Introduction
Polycystic ovary syndrome (PCOS) is a very common
reproductive endocrinological disorder seen in women, affecting
5–20% of the reproductive age women globally (1). Insulin resistance (IR) and associated
metabolic abnormalities appear to play a significant role in the
development of PCOS and in sustaining this disorder (2,3). A vast
majority of the affected women also show hyperinsulinemia,
developed as a compensatory physiological body need, which in
itself contributes to several problems including overweight.
Hyperinsulinemia in these patients contributes to the development
of metabolic syndrome (MetS), which is a composite of type 2
diabetes, atherosclerosis, obesity and cardiovascular disorders
(4,5). The precise etiology of PCOS remains
unclear. However, it is suggested that the primary defect lies at
the ovarian level or may be a manifestation of hyperinsulinemia
that drives elevated androgen production (6). Hyperandrogenism in association with
ovulatory dysfunction and polycystic ovarian morphology (PCOM) are
common features of PCOS, with the ovaries producing large
quantities of androgens (1). This is
also accompanied by menstrual disorders (oligo-amenorrhea)
(5). The following criteria have
been established by several health agencies across the world
(National Institutes of Health, European Society of Human
Reproduction and Embryology, and American Society of Reproduction
Medicine) for the proper diagnosis of PCOS, after eliminating the
possibility of other diseases. On the basis of these
recommendations, at least two of the following three diagnostic
criteria are required for diagnosing PCOS: hyperandrogenism,
oligo-anovulation, and polycystic morphology of at least one ovary,
as ascertained by ultrasound (minimum 12 follicles of 2–9 mm in
diameter or ≥10 cm3 ovarian volume). Depending on the
presence or absence of ovulation disorders, the phenotypes of PCOS
have been separated as the classic PCOS (hyperandrogenism and
chronic anovulation, and presence or absence of PCOS) and PCOS with
ovulation disorders and polycystic morphology, with IR being
evident in both phenotypes (1,5,7).
Apparently, the incidence of MS among PCOS patients
seems to be affected also by the geographical region as well as the
habits of the patients as it has been recently shown that in Iran
the incidence of MS in the Iranian PCOS patients (19.7%) is less
than that seen in United States (33–46%) (8), India (9)
and Brazil (10) and its incidence
increases with age and body mass index (BMI), with the most
prevalent condition being low/high density lipoprotein-cholesterol
(11). On the other hand, the
incidence of MS was reported to be lower among European women with
PCOS (12,13). It has been suggested that these
differences may be due to differences in the criteria used to
diagnose MS in these studies. In this review, we have summarized
the current knowledge regarding the association of MetS and PCOS
and the resulting complications in pregnancy.
PCOS and obesity
It is well-known that there is elevated risk for
type 2 diabetes mellitus, gestational diabetes and other
pregnancy-related complications including venous thromboembolism,
cerebrovascular and cardiovascular events and endometrial cancer in
patients with PCOS (1). The chances
of developing MS in PCOS women was shown (8) to increase by almost 14-fold in patients
with BMI in the highest quartile (≥30) as compared to those with
BMI in the lowest quartile (<25). Fasting insulin level was
found to be elevated even in PCOS women without evident MS and it
was suggested that the elevated insulin contributes to the elevated
androgen production by the ovaries and other complications. Several
studies indicated that as much as 60–95% of PCOS women show IR,
which becomes aggravated if accompanied by increased abdominal fat
(14,15). However, IR in PCOS women cannot be
completely explained by abdominal adiposity and several other
factors such as defective glucose, lipid and steroid metabolism,
dysregulated insulin signaling and altered adipokine secretion also
likely contribute to IR (16). IR
and elevated circulating insulin were found to stimulate the theca
cells of ovaries to produce and secrete androgens and also to
enhance the responsiveness of ovaries to luteinizing hormone (LH)
to produce androgens (5,17). In fact, it has been noted that even
in the absence of overt obesity, there can be preferential
deposition of fat intra-abdominally in PCOS women with normal body
weight. This intra-abdominal fat leads to elevated number of small
subcutaneous abdominal adipocytes, which contribute to impaired
insulin action and thus functional IR and hyperandrogenism
(18). Decreased ability of
intra-abdominal subcutaneous adipocytes to store and sequester fat
in normal weight PCOS women leads to ectopic fat deposition in
other tissues such as muscle and liver, and this exerts
lipotoxicity and associated IR, contributing to hyperandrogenism
(19,20). On the other hand, in overweight PCOS
women, adipocytes in the subcutaneous abdominal adipose are large
and are not responsive to insulin regulated glucose utilization and
also to catecholamine controlled lipolysis and these changes are
thought to be androgen-mediated (21).
