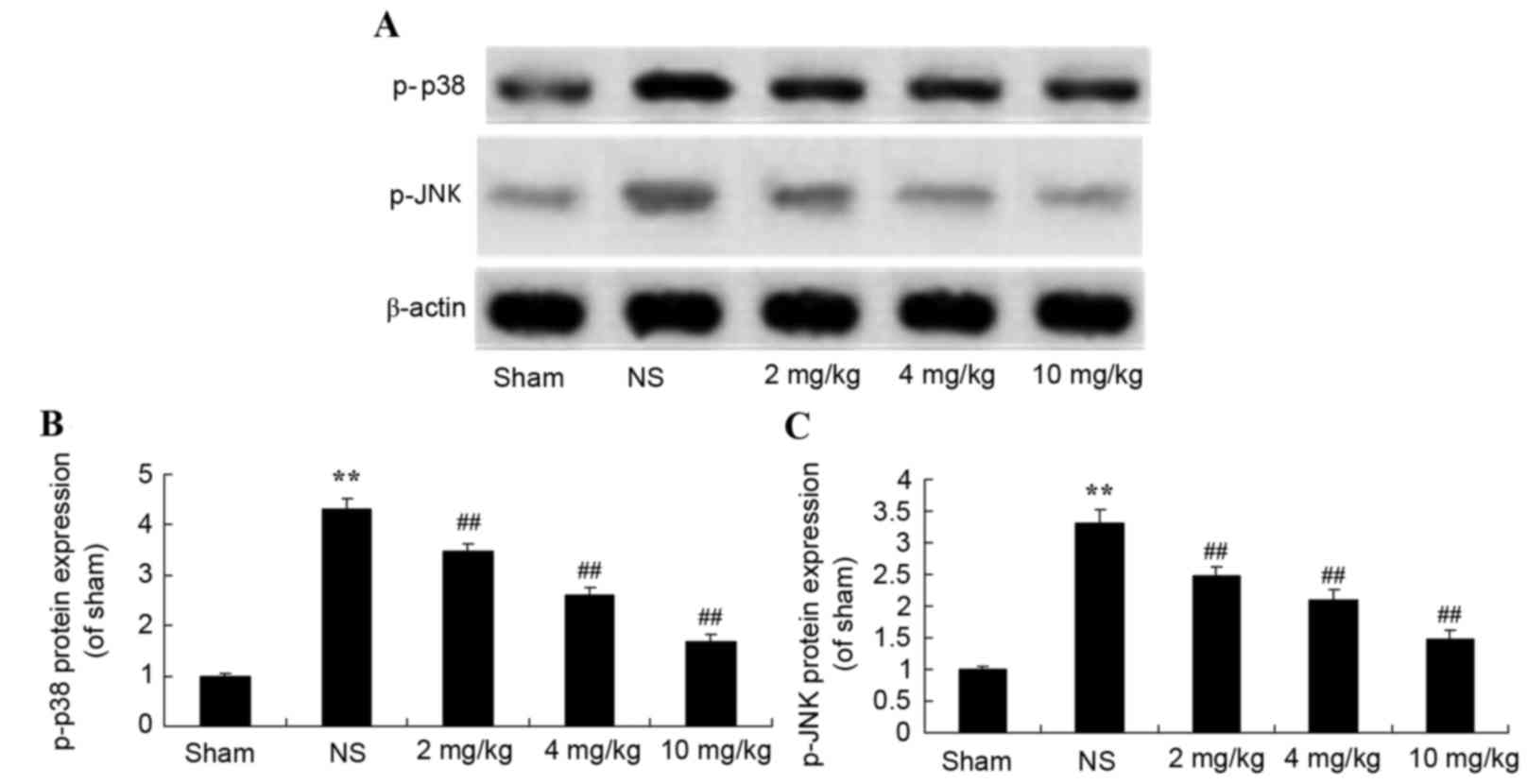
Protective effect of glycyrrhizin on
p38 and JNK protein expression in rats with myocardial I/R
injury
To further demonstrate the protective effect of
glycyrrhizin on myocardial I/R injury, the effect on the
mitogen-activated protein kinase (MAPK) signaling pathway was
explored using western blotting. Protein expression levels of p-p38
and p-JNK were significantly increased after myocardial I/R injury
when compared with the sham group (P<0.01; Fig. 9). Pre-treatment with 2–10 mg/kg
glycyrrhizin significantly alleviated these changes in myocardial
I/R injury rats when compared with the NS group (P<0.01;
Fig. 9).
