Introduction
Glaucoma is one of the prominent eye diseases
characterized by continuous increased intraocular tension.
Persistent high intraocular tension could damage the eyeball and
visual function partially. The patients could even lose complete
vision, if left untreated (1).
Glaucoma leads to blindness, with total incidence rate of 1%, which
gradually increases with age (2). As
a severe eye disease, the increased intraocular tension causes
optic disk (named previously as optic nerve head) depression and
visual field defect, leading to blindness. The normal intraocular
tension ranges from 10–21 mmHg (Schiotz tononometer) and above 24
mmHg is the clear indication of pathological phenomenon. Increased
intraocular tension could cause visual function damage with visible
typical glaucomatous changes in view (3). The longer the time of increased
intraocular tension lasts, the more serious is the visual function
damage. The prime reason is the disruption of dynamic equilibrium
of the aqueous fluid circulation (4). Heat shock proteins (HSPs) are a kind of
heat stress protein among germs and mammals (5). When an organism is exposed to high
temperature, the proteins would be compounded and activated for
protection. A recent research found that the expression level of
HSPs in retina may increase or decrease during the course of
glaucoma (6), however, the study of
heat shock protein 72 (HSP72) is not clear. The present study
analyzed and measured the HSP72 expression of retinal ganglion
cells (RGCs) from normal and glaucoma models in rats. It also
further verified the specific expression of HSP72 in glaucoma model
in rats in terms of protein levels and explored the potential
relation between HSP and glaucomatous optic neuropathy.
Materials and methods
Rats and groups
A total of 50 Wistar rats (Animal Center of Wuhan
University, Wuhan, China) of either sex that weighed between
200–300 g were selected and randomly divided into the high
intraocular tension group (25 rats) and the sham control group (25
rats). The right eyes were regarded as experimental eyes, while the
left eyes as blank control eyes.
Main experimental apparatus
Zeiss ophthalmic operating microscope (Carl Zeiss
AG, Oberkochen, Germany), intraocular microscissors, microscopical
toothed forceps, underwater electrocoagulator were procured from
Karl-Storz GmbH & Co. KG (Tuttlingen, Germany). Anterior
chamber stab knife was from Alcon Laboratories, Inc. (Fort Worth,
TX, USA) and TONO-PEN XL Tonometer was procured from Mentor Co.
(New York, NY, USA). Leica microtome (Leica, Mannheim, Germany) was
used with a special in-house no. 9 infusion needle.
Main reagents
HSP72 monoclonal antibody, ready-to-use
immunohistochemical Elivision™Plus kits of the second generation
and diaminobenzidine (DAB) chromogenic kit, were procured from
Fuzhou Maixin Biological Technology Co., Ltd. (Fuzhou, China).
Establishment of glaucoma models
Related surgical instruments were treated for
conventional disinfection. The rats were kept in a quiet and dark
environment for a week. Surgical methods: a total of 30
g·l−1 pentobarbital sodium was used for intra-peritoneal
injection anesthesia by 30 mg·kg−1. After anesthesia
took effect, the rats were fastened to operating table. A total of
5 g·l−1 dicaine was dropped to the operative eyes and
bulbar conjunctiva was cut 720° along corneal limbus in order to
separate and expose the veins of scleral surface. In the high
intraocular tension group, at least three groups of veins from rat
sclera surface and corneal limbus peripheral vessels were conducted
with electrocoagulation by underwater electrocoagulator, until
vascular tissue became white. In the sham control group, cut bulbar
conjunctiva without the electrocoagulation of sclera surface veins
and corneal limbus peripheral vessels. The operative eyes were
smeared with erythromycin eye ointment. After operation, the rats
were placed at room temperature and were allowed to wake up
naturally. Five days after operation, 2.5 g·l−1
chloramphenicol eye drops (3 times/day) and erythromycin eye
ointment (once a day) were used daily. This study was approved by
the Animal Ethics Committee of Animal Center of Wuhan
University.