Hyperandrogenism and IR in PCOS
The association between hyperandrogenism and PCOS
stemmed from the observations that elevated levels of free
testosterone in plasma of hirsute amenorrheic women actually
originate from ovaries (22) and
that administration of testosterone resulted in polycystic ovaries
in female-to-male transsexuals (23). Those findings led to the hypothesis
that hyperandrogenism leads to PCOS. In addition, evidence was
presented in some studies that IR is related to hyperandrogenism
(24) and that insulin addition to
ovaries, in vitro, stimulates them to produce androgens
(25) as well as LH (26). These results led to the proposal that
hyperinsulinemia as seen in IR conditions contributes to excess
androgen production by ovaries. Many of the hyperandrogenic women
with classic PCOS display ovarian steroid hyper-responsiveness
without any steroidogenic block and also dysregulation of
cytochrome p450-c17α-hydroxylase (27). This type of ovarian dysfunction is
known as ‘functional ovarian hyperandrogenism’, as the
steroidogenic response is gonadotropin-dependent (28). Notably, even though the peripheral
tissues such as muscle and liver are insulin resistant in PCOS
women, the ovaries are very much responsive to both
hyperinsulinemia and LH to produce androgens.
Functional ovarian hyperandrogenism was suggested to
be a consequence of IR, which causes elevated oxidative stress
accompanied by lower antioxidant status in ovaries (29). Increased oxidative stress was found
to correlate directly with IR as well as serum testosterone and
androstenedione levels (30). IR
appears to activate the critical enzyme responsible for the
synthesis of androgens, cytochrome p450-c17α-hydroxylase, in
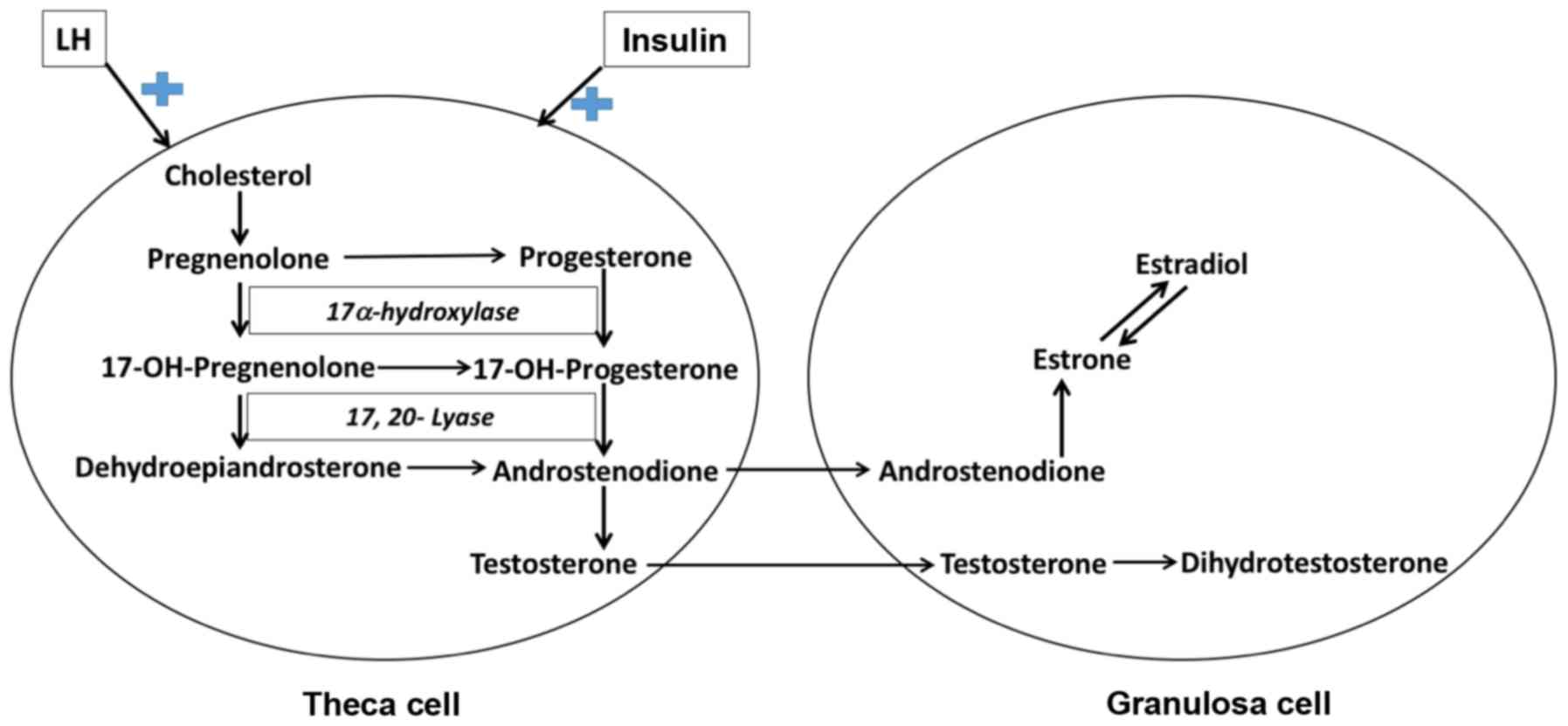
ovarian theca cells (Fig. 1),
resulting in hyperandrogenism, despite elevated or normal LH
secretion (31). Theca cells
isolated from polycystic ovaries of classic PCOS patients display
elevated expression of several steroidogenic enzymes, specifically,
cytochrome P450c17 following long-term cell culture, and also show,
that it is characteristic of functional ovarian hyperandrogenism
(32). Formation of androgens is
controlled by the cytochrome P450c17 enzyme in gonads and also
