Introduction
Acute myocardial infarction (MI) is a leading cause
of mortality and morbidity worldwide. When acute MI occurs, the
rapid restoration of coronary artery blood flow via thrombolytic
therapy or percutaneous coronary intervention is essential.
However, reperfusion itself can lead to myocardial injury and an
inflammatory response, which is called ischemia reperfusion (I/R)
injury (1). Therefore, attenuating
myocardial I/R injury is important in the treatment of acute
MI.
Hypoxia inducible factor (HIF)-1α is an important
transcription factor that serves an essential role in cellular
adaption to conditions of hypoxia and ischemia, which enables cells
to differentiate and survive under low oxygen conditions (2). Furthermore, HIF-1α can restore oxygen
homeostasis through the induction of glycolysis, erythropoiesis and
angiogenesis (3). A previous study
by our group demonstrated that increased myocardial expression of
HIF-1α is associated with cardioprotection against I/R injury
(4). Therefore, increasing
myocardial expression of HIF-1α may attenuate myocardial injury
following myocardial I/R.
High mobility group box 1 protein (HMGB1) is widely
expressed in various tissues, inclduing the liver, brain, spleen,
lung, heart and kidney, and can induce the production of
proinflammatory cytokines, including tumor necrosis factor (TNF)-α
and interleukin (IL)-6, in addition to acting as a proinflmmatory
cytokine itself (5). HMGB1 serves a
role in numerous cardiovascular diseases, inclduing
atherosclerosis, myocardial I/R injury, heart failure and MI
(6–10). Extracellular HMGB1 can recognize
tissue damage and initiate reparative responses, in addition to
participating in the pathogenesis of inflammation and enhancing
myocardial I/R injury (11,12).
p38 mitogen-activated protein kinase (p38 MAPK)
serves a role in the regulation of various cellular functions.
Phosphorylated p38 MAPK (P-p38 MAPK) is the active form of p38
MAKP. Inhibition of p38 MAPK activity can protect the heart from
I/R injury (13). However, the
association between p38 MAPK and HMGB1 in myocardial I/R injury is
not yet clear.
Accumulating evidence (14–16) has
demonstrated that the administration of HMGB1 after MI or acute
global I/R improves left ventricular function via cardiomyocyte
regeneration. Our group recently reported that intravenous HMGB1
protect the heart from I/R injury (17). However, a previous study identified
that increasing the dose of HMGB1 did not further recover heart
function, although it did inhibit inflammatory reactions (18). The majority of previous studies
(15,16) administered HMGB1 via direct
intramyocardial injection in various animal models.
Whether the cardioprotective effects of intravenous
infusion of HMGB1 on myocardial I/R injury are associated with the
myocardial expression of HIF-1α remains unclear. Furthermore, the
underlying molecular mechanisms by which intravenous HMGB1 protects
the heart from I/R injury remain to be identified. Thus, the
present study aimed to evaluate the effect of HMGB1 pretreatment on
the myocardial expression of HIF-1α and investigate the underlying
mechanisms of this effect in a rat model.
Materials and methods
Animal groups
All animal procedures were performed according to
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (19), and were approved by the Institutional
Review Board of Liaocheng People's Hospital (Liaocheng, China). The
rats were housed in a temperature controlled room (temperature,
22±1°C) under a 12 h light/dark cycle with free access to food and
water. Male Wistar rats, (n=50; weight, 250–300 g; aged 5–11 weeks)
were provided by Shandong Lukang Pharmaceutical Co., Ltd. (Jining,
China). The rats were divided into five groups (n=10/group) as
follows: i) Sham operation group (sham; administered 0.5 ml normal
saline intravenously only); ii) I/R group (administered 0.5 ml
normal saline intravenously; other treatment described below); iii)
HMGB50 group (50 ng/kg recombinant HMGB1 administered intravenously
30 min before ischemia; the recombinant HMGB1 was purchased in Sino
Biological Inc. (Beijing, China; 10326-H08H-50); iv) HMGB100 group
(100 ng/kg recombinant HMGB1 administered intravenously 30 min
before ischemia); and v) HMGB200 group (200 ng/kg recombinant HMGB1
administered intravenously 30 min before ischemia). All the HMGB
groups underwent the process of I/R.
Animal model of I/R
The rat I/R model was established according to the
method previously reported by our group (20). Briefly, after anesthesia with sodium
pentobarbital (60 mg/kg intraperitoneally), the rats were
artificially ventilated (55 breaths/min). A thermal pad was used to
maintain the rat's body temperature at 37±0.5°C. The rats then
underwent total ligation of the left anterior descending coronary
artery (LAD) for 30 min and subsequent reperfusion for 180 min.