Measurement of intraocular
tension
The intraocular tension of all rats was measured
after 1, 2, 3, 4 and 8 weeks of operation, respectively. Inspection
methods: a total of 30 g·l−1 pentobarbital sodium was
used for intraperitoneal injection for anesthesia. After anesthesia
took effect, the nib of tonometer TONO-PEN XL impinged on cornea
and the readings were recorded 3–5 times. The unreliable readings
of contact or departure from cornea were discarded and the average
was taken as the measured value.
Eyecup preparation
After anesthesia, chest skin and thoracic tissue
were cut to expose the heart. Left ventricular was cut off and a
no. 9 infusion needle was inserted to aorta ascendens for
perfusion. Aorta was clipped by haemostatic forceps and fixed with
an infusion needle together. At first, 100 ml normal saline (NS) of
9 g·l−1 was injected at the speed of 40
ml·min−1, and then about 200 ml (avoiding RGC
degeneration) triformol of 40 g·l−1 was injected quickly
until the rats became stiff. Complete eyeballs of rats were taken
out and placed into the penicillin bottle with numbers that
contained triformol at a dose rate of 40 g/l. After 30 min,
anterior chamber was punctured by stab knife. After 2 h, cornea and
crystalline lens were picked out (rats with few vitreous body) to
make eyecups. Obvious amotio retinae were avoided when taking out
the crystalline lens. Eyecups were put into the same bottle and
fixed for 4–6 h. After eyecups were picked out, graded alcohol
dehydration, xylene transparency and paraffin embedding were
conducted successively for H&E staining and
immunohistochemistry.
H&E staining
Paraffin sections were cut vertically along optic
nerve long axis with section thickness of 5 µm. One section was cut
from each paraffin block for H&E conventional staining. The
organization structure of retina in rats was observed under an
optical microscope.
Immunohistochemical staining
Paraffin section proceeded with deparaffination and
hydration, which was incubated at room temperature for 10 min after
adding 30 gl−1 H2O2, to interdict
the activity of endogenous peroxidase. The ready-to-use HSP72
monoclonal primary antibodies were followed by addition of PBS and
incubation under 37°C for 60 min. Polymer enhancer was added and
incubated at room temperature for 20 min. Enzyme-labeled
anti-rat/rabbit polymer was added and incubated at room temperature
for 30 min. Freshly prepared DAB developing solution was added and
color development was performed for 3–10 min, Then followed the
treatment with hematoxylin for 40 sec; 1g·l−1
hydrochloric acid alcohol was used for differentiation for 2 sec,
then washing in water for 15 min. After washing, gradient alcohol
dehydration, xylene transparency and neutral balsam mounting were
performed step by step.
Counting methods of HSP72 positive
cells in retina
The main signal of HSP72 positive expression is
brown cytoplasm. Three sections were taken from each paraffin block
under 40X objective lens and 5–8 views of retina cells were evenly
distributed and selected from each section. Two examiners in the
pathological teaching and research office were responsible for the
counting of positive and negative cells and for the calculation of
positive cell rates.
Western blot analysis
With removed eyeballs and peeled retina, the protein
of retina was extracted from the homogenate, and western blot
analysis was performed according to the standard protocol. The
densities of light bands were analyzed quantitatively by Scion
image software (Scion Corp., Frederick, MD, USA).
Statistical analysis
Intraocular tension results were analyzed by SPSS
10.0 (SPSS, Inc., Chicago, IL, USA) one-way ANOVA, and the positive
cell rate of retina was detected by χ2. P<0.05 is
considered to be statistically significant.
Results
Detection of intraocular tension after
model creation
One week after operation, the intraocular tension
was stable, 1, 2, 3, 4 and 8 weeks after operation, the comparison
of intraocular tension among the right eyes of the model group
revealed no significant difference (P>0.05); postoperative
right-eye intraocular tensions of the sham control group at
different time points were compared with those of its left eyes,
without significant difference (P>0.05). At least three groups
of veins of rat sclera surface and corneal limbus peripheral
vessels were applied for electrocoagulation. The venous return of
aqueous humor was reduced and intraocular tension was increased
persistently. In this way, the glaucoma model was created
successfully (Table I).
 | Table I.Intraocular tensions of different
groups of rats (mmHg). |
Table I.