Discussion
In recent years, with the development of the economy
and the advancement of internalization, mental states, dietary
structures and physical labor intensities have undergone
fundamental changes (4).
Unfortunately, these changes are not always beneficial to the
health of the population. Consequently, the morbidity rates
associated with hypertension, hyperlipidemia, diabetes and
cardia-cerebrovascular diseases are increasing each year (14). As far as myocardial infarction of
ST-elevation type is concerned, approximately two million
individuals in China are diagnosed with myocardial infarction and
500,000 new cases present with myocardial infarction (15). The number of cases of acute
myocardial infarction in 1991 (1,492) were 2.47 times greater than
the cases noted in 1972 (604) in 16 large- and medium-sized
hospitals located in Beijing (16).
In the present study, the protective effect of glycyrrhizin notably
inhibited infarct size and plasma AST, LDH, ALT and CK levels in
rats with myocardial I/R injury. Zhang et al (17) indicated that glycyrrhizin protected
the brain against ischemia-reperfusion injury in mice. Zhai et
al demonstrated that glycyrrhizin protected the heart against
I/R injury, through the blockade of HMGB1 and the phospho-JNK
pathway (18).
Neutrophil granulocytes are ‘the power plant’ of
oxygen radicals, with damaging effects that exceed the protective
effect of myocardium (19). Infusion
of hemameba into the heart with isolated perfusion has been
demonstrated to result in I/R (20).
These findings support the theory that neutrophil granulocytes have
pathogenic effects in myocardial I/R injury (21). To reduce the effects of myocardial
infiltration, neutrophils may predominantly reduce the discharge of
oxygen radicals. This effect may relieve stunned myocardium after
ischemia and shorten the duration of stunned myocardium, which
would relieve the degrees of myocardial I/R injury and shorten the
duration required to protect myocardial I/R injury (22). The present study demonstrated that
pretreatment with 2–10 mg/kg glycyrrhizin significantly elevated
plasma GSH and GSH-PX levels in rats with myocardial I/R injury.
Wang et al reported that glycyrrhizic acid attenuated
reactive oxygen species production in the kidneys of diabetic mice
(23). Rahman and Sultana (10) reported that glycyrrhizin inhibited
12-O-tetradecanoyl phorbol-13-acetate-induced cutaneous oxidative
stress in Swiss albino mice.
iNOS is not calcium-dependent and rarely exhibits
expression in normal tissues (24).
When stimulated by inflammation, iNOS maybe expressed in any cells,
not only immuno-reactive cells, such as mononuclear cells,
mastocytes and neutrophils, but also in cancer and cardiovascular
cells (25). Within the
cardiovascular system, iNOS enzyme is predominantly expressed in
blood vessel endothelium, myocardium and vascular smooth cells.
When endothelial cells are stimulated by cytokines as a result of
inflammation, expression of endothelial NOS is inhibited (26). Subsequently, high concentrations of
NO may be catalyzed (27). Previous
findings have revealed that the production of NO by iNOS is
important in the development of cellular damages in various
inflammatory diseases (26). In the
present study, I/R-induced myocardial injury of rat hearts
subjected to pre-treatment with glycyrrhizin revealed significantly
decreased iNOS miRNA expression levels. Similarly, Kim et al
concluded that glycyrrhizin reduced HMGB1 secretion, reduced
hepatic injury and the expression of iNOS (28).
Previous studies have indicated that TNF-α levels
after myocardial ischemia are significantly increased (29). For patients with acute myocardial
infarction or unstable angina, levels of IL-1β, SIL-2R, IL-6 and
TNF-α are significantly increased. Following four months of
follow-up, these levels are significantly decreased (30). For patients with acute or chronic
coronary artery diseases, both IFN-γ and TNF-α are significantly
increased. Intervention of TNF-α may improve myocardial ischemia,
with the time of intervention being either before reperfusion or at
the beginning of reperfusion (31).
Intervention at the beginning of reperfusion was discovered to have
an influential role in the recovery of myocardium (31). It is therefore feasible to consider
the inflammatory factor TNF-α, as a useful treatment at the
beginning of reperfusion (29).
TNF-α levels are increased in MI/RI and the process of percutaneous
coronary intervention, thus MI/RI may be protected by a mechanism
involving TNF-α (29). In the
present study, pre-treatment with glycyrrhizin significantly
inhibited plasma NF-κB-p65 TNF-α, IL-1β and IL-6 levels in rats
with myocardial I/R injury. Michaelis et al (32) reported that glycyrrhizin may have
anti-oxidative and anti-inflammatory effects in H5N1 influenza A
virus-infected cells, through reducing the activation of JNK and
p38. HMGB-1 is a highly conservative nucleoprotein that is released
by necrotic or injured cells and secreted by mononuclear cells,
and/or macrophages, which react with endogenous and exogenous
non-inflammatory stimuli (33).