adrenal cortex and its expression is dependent on LH stimulation in
ovaries and ACTH in adrenal cortex. Cytochrome P450c17 possesses
two activities essential for the generation of androgens:
17-hydroxylase, which converts pregnenolone to
17-hydroxypregnenolone, which is then converted by the 17,20 lyase
activity to dehydroepiandrosterone. Dehydroepiandrosterone in turn
gives rise to androstenodione and sex steroids. In the theca cells
of ovary, cytochrome P450c17 can also convert progesterone to
androstenodione and sex steroids (32). A recent study indicated that in PCOS
women with functional ovarian hyperandrogenism, elevated LH:FSH
ratio, enhanced oxidative stress and increased levels of free (not
total) testosterone correlate with each other positively and that
the elevated LH:FSH ratio is a better predictive biomarker for the
onset of PCOS in women with functional ovarian hyperandrogenism
(30). Elevated levels of androgens
cause an inhibition of folliculogenesis that in turn leads to
polyfollicular morphology, disturbing menstrual cycle and
anovulatory infertility (33).
Apparently, androgens have complex effects on folliculogenesis at a
concentration of 10 ng/ml, testosterone positively affects
preantral follicle growth, whereas, when testosterone concentration
is elevated to 50 ng/ml, levels seen in the hyperandrogenemia
conditions, there is a strong blockade of the follicle growth
(34). Testosterone and other
androgens can also be synthesized by adrenal cortex, under the
stimulation of ACTH and approximately 25% of the functional ovarian
hyperandrogenism cases are actually due to primary functional
adrenal hyperandrogenism and contribute to PCOS (35). Thus overall evidence suggests that
functional ovarian hyperandrogenism is the underlying causative
factor for PCOS and accounts for many of the clinical features of
PCOS such as anovulation, hirsutism and polycystic ovaries. IR and
associated hyperinsulinemia aggravate the pathogenic effects of
hyperandrogenism. The elevated levels of LH, during the early
stages of hyperandrogenemia, accelerate the ovarian functional
deterioration, which is further aggravated by accompanying
hyperinsulinemia (Fig. 2) (32).