Intraocular tensions of different
groups of rats (mmHg).
|
| Increased intraocular
tension (n=25) | Sham operation group
(n=25) |
|---|
|
|
|
|
|---|
| Operation time
(week) | Right eyes | Left eyes | Right eyes | Left eyes |
|---|
| Baseline | 15.89±3.16 | 15.83±2.83 | 16.38±2.87 | 15.88±2.63 |
| 1 |
30.12±5.18a | 15.93±3.33 | 16.33±2.88 | 15.87±2.97 |
| 2 |
32.51±5.56a | 16.12±3.11 | 16.42±3.28 | 15.86±3.14 |
| 3 |
31.77±4.43a | 16.32±3.48 | 16.33±3.96 | 15.94±2.99 |
| 4 |
33.07±5.89a | 15.78±2.48 | 17.12±2.92 | 16.15±3.23 |
| 8 |
34.22±7.28a | 16.13±1.87 | 17.11±3.37 | 16.77±3.28 |
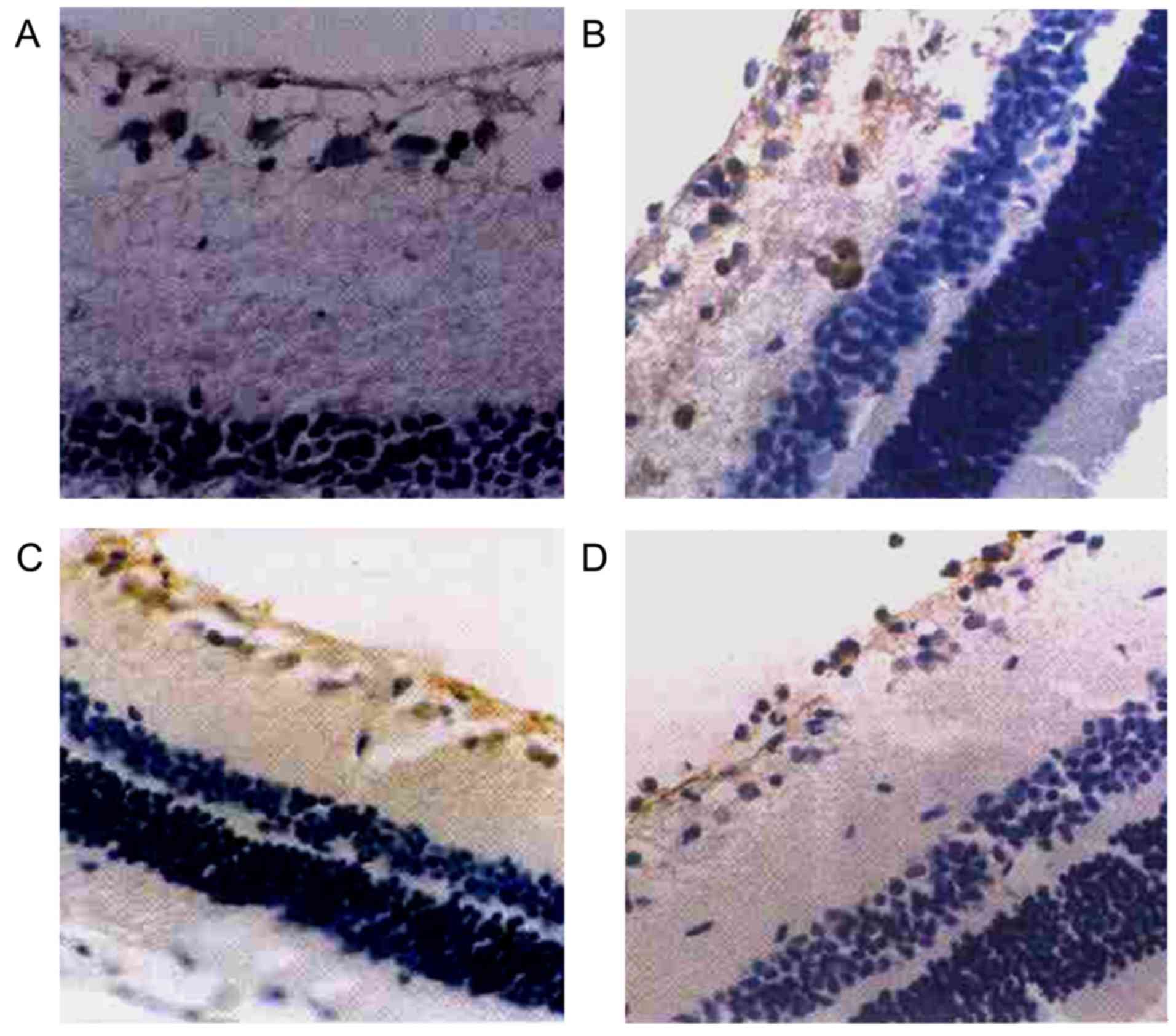
Positive expression of HSP72 in retina
as detected by immunohistochemical staining
As intraocular tension increased and high
intraocular tension persisted, the positive expression of HSP72 in
the retina of the right eyes in the high intraocular tension group