HMGB-1 is secreted out of cells by non-classical and mediated
secretory pathways, with HMGB-1 secretion occurring later than
pro-inflammatory cytokines, including tumor necrosis factors (such
as IL-1). HMGB-1 is considered to be an important late
pro-inflammatory cytokine (34).
Extracellular HMGB-1 has dual roles in the immune response during
inflammation (35). While necrotic
cells may release HMGB-1 and activate early inflammatory responses
to eliminate foreign matters and therefore promote the repair of
injured tissues or organs (36),
mononuclear macrophages may secrete HMGB-1 to activate late
non-inflammatory responses (37).
Affected by chemotactic factors, more inflammatory cells
subsequently infiltrate injured tissues and aggravate pathological
injuries. Extracellular HMGB-1 requires association with
corresponding cell-membrane receptors to exert its biological
effects (38). Notably, the present
study indicated that treatment with glycyrrhizin effectively
decreased HMGB1 miRNA expression levels in rats with myocardial I/R
injury. However, Ni et al (39) reported that the neuroprotective
effect of glycyrrhizin prevents spinal cord I/R injury, through
inflammatory cytokines and HMGB-1 expression.
The morbidity rates associated with cardiovascular
disease are increasing each year, and this disease accounts for one
of the highest mortality rates (40). In the occurrence and development of
various cardiovascular diseases, including diabetic cardiomyopathy,
MI/RI and drug-induced cardiomyopathy, the activation of MAPK has
an essential role. This is due to the fact that various stimuli
regulate genetic expression levels and changes of protein functions
by activating signal transduction pathways in MAPK, thus myocardial
damages may be induced or aggravated (41). In the present study, glycyrrhizin
significantly alleviated p38 and JNK protein expression levels and
did not exhibit notable effects on ERK protein expression in
myocardial I/R injury rats. Michaelis et al (32) reported that glycyrrhizin induced
anti-oxidative and anti-inflammatory effects in H5N1 influenza A
virus-infected cells by reducingthe activation of JNK and p38.
Furthermore, Honda et al (42) revealed that glycyrrhizin suppressed
LPS-induced activation by inhibiting the activation of MAPKs (JNK
and p38) signaling via a different manner.
In conclusion, the present results demonstrated that
the protective effect of glycyrrhizin attenuated myocardial I/R
injury in rats. The protective effect of glycyrrhizin on oxidative
stress, iNOS and inflammatory reactions was revealed in
vivo, through triggering HMGB1 and the blockage of the p38 and
JNK pathways. These data suggest a novel therapeutic approach for
the treatment of ischemic stroke with glycyrrhizin.
References
|
1
|
Yao T, Lu W, Zhu J, et al: Role of
CD11b+Gr-1+ myeloid cells in AGEs-induced myocardial injury in a
mice model of acute myocardial infarction. Int J Clin Exp Pathol.
8:3238–3249. 2015.PubMed/NCBI
|
|
2
|
Valls N, Gormaz JG, Aguayo R, González J,
Brito R, Hasson D, Libuy M, Ramos C, Carrasco R, Prieto JC, et al:
Amelioration of persistent left ventricular function impairment
through increased plasma ascorbate levels following myocardial
infarction. Redox Rep. 21:75–83. 2016.PubMed/NCBI
|
|
3
|
Sun Z, Zeng J and Huang H: Intracoronary
injection of tirofiban prevents microcirculation dysfunction during
delayed percutaneous coronary intervention in patients with acute
myocardial infarction. Int J Cardiol. 208:137–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulla ZD, Wilson B, Abedin Z, Hernandez LL
and Plavsic SK: Acute myocardial infarction in pregnancy: A
statewide analysis. J Registry Manag. 42:12–17. 2015.PubMed/NCBI
|
|
5
|
Bergmeijer TO, Janssen PW, Schipper JC,
Qaderdan K, Ishak M, Ruitenbeek RS, Asselbergs FW, van 't Hof AW,
Dewilde WJ, Spanó F, et al: CYP2C19 genotype-guided antiplatelet
therapy in ST-segment elevation myocardial infarction
patients-Rationale and design of the Patient Outcome after primary
PCI (POPular) Genetics study. Am Heart J. 168:16–22.e1. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ota S, Tanimoto T, Hirata K, Orii M,
Shiono Y, Shimamura K, Ishibashi K, Yamano T, Ino Y, Kitabata H, et
al: Assessment of circumferential endocardial extent of myocardial
edema and infarction in patients with reperfused acute myocardial
infarction: A cardiovascular magnetic resonance study. Int Heart J.