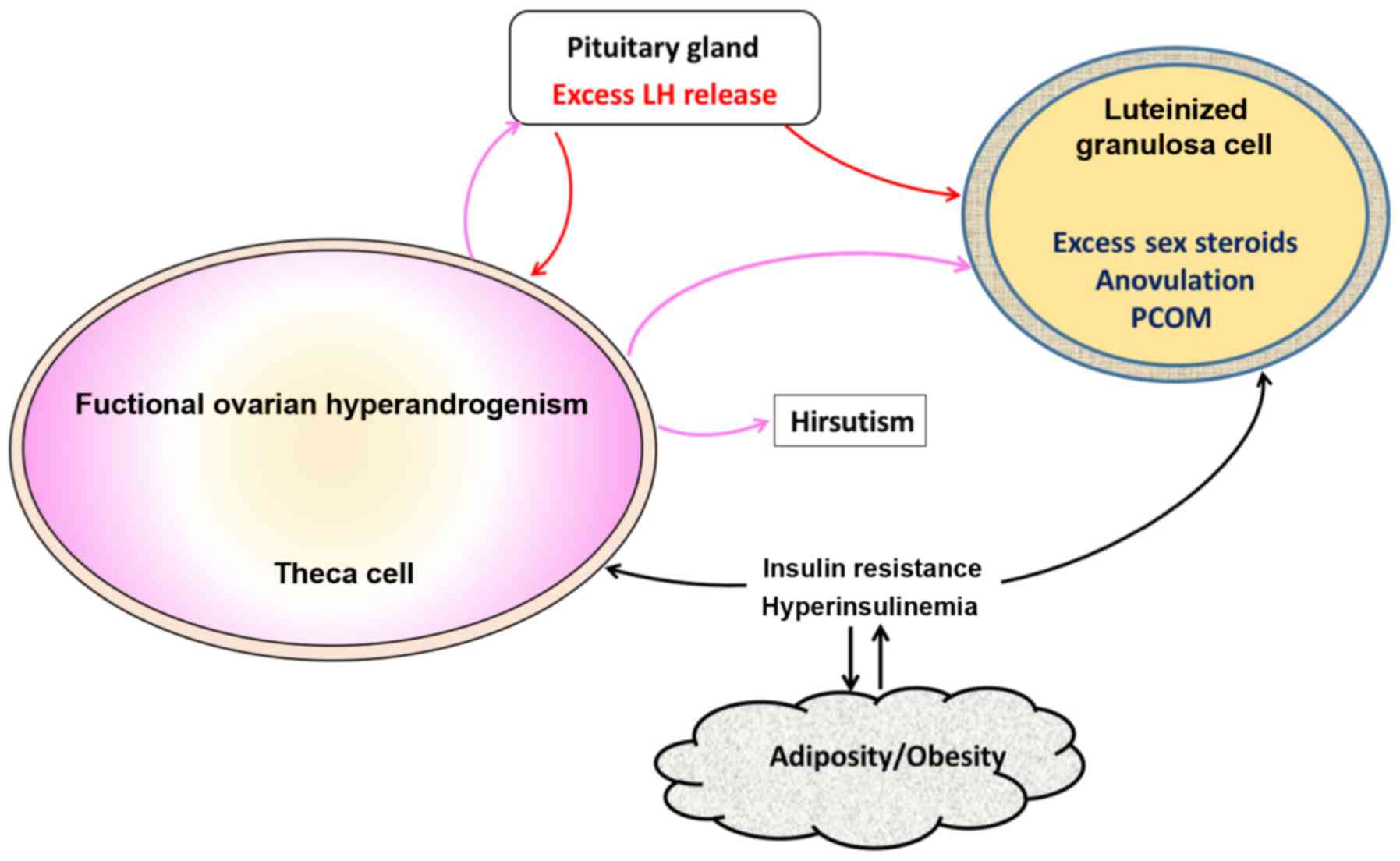 | Figure 2.Pathological events in PCOS. Ovarian
hyperandrogenism is very common in PCOS and contributes to several
abnormalities including hirsutism, oligo-anovulation, and PCOM. LH
secretion from pituitary gland is needed for the ovarian androgen
production, but other factors such as hyperinsulinism and obesity
are also necessary for full-blown pathogenesis of PCOS. Insulin
resistance, which is very common in PCOS leads to hyperinsulinemia,
which stimulates theca cells and aggravates hyperandrogenism.
Excess insulin along with androgen, luteinize granulosa cells
prematurely. Adipogenesis is another abnormality resulting from
hyperinsulinism. Elevated androgens coming from theca cells in turn
stimulate pituitary and cause LH excess, which worsens
hyperandrogenism. These changes in granulosa cells further
exacerbate PCOM and lead to oligo-anovulation. PCOS, polycystic
ovary syndrome; PCOM, polycystic ovarian morphology; LH,
luteinizing hormone. |
PCOS and gestational diabetes
Inasmuch as significant populations of PCOS women
are insulin resistant and hyperinsulinemic, they are highly prone
to develop type 2 diabetes. In fact, a major complication of PCOS
diagnosis in pregnancy is gestational diabetes (36) and several studies demonstrated
increased incidence of gestational diabetes in PCOS women (37,38) and
vice versa (39). However, few
studies suggested increased BMI to be a better predictor of
gestational diabetes than PCOS, raising questions on cause and
effect relationship between PCOS and gestational diabetes (40). The risk of developing gestational
diabetes in PCOS women is approximately three times greater, as
compared to non-PCOS women (41).