mainly manifested in RGCs and retinal nerve fiber layer (RNFL). RGC
cytoplasm showed as clay bank and a few RGC colored karyon. There
was no obvious positive expression of HSP72 in other layers of
retina (Table II, Fig. 1).
 | Table II.Positive expression of HSP72 in retina
detected by immunohistochemical staining. |
Table II.
Positive expression of HSP72 in retina
detected by immunohistochemical staining.
|
| Right
eyesa | Left
eyesa | Right
eyesb | Left
eyesb |
|---|
|
|
|
|
|
|
|---|
| Operation time
(week) | Positive cell | Positive cell rate
(%) | Positive cell | Positive cell rate
(%) | Positive cell | Positive cell rate
(%) | Positive cell | Positive cell rate
(%) |
|---|
| 1 | 252 | 16.45c | 26 | 1.76 | 17 | 0.97 | 14 | 0.88 |
| 2 | 367 | 17.91c | 34 | 2.15 | 19 | 0.99 | 16 | 0.93 |
| 3 | 468 | 29.83c | 45 | 2.58 | 16 | 1.09 | 17 | 0.96 |
| 4 | 434 | 31.22c | 49 | 2.65 | 21 | 1.35 | 20 | 1.12 |
| 8 | 523 | 41.16c | 53 | 3.12 | 22 | 1.6 | 21 | 1.73 |
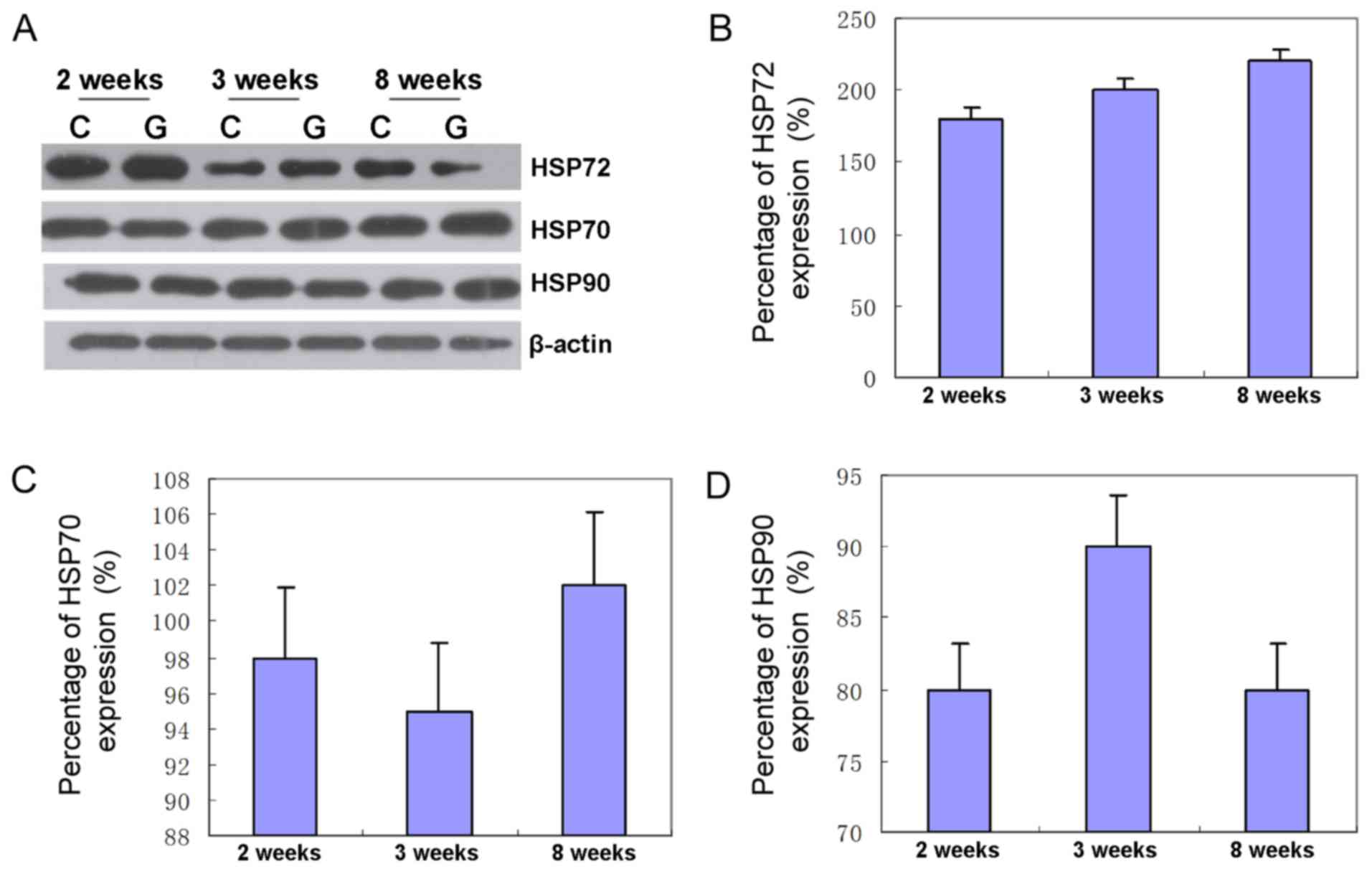
Western blot analysis
The expression of HSP72 in high intraocular tension
increased by 186% for the second week, 196% for the third week and
208% for the eighth week in comparison with the sham group
(Fig. 2). Furthermore, the
expression of HSP70 in high intraocular tension increased by 95%
for the second week, 91% for the third week and 109% for the eighth
week as compared to the sham control group. Protein levels of HSP70
in retina increased by 98.3% on average, showing that the protein
level of HSP70 was unchanged (Fig.
2). Compared with the sham control group, the expression of
HSP90 in high intraocular tension increased by 82% for the second
week, 87% for the third week and 87% for the eighth week. Protein
levels of HSP90 in retina increased by 82.6% on average, showing
that the protein level of HSP90 was unchanged (Fig. 2).