55:234–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liehn EA, Tuchscheerer N, Kanzler I,
Drechsler M, Fraemohs L, Schuh A, Koenen RR, Zander S, Soehnlein O,
Hristov M, et al: Double-edged role of the CXCL12/CXCR4 axis in
experimental myocardial infarction. J Am Coll Cardiol.
58:2415–2423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu PY, Wang YW, Pang XH and Wang HW: T174M
polymorphism in the angiotensinogen gene and risk of myocardial
infarction: A meta-analysis. Genet Mol Res. 14:3767–3774. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jose R, Sajitha GR and Augusti KT: A
review on the role of nutraceuticals as simple as se(2+) to complex
organic molecules such as glycyrrhizin that prevent as well as cure
diseases. Indian J Clin Biochem. 29:119–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahman S and Sultana S: Glycyrrhizin
exhibits potential chemopreventive activity on 12-O-tetradecanoyl
phorbol-13-acetate-induced cutaneous oxidative stress and tumor
promotion in Swiss albino mice. J Enzyme Inhib Med Chem.
22:363–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He SQ, Gao M, Fu YF and Zhang YN:
Glycyrrhizic acid inhibits leukemia cell growth and migration via
blocking AKT/mTOR/STAT3 signaling. Int J Clin Exp Pathol.
8:5175–5181. 2015.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng ZH, Huang L, Song MB and Song YM:
Protective effect of a polysaccharide from Salvia miltiorrhiza on
isoproterenol (ISO)-induced myocardial injury in rats. Carbohydr
Polym. 132:638–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Husebye T, Eritsland J, Arnesen H,
Bjørnerheim R, Mangschau A, Seljeflot I and Andersen GØ:
Association of interleukin 8 and myocardial recovery in patients
with ST-elevation myocardial infarction complicated by acute heart
failure. PLoS One. 9:e1123592014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tilling L, Hunt J, Donald A, Clapp B and
Chowienczyk P: Darbepoetin enhances endothelium-dependent vasomotor
function in patients with stable coronary artery disease only after
preceding ischaemia/reperfusion. Clin Sci (Lond). 122:329–336.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang XC, Liu Y, Wang LF, Cui L, Wang T, Ge
YG, Wang HS, Li WM, Xu L, Ni ZH, et al: Reduction in myocardial
infarct size by postconditioning in patients after percutaneous
coronary intervention. J Invasive Cardiol. 19:424–430.
2007.PubMed/NCBI
|
|
17
|
Zhang J, Wu Y, Weng Z, Zhou T, Feng T and
Lin Y: Glycyrrhizin protects brain against ischemia-reperfusion
injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain
Res. 1582:176–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhai CL, Zhang MQ, Zhang Y, Xu HX, Wang
JM, An GP, Wang YY and Li L: Glycyrrhizin protects rat heart
against ischemia-reperfusion injury through blockade of
HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin.
33:1477–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong W, Zhou M, Dong M, Pan B, Liu Y, Shao
J, Gu X, Huang Y, Li G, Wang Y and Sun H: Keto acid metabolites of
branched-chain amino acids inhibit oxidative stress-induced
necrosis and attenuate myocardial ischemia-reperfusion injury. J
Mol Cell Cardiol. 101:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan W, Taylor AJ, Ellims AH, Lefkovits L,
Wong C, Kingwell BA, Natoli A, Croft KD, Mori T, Kaye DM, et al:
Effect of iron chelation on myocardial infarct size and oxidative
stress in ST-elevation-myocardial infarction. Circ Cardiovasc
Interv. 5:270–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang
L, Chen L, Feng W, Shi H, Yu X, et al: bFGF attenuates endoplasmic
reticulum stress and mitochondrial injury on myocardial
ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J
Cell Mol Med. 19:595–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mura M, Hopkins TG, Michael T, Abd-Latip
N, Weir J, Aboagye E, Mauri F, Jameson C, Sturge J, Gabra H, et al:
LARP1 post-transcriptionally regulates mTOR and contributes to
cancer progression. Oncogene. 34:5025–5036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZH, Hsieh CH, Liu WH and Yin MC:
Glycyrrhizic acid attenuated glycative stress in kidney of diabetic
mice through enhancing glyoxalase pathway. Mol Nutr Food Res.