Treatment of pregnant PCOS women with metformin, an insulin
sensitizer drug, commonly used in type 2 diabetic patients, was
found to be beneficial as it reduced the high rates of miscarriage
usually seen in PCOS women and also the incidence of gestational
diabetes (42,43). Although its continuous use is
controversial (44), metformin is
prescribed to pregnant PCOS women to correct not only the metabolic
abnormalities and hyperinsulinemia, but also endocrine
disturbances, such as lowering LH and sex-hormone binding globulin
levels (45). Metformin is useful as
the first-line therapy in PCOS women for inducing ovulation
(46). Metformin was suggested to
protect against early pregnancy loss in PCOS women, by lowering
plasma androgen levels, probably secondary to reduction in insulin
levels (47). There is a need to
conduct randomized clinical trials with appropriate placebo
controls and blinding, with large cohorts, in order to ascertain
the beneficial effects of metformin in PCOS women, particularly
because this drug does not have any teratogenic effects and has no
adverse effects (45,46). It has been suggested that PCOS be
considered as a ‘prediabetic’ condition that is associated with
impaired glucose tolerance (with a prevalence of approximately 33%)
and because PCOS women with impaired glucose tolerance develop type
2 diabetes at 5- to 10-fold higher rate than women without PCOS
(48).
PCOS and MetS
MetS, also known as Syndrome X is a combination of
multiple conditions including central abdominal obesity,
hypertension, dyslipidemia and hyperglycemia, all of which are the
prime risk factors for cardiovascular diseases. People suffering
from MetS display varying degrees of these abnormalities, which
primarily result from complex multi-organ interactions of IR,
obesity and age (49). Inasmuch as
IR is almost globally present in PCOS women, it has been reported
that nearly 33% of adolescents with PCOS develop MetS and this
incidence increase to 50% with age in adults with PCOS (50,51). A
recent clinical study with 100 newly diagnosed PCOS women observed
that presence of at least two of the following three criteria,
viz, hyperandrogenism, oligo/anovulation and polycystic
ovaries, poses the highest risk for the development of MetS
(52). A recent study has proposed
that lipid accumulation product and visceral adiposity index are
better markers of IR risk for cardiovascular disease than simple
lipid ratios (53). There is a
school of thought that suggests that the metabolic abnormalities
associated with IR and obesity are probably more important,
mechanistically, than hyperandrogenemia for the anovulation in PCOS
women (54,55). There is some evidence suggesting
women with typical PCOS, i.e., associated hyperandrogenemia, have a
heritable component for β-cell defect and impaired glucose
tolerance (56). In PCOS women the
extent of IR is often much higher than what is anticipated on the
basis of existing adiposity in them. The tissue selective IR
together with hyperinsulinism is a major extraovarian contributory
factor for the pathogenic alterations seen in PCOS ovaries. Insulin
function is preserved in the ovaries of PCOS women despite a state
of IR in the body and insulin signaling via insulin receptor in the
theca cells appears to mediate the steroidogenic and androgenic
effects of insulin (57).
Besides the known association of type 2 diabetes
with PCOS as mentioned above, type 1 diabetes also appears to be
associated with PCOS and this is particularly because of the very
high doses of insulin administered to these patients systemically,
for controlling hyperglycemia (58).
Almost all the approaches that are commonly employed to correct
insulin homeostasis in MS and obese patients, such as lifestyle
modification for weight reduction, bariatric surgery,
thiazolidinediones and metformin have beneficial effects on
ovulation and also control hyperandrogenemia in PCOS women
(32). Besides these drugs, of which
thiazolidinediones are seldom used, there are inconsistent reports
showing efficacy of myo-inositol in improving ovulation and other
ovarian functions in PCOS women (59). An important characteristic of MS, the
central abdominal obesity, and also occasionally pseudo-Cushing
syndrome are noted in PCOS women and appear to be due to
hyperinsulinism and possibly also through stimulation of
glucocorticoid action and associated β-cell dysfunction (60). Thus, the overall interrelationship
between obesity, IR and hyperandrogenemia together with LH drive
the pathogenesis of PCOS.
Conclusion
PCOS is a very common endocrine disorder affecting a
significant proportion of women worldwide and yet there is no
effective treatment for this disease. PCOS poses significant risk
to pregnant women for loss of pregnancy and/or other associated
disorders such as pre-eclampsia and gestational diabetes. PCOS
encompasses disturbances in several hormones, including insulin,
androgens, and LH. Functional ovarian hyperandrogenism of ovaries,
is a consequence of IR, and develops by the activation of androgen
synthesis and contributes to PCOS pathogenesis. Despite several
common conditions such as diabetes, IR and MetS, seen in non-PCOS
obese women, the treatment options available for non-PCOS women do
not always work effectively in PCOS women, because of the
complexity of the disease. Typical insulin sensitizing drugs such
as metformin, have been tried to curtail IR and hyperinsulinemia in
pregnant PCOS women, with varying results indicating the complexity
of the disease and the need for better controlled studies and
additional efforts for PCOS specific drug discovery.