Discussion
Glaucoma is a serious optic neuropathy. Among the
patients with glaucoma, reduction of the intraocular tension could
improve the clinical symptom, but optic neuropathy may continue to
develop. Research has found that the death mechanism of RGC is RGC
apoptosis. Moreover, lack of multiple trophic factors, ischemia and
the toxic effect of excitatory amino acid may be the pathology and
pathophysiology factors that induce apoptosis (2). Many researchers are seeking for a
method that could block or delay the primary and/(or) secondary
damage of ganglion cells. At present, the therapeutic methods of
glaucoma optic nerve protection include glutamic acid antagonist,
calcium antagonist, antioxidant, NO enzyme inhibitor, neuro-trophic
factors, vaccination and ganglion cell apoptosis inhibition
(7).
HSP is a group of polypeptides with a highly
conservative structure. When an organism encounters various stress
stimulation, such as ischemia, high temperature, tissue injury and
excitatory toxin, leading to the change of gene expression, it
induces the generation of HSP, so that the endogenous protection
mechanism could be triggered (4).
Therefore, HSP is identified as a neuro-protective agent. However,
the exact neuro-protective mechanism induced by stress is not
clear. According to molecular weight, HSP could be divided into
HSP70, HSP90, and micromolecule HSP (8–11). The
molecular weight of inducible HSP72 is 72 kDa, so it is also known
as HSP72, with low expression level in non-stress state, but the
expression level would markedly increase in stress state to protect
during injury or repair (4,12,13). It
has been shown (14) that HSP72
could protect neural pathways by interdicting the activity of
apoptosis pathway SAPK/JNK in RGCs and lateral geniculate body
nerve cells (5). HSP72 is considered
as an apoptosis inhibitor. Pathological change, namely increased
intraocular tension is regarded as one of the main risk factors of
glaucoma. There are three methods to increase the intraocular
tension of glaucoma models experimentally (6). However, most of them could cause
obvious inflammatory reaction, with sudden increment for short
persistent period. This study applied at least three groups of
vortex veins of scleral surface and corneal limbus peripheral
vessels by underwater electrocoagulator, so as to observe the
influence of intraocular tension on HSP72 as much as possible.
After operation, the inflammatory response of operative eyes was
light. In addition, the duration of high intraocular tension was
long and stable. This basically coincided with the results of
increased intraocular tension that applied scleral surface and
corneal limbus peripheral vessels by laser photocoagulation
(15,16). As intraocular tension increased, high
intraocular tension persisted. The high intraocular tension group
was found with increased HSP72 expression in RGC of the right eyes
and positive cells of RGC (1), but
there was no significant difference of HSP72 expression in glaucoma
with normal intraocular tension and primary open angle glaucoma.
So, the increased expression of HSP72 in glaucoma retina had no
relation with intraocular tension. However, it is associated with
damage of optic nerve tissue during glaucoma. So, it could be
suggested that HSP72 may play a protective role in the injury of
glaucoma optic nerve for cells (1).
Moreover, the intensity of HSP72 immuno-staining had regional
difference in retina, as well as in single RGC. It seems that the
priority reaction to stress is in accordance with the mode of optic
nerve defect of glaucoma. In the study conducted by Qing et
al, the solution of exogenous HSP72 was injected to vitreous
cavity of the Wistar rats of ischemia for 60 min and HSP72 was
imported to RGC by electroporation (17). It was observed that exogenous HSP72
could enhance the ability to resist RGC apoptosis induced by
ischemia and reperfusion injury. The processing of rat cells with
zinc induced generation and secretion of HSP72. This played an
important role in rat acute glaucoma model RGCs. So, it could serve
as one of the clinical treatment methods for acute glaucoma, and
its effective mechanism lies in increasing the expression level of
endogenous HSP72.
By creating a glaucoma animal model, this study
verified that the expression of HSP72 mainly increased in RGC.
Furthermore, the nerve fiber layer of glaucoma may play a
protective role for RGC in glaucoma, which will undoubtedly become
a significant adjuvant therapeutic measure for glaucoma. Further
study of the neuroprotective effect of HSP72 in glaucomatous optic
neuropathy may bring about a broad prospect for the effective
treatment of glaucoma. Collectively, we believed that the enhanced
expression of endogenous HSP72 may play an important role in
glaucomatous optic neuro-protection.