58:1426–1435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zaitone SA and Abo-Gresha NM: Rosuvastatin
promotes angiogenesis and reverses isoproterenol-induced acute
myocardial infarction in rats: Role of iNOS and VEGF. Eur J
Pharmacol. 691:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye Y, Perez-Polo JR and Birnbaum Y:
Protecting against ischemia-reperfusion injury: Antiplatelet drugs,
statins and their potential interactions. Ann N Y Acad Sci.
1207:76–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen TH, Liao FT, Yang YC and Wang JJ:
Inhibition of inducible nitric oxide synthase ameliorates
myocardial ischemia/reperfusion injury-induced acute renal injury.
Transplant Proc. 46:pp. 1123–1126. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Q, Zhou D and Li X, Yang N, Guo P, Xu D
and Li X: Renoprotective effects of propofol on the expression of
iNOS protein in rats with ischemia reperfusion injury. Int J Clin
Exp Med. 8:776–780. 2015.PubMed/NCBI
|
|
28
|
Kim YM, Kim HJ and Chang KC: Glycyrrhizin
reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7
cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1.
Int Immunopharmacol. 26:112–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleinbongard P, Schulz R and Heusch G:
TNFα in myocardial ischemia/reperfusion, remodeling and heart
failure. Heart Fail Rev. 16:49–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yung MM, Chan DW, Liu VW, Yao KM and Ngan
HY: Activation of AMPK inhibits cervical cancer cell growth through
AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 13:3272013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu G, Huang X, Zhang K, Jiang H and Hu X:
Anti-inflammatory effect of B-type natriuretic peptide
postconditioning during myocardial ischemia-reperfusion:
Involvement of PI3K/Akt signaling pathway. Inflammation.
37:1669–1674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michaelis M, Geiler J, Naczk P, Sithisarn
P, Leutz A, Doerr HW and Cinatl J Jr: Glycyrrhizin exerts
antioxidative effects in H5N1 influenza A virus-infected cells and
inhibits virus replication and pro-inflammatory gene expression.
PLoS One. 6:e197052011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong J, Wang Q, Xue FS, Yuan YJ, Li S,
Liu JH, Liao X and Zhang YM: Comparison of cardioprotective and
anti-inflammatory effects of ischemia pre- and postconditioning in
rats with myocardial ischemia-reperfusion injury. Inflamm Res.
60:547–554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nogueira-Machado JA and de Oliveira Volpe
CM: HMGB-1 as a target for inflammation controlling. Recent Pat
Endocr Metab Immune Drug Discov. 6:201–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dejean E, Foisseau M, Lagarrigue F, Lamant
L, Prade N, Marfak A, Delsol G, Giuriato S, Gaits-Iacovoni F and
Meggetto F: ALK+ALCLs induce cutaneous, HMGB-1-dependent IL-8/CXCL8
production by keratinocytes through NF-κB activation. Blood.
119:4698–4707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu J, Nishimura M, Wang Y, Sims JR, Qiu
S, Savitz SI, Salomone S and Moskowitz MA: Early release of HMGB-1
from neurons after the onset of brain ischemia. J Cereb Blood Flow
Metab. 28:927–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frost RA, Nystrom G, Burrows PV and Lang
CH: Temporal differences in the ability of ethanol to modulate
endotoxin-induced increases in inflammatory cytokines in muscle
under in vivo conditions. Alcohol Clin Exp Res. 29:1247–1256. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radic Biol Med. 84:103–115.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ni B, Cao Z and Liu Y: Glycyrrhizin
protects spinal cord and reduces inflammation in spinal cord
ischemia-reperfusion injury. Int J Neurosci. 123:745–751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pando R, Cheporko Y, Haklai R,
Maysel-Auslender S, Keren G, George J, Porat E, Sagie A, Kloog Y
and Hochhauser E: Ras inhibition attenuates myocardial
ischemia-reperfusion injury. Biochem Pharmacol. 77:1593–1601. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS
and Kim SJ: Curcumin protects against regional myocardial
ischemia/reperfusion injury through activation of RISK/GSK-3β and
inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther.
17:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Honda H, Nagai Y, Matsunaga T, Saitoh S,
Akashi-Takamura S, Hayashi H, Fujii I, Miyake K, Muraguchi A and
Takatsu K: Glycyrrhizin and isoliquiritigenin suppress the LPS
sensor toll-like receptor 4/MD-2 complex signaling in a different
manner. J Leukoc Biol. 91:967–976. 2012. View Article : Google Scholar : PubMed/NCBI
|