Acknowledgements
This study was suppoted by Scientific Research
Project of Sichuan Medical Association (S16053).
References
|
1
|
Azziz R, Carmina E, Chen Z, Dunaif A,
Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ and
Yildiz BO: Polycystic ovary syndrome. Nat Rev Dis Primers.
2:160572016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amato MC, Vesco R, Vigneri E, Ciresi A and
Giordano C: Hyperinsulinism and polycystic ovary syndrome (PCOS):
role of insulin clearance. J Endocrinol Invest. 38:1319–1326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diamanti-Kandarakis E and Dunaif A:
Insulin resistance and the polycystic ovary syndrome revisited: an
update on mechanisms and implications. Endocr Rev. 33:981–1030.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ford ES: The metabolic syndrome and
mortality from cardiovascular disease and all-causes: findings from
the National Health and Nutrition Examination Survey II Mortality
Study. Atherosclerosis. 173:309–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polak K, Czyzyk A, Simoncini T and
Meczekalski B: New markers of insulin resistance in polycystic
ovary syndrome. J Endocrinol Invest. 40:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kauffman RP, Baker VM, DiMarino P and
Castracane VD: Hyperinsulinemia and circulating
dehydroepiandrosterone sulfate in white and Mexican American women
with polycystic ovary syndrome. Fertil Steril. 85:1010–1016. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fauser BC, Tarlatzis BC, Rebar RW, Legro
RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, et
al: Consensus on women's health aspects of polycystic ovary
syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS
consensus workshop group. Fertil Steril. 97:28–38.e25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrmann DA, Liljenquist DR, Kasza K, Azziz
R, Legro RS and Ghazzi MN; PCOS/Troglitazone Study Group, :
Prevalence and predictors of the metabolic syndrome in women with
polycystic ovary syndrome. J Clin Endocrinol Metab. 91:48–53. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhattacharya SM: Prevalence of metabolic
syndrome in women with polycystic ovary syndrome, using two
proposed definitions. Gynecol Endocrinol. 26:516–520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soares EM, Azevedo GD, Gadelha RG, Lemos
TM and Maranhão TM: Prevalence of the metabolic syndrome and its
components in Brazilian women with polycystic ovary syndrome.
Fertil Steril. 89:649–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madani T, Hosseini R, Ramezanali F,
Khalili G, Jahangiri N, Ahmadi J, Rastegar F and Zolfaghari Z:
Metabolic syndrome in infertile women with polycystic ovarian
syndrome. Arch Endocrinol Metab. 60:199–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vural B, Caliskan E, Turkoz E, Kilic T and
Demirci A: Evaluation of metabolic syndrome frequency and premature
carotid atherosclerosis in young women with polycystic ovary
syndrome. Hum Reprod. 20:2409–2413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmina E, Napoli N, Longo RA, Rini GB and
Lobo RA: Metabolic syndrome in polycystic ovary syndrome (PCOS):
lower prevalence in southern Italy than in the USA and the
influence of criteria for the diagnosis of PCOS. Eur J Endocrinol.
154:141–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stepto NK, Cassar S, Joham AE, Hutchison
SK, Harrison CL, Goldstein RF and Teede HJ: Women with polycystic
ovary syndrome have intrinsic insulin resistance on
euglycaemic-hyperinsulaemic clamp. Hum Reprod. 28:777–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moghetti P, Tosi F, Bonin C, Di Sarra D,
Fiers T, Kaufman JM, Giagulli VA, Signori C, Zambotti F, Dall'Alda
M, et al: Divergences in insulin resistance between the different
phenotypes of the polycystic ovary syndrome. J Clin Endocrinol
Metab. 98:E628–E637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumesic DA, Oberfield SE, Stener-Victorin
E, Marshall JC, Laven JS and Legro RS: Scientific statement on the
diagnostic criteria, epidemiology, pathophysiology, and molecular
genetics of polycystic ovary syndrome. Endocr Rev. 36:487–525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baptiste CG, Battista MC, Trottier A and
Baillargeon JP: Insulin and hyperandrogenism in women with
polycystic ovary syndrome. J Steroid Biochem Mol Biol. 122:42–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumesic DA, Akopians AL, Madrigal VK,
Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R,
Schooler TA, et al: Hyperandrogenism accompanies increased
intra-abdominal fat storage in normal weight polycystic ovary
syndrome women. J Clin Endocrinol Metab. 101:4178–4188. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Zegher F, Lopez-Bermejo A and Ibáñez L:
Adipose tissue expandability and the early origins of PCOS. Trends
Endocrinol Metab. 20:418–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unger RH, Clark GO, Scherer PE and Orci L:
Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim
Biophys Acta. 1801:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mannerås-Holm L, Leonhardt H, Kullberg J,
Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G,
Stener-Victorin E, et al: Adipose tissue has aberrant morphology
and function in PCOS: enlarged adipocytes and low serum
adiponectin, but not circulating sex steroids, are strongly
associated with insulin resistance. J Clin Endocrinol Metab.