References
|
1
|
Sleath B, Sayner R, Vitko M, Carpenter DM,
Blalock SJ, Muir KW, Giangiacomo AL, Hartnett ME and Robin AL:
Glaucoma patient-provider communication about vision
quality-of-life. Patient Educ Couns. Nov 22–2016.(Epub ahead of
print).
|
|
2
|
Shim MS, Kim KY and Ju WK: Role of cyclic
AMP in the eye of glaucoma. BMB Rep. 50:60–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giannaccare G, Vagge A, Gizzi C, Bagnis A,
Sebastiani S, Del Noce C, Fresina M, Traverso CE and Campos EC:
High-intensity focused ultrasound treatment in patients with
refractory glaucoma. Graefes Arch Clin Exp Ophthalmol. 255:599–605.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tosaka K, Mashima Y, Funayama T, Ohtake Y
and Kimura I; Glaucoma Gene Research Group, : Association between
open-angle glaucoma and gene polymorphism for heat-shock protein
70–1. Jpn J Ophthalmol. 51:417–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak A, Szaflik JP, Gacek M,
Przybylowska-Sygut K, Kamińska A, Szaflik J and Majsterek I: BDNF
and HSP gene polymorphisms and their influence on the progression
of primary open-angle glaucoma in a Polish population. Arch Med
Sci. 10:1206–1213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urbak L and Vorum H: Heat shock proteins
in the human eye. Int J Proteomics. 2010:4795712010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokoyama A, Oshitari T, Negishi H, Dezawa
M, Mizota A and Adachi-Usami E: Protection of retinal ganglion
cells from ischemia-reperfusion injury by electrically applied
Hsp27. Invest Ophthalmol Vis Sci. 42:3283–3286. 2001.PubMed/NCBI
|
|
8
|
Birnbaum G: Stress proteins: Their role in
the normal central nervous system and in disease states, especially
multiple sclerosis. Springer Semin Immunopathol. 17:107–118. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehlen P, Schulze-Osthoff K and Arrigo AP:
Small stress proteins as novel regulators of apoptosis. Heat shock
protein 27 blocks Fas/APO-1- and staurosporine-induced cell death.
J Biol Chem. 271:16510–16514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yenari MA, Giffard RG, Sapolsky RM and
Steinberg GK: The neuroprotective potential of heat shock protein
70 (HSP70). Mol Med Today. 5:525–531. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
WoldeMussie E, Ruiz G, Wijono M and
Wheeler LA: Neuroprotection of retinal ganglion cells by
brimonidine in rats with laser-induced chronic ocular hypertension.
Invest Ophthalmol Vis Sci. 42:2849–2855. 2001.PubMed/NCBI
|
|
12
|
Yamashima T: Hsp70.1 and related lysosomal
factors for necrotic neuronal death. J Neurochem. 120:477–494.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sohn S, Im JE, Kim TE and Kee C: Effect of
heat shock protein 72 expression on etoposide-induced cell death of
rat retinal ganglion cells. Korean J Ophthalmol. 27:48–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Li Y and Duan X: Heat shock protein
72 confers protection in retinal ganglion cells and lateral
geniculate nucleus neurons via blockade of the SAPK/JNK pathway in
a chronic ocular-hypertensive rat model. Neural Regen Res.
9:1395–1401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tezel G, Hernandez R and Wax MB:
Immunostaining of heat shock proteins in the retina and optic nerve
head of normal and glaucomatous eyes. Arch Ophthalmol. 118:511–518.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nickells RW: Retinal ganglion cell death
in glaucoma: The how, the why, and the maybe. J Glaucoma.
5:345–356. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qing G, Duan X and Jiang Y: Induction of
heat shock protein 72 in RGCs of rat acute glaucoma model after
heat stress or zinc administration. Yan Ke Xue Bao. 20:30–3.
2004.PubMed/NCBI
|