96:E304–E311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosenfield RL, Ehrlich EN and Cleary RE:
Adrenal and ovarian contributions to the elevated free plasma
androgen levels in hirsute women. J Clin Endocrinol Metab.
34:92–98. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Futterweit W and Deligdisch L:
Histopathological effects of exogenously administered testosterone
in 19 female to male transsexuals. J Clin Endocrinol Metab.
62:16–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dunaif A, Segal KR, Futterweit W and
Dobrjansky A: Profound peripheral insulin resistance, independent
of obesity, in polycystic ovary syndrome. Diabetes. 38:1165–1174.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbieri RL, Makris A, Randall RW, Daniels
G, Kistner RW and Ryan KJ: Insulin stimulates androgen accumulation
in incubations of ovarian stroma obtained from women with
hyperandrogenism. J Clin Endocrinol Metab. 62:904–910. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cara JF and Rosenfield RL: Insulin-like
growth factor I and insulin potentiate luteinizing hormone-induced
androgen synthesis by rat ovarian thecal-interstitial cells.
Endocrinology. 123:733–739. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenfield RL, Barnes RB, Cara JF and
Lucky AW: Dysregulation of cytochrome P450c 17 alpha as the cause
of polycystic ovarian syndrome. Fertil Steril. 53:785–791. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ehrmann DA, Rosenfield RL, Barnes RB,
Brigell DF and Sheikh Z: Detection of functional ovarian
hyperandrogenism in women with androgen excess. N Engl J Med.
327:157–162. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
González F, Rote NS, Minium J and Kirwan
JP: Reactive oxygen species-induced oxidative stress in the
development of insulin resistance and hyperandrogenism in
polycystic ovary syndrome. J Clin Endocrinol Metab. 91:336–340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suresh S and Vijayakumar T: Correlations
of insulin resistance and serum testosterone levels with LH:FSH
ratio and oxidative stress in women with functional ovarian
hyperandrogenism. Indian J Clin Biochem. 30:345–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nisenblat V and Norman RJ: Androgens and
polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes.
16:224–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenfield RL and Ehrmann DA: The
pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of
pcos as functional ovarian hyperandrogenism revisited. Endocr Rev.
37:467–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azziz R: Diagnostic criteria for
polycystic ovary syndrome: a reappraisal. Fertil Steril.
83:1343–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodrigues JK, Navarro PA, Zelinski MB,
Stouffer RL and Xu J: Direct actions of androgens on the survival,
growth and secretion of steroids and anti-Müllerian hormone by
individual macaque follicles during three-dimensional culture. Hum
Reprod. 30:664–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azziz R, Carmina E, Dewailly D,
Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen
OE, Legro RS, Norman RJ, Taylor AE, et al: Task Force on the
Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess
and PCOS Society: The androgen excess and PCOS society criteria for
the polycystic ovary syndrome: the complete task force report.
Fertil Steril. 91:456–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Homburg R: Pregnancy complications in
PCOS. Best Pract Res Clin Endocrinol Metab. 20:281–292. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bjercke S, Dale PO, Tanbo T, Storeng R,
Ertzeid G and Abyholm T: Impact of insulin resistance on pregnancy
complications and outcome in women with polycystic ovary syndrome.
Gynecol Obstet Invest. 54:94–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weerakiet S, Srisombut C, Rojanasakul A,
Panburana P, Thakkinstian A and Herabutya Y: Prevalence of
gestational diabetes mellitus and pregnancy outcomes in Asian women
with polycystic ovary syndrome. Gynecol Endocrinol. 19:134–140.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anttila L, Karjala K, Penttilä RA,
Ruutiainen K and Ekblad U: Polycystic ovaries in women with
gestational diabetes. Obstet Gynecol. 92:13–16. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turhan NO, Seçkin NC, Aybar F and Inegöl
I: Assessment of glucose tolerance and pregnancy outcome of
polycystic ovary patients. Int J Gynaecol Obstet. 81:163–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD
and Chen HY: Obstetric complications in women with polycystic ovary
syndrome: a systematic review and meta-analysis. Reprod Biol
Endocrinol. 11:562013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Glueck CJ, Wang P, Goldenberg N and
Sieve-Smith L: Pregnancy outcomes among women with polycystic ovary
syndrome treated with metformin. Hum Reprod. 17:2858–2864. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Glueck CJ, Wang P, Kobayashi S, Phillips H
and Sieve-Smith L: Metformin therapy throughout pregnancy reduces
the development of gestational diabetes in women with polycystic
ovary syndrome. Fertil Steril. 77:520–525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghazeeri GS, Nassar AH, Younes Z and Awwad
JT: Pregnancy outcomes and the effect of metformin treatment in
women with polycystic ovary syndrome: an overview. Acta Obstet
Gynecol Scand. 91:658–678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nawaz FH, Khalid R, Naru T and Rizvi J:
Does continuous use of metformin throughout pregnancy improve
pregnancy outcomes in women with polycystic ovarian syndrome? J
Obstet Gynaecol Res. 34:832–837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Costello MF and Eden JA: A systematic
review of the reproductive system effects of metformin in patients
with polycystic ovary syndrome. Fertil Steril. 79:1–13. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jakubowicz DJ, Iuorno MJ, Jakubowicz S,
Roberts KA and Nestler JE: Effects of metformin on early pregnancy
loss in the polycystic ovary syndrome. J Clin Endocrinol Metab.
87:524–529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ben-Haroush A, Yogev Y and Hod M:
Epidemiology of gestational diabetes mellitus and its association
with type 2 diabetes. Diabet Med. 21:103–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rossi B, Sukalich S, Droz J, Griffin A,
Cook S, Blumkin A, Guzick DS and Hoeger KM: Prevalence of metabolic
syndrome and related characteristics in obese adolescents with and
without polycystic ovary syndrome. J Clin Endocrinol Metab.
93:4780–4786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Apridonidze T, Essah PA, Iuorno MJ and
Nestler JE: Prevalence and characteristics of the metabolic
syndrome in women with polycystic ovary syndrome. J Clin Endocrinol
Metab. 90:1929–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bil E, Dilbaz B, Cirik DA, Ozelci R,
Ozkaya E and Dilbaz S: Metabolic syndrome and metabolic risk
profile according to polycystic ovary syndrome phenotype. J Obstet
Gynaecol Res. 42:837–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Abruzzese GA, Cerrrone GE, Gamez JM,
Graffigna MN, Belli S, Lioy G, Mormandi E, Otero P, Levalle OA and
Motta AB: Lipid accumulation product (LAP) and visceral adiposity
index (VAI) as markers of insulin resistance and metabolic
associated disturbances in young argentine women with polycystic
ovary syndrome. Horm Metab Res. 49:23–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Turkmen S, Ahangari A and Bäckstrom T:
Roux-en-y gastric bypass surgery in patients with polycystic ovary
syndrome and metabolic syndrome. Obes Surg. 26:111–118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Legro RS, Dodson WC, Kris-Etherton PM,
Kunselman AR, Stetter CM, Williams NI, Gnatuk CL, Estes SJ, Fleming
J, Allison KC, et al: Randomized controlled trial of preconception
interventions in infertile women with polycystic ovary syndrome. J
Clin Endocrinol Metab. 100:4048–4058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Colilla S, Cox NJ and Ehrmann DA:
Heritability of insulin secretion and insulin action in women with
polycystic ovary syndrome and their first degree relatives. J Clin
Endocrinol Metab. 86:2027–2031. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu S, Divall S, Nwaopara A, Radovick S,
Wondisford F, Ko C and Wolfe A: Obesity-induced infertility and
hyperandrogenism are corrected by deletion of the insulin receptor
in the ovarian theca cell. Diabetes. 63:1270–1282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Codner E and Escobar-Morreale HF: Clinical
review: hyperandrogenism and polycystic ovary syndrome in women
with type 1 diabetes mellitus. J Clin Endocrinol Metab.
92:1209–1216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Facchinetti F, Bizzarri M, Benvenga S,
D'Anna R, Lanzone A, Soulage C, Di Renzo GC, Hod M, Cavalli P, Chiu
TT, et al: Results from the International Consensus Conference on
myo-inositol and d-chiro-inositol in obstetrics and gynecology: the
link between metabolic syndrome and PCOS. Eur J Obstet Gynecol
Reprod Biol. 195:72–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ehrmann DA, Breda E, Corcoran MC, Cavaghan
MK, Imperial J, Toffolo G, Cobelli C and Polonsky KS: Impaired
beta-cell compensation to dexamethasone-induced hyperglycemia in
women with polycystic ovary syndrome. Am J Physiol Endocrinol
Metab. 287:E241–E246. 2004. View Article : Google Scholar : PubMed/NCBI
|