Electrocardiography was used to record changes in the heart rate
and rhythm. The LADs of rats in the sham group were not occluded,
with only a suture placed at the origin of the LAD.
Biochemical analysis
Blood samples were collected from the femoral vein
and centrifuged at 1000 × g for 10 min at 4°C, and the serum
obtained was stored at −80°C until required. Serum cardiac troponin
I (cTnI), TNF-α and IL-6 levels were determined using ELISA kits
(cTnI, KL15219; Shanghai Kanglang Biotechnology Co., Ltd.,
Shanghai, China) (TNF-α, 69-30484; IL-6, 69-30490; Wuhan Moschak
Biotechnology Co., Ltd., Wuhan, China).
Measurement of myocardial
malondialdehyde (MDA) and superoxide dismutase (SOD) levels
The rats were sacrificed by decapitation after
anesthetization via intraperitoneal injection of phenobarbital (60
mg/kg). The hearts were harvested and washed with normal saline.
Ischemic heart tissue (0.5 g) was then homogenized at 0–4°C and the
homogenate was centrifugated at 1,200 × g for 30 min at 4°C. The
supernatant was obtained and stored at −80°C until required. The
thiobarbituric acid reactive substances assay was used to determine
the level of MDA and the xanthine oxide method was used to
determine SOD activity. The MDA assay kit (A003-1) and SOD assay
kit (A001-3) were purchased from NanJing JianCheng Bioengineering
Institute (Nanjing, China) and used according to the manufacturer's
protocol.
Assessment of infarction size
(IS)
IS was assessed by Evans blue dye and
2,3,5-triphenyltetrazolium chloride (TTC) staining methods as
described previously (21). Briefly,
after reperfusion, the LAD was occluded again and 1 ml 2.0% Evans
blue dye was injected intravenously. The heart was excised, rinsed
and the atria were trimmed off. The left ventricle was sliced
horizontally into five slices from apex to base. The thickness of
the slices were ~2 mm. The sections were incubated in 1% TTC for 15
min at 37°C. Impaired, infarcted and normal myocardium was stained
red, white and blue, respectively. The borders of the infarcted,
ischemic and nonischemic areas of the heart on the images captured
were traced and measured using Image-Pro Plus software (version
3.0; Media Cybernetics, Inc., Rockville, MD, USA). IS was expressed
as a percentage of the risk area volume as follows: IS (%)=IS/risk
area. Risk area referred to the myocardium that was stained red by
TTC.
Assessment of left ventricular
function
A total of 4 weeks following I/R, all rats underwent
a transthoracic echocardiography using a Philips Sosnos 7500
ultrasound machine (Philips Healthcare, Amsterdam, The Netherlands;
probe frequency 10 MHz). Left ventricular ejection fraction (LVEF),
left ventricular end diastolic diameter (LVEDD) and left
ventricular fractional shortening (LVFS) were measured.
Western blotting for HIF-1α and
P-p38MAPK
Expression of HIF-1α and P-p38MAPK protein was
determined using western blotting. The rats were sacrificed by
decapitation following anesthetization via intraperitoneal
injection of phenobarbital (Tianjin Jinyao Pharmaceutical Co.,
Ltd., Tianjing, China; 60 mg/kg). The hearts were harvested and
washed with normal saline. Ischemic heart tissue (0.5 g) was then
homogenized at 0–4°C. Homogenate were centrifuged at 780 × g at
4°C. The bicinchoninic acid protein assay kit (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was used to determine protein levels
according to the manufacturer's protocol. Proteins (50 µg/lane)
were separated via SDS-PAGE (10% separation gel, 5% spacer gel) and
transferred onto polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
for 60 min at room temperature in 5% non-fat milk in Tris-buffered
saline-Tween-20 (TBST). Subsequently, the membranes were incubated
with the following primary rabbit polyclonal antibodies overnight
at 4°C: anti-HIF-1α (1:2,000; 14179), anti P-p38 MAPK (1:4,000;
9212) and anti-β-actin (1:5,000; 4967; all Cell Signaling
Technology, Inc., Danvers, MA, USA). The membranes were then
incubated with IRDye680® goat anti-rabbit IgG secondary antibodies
(1:2,000; 926-68029; LI-COR Bioscience, Lincoln, NE, USA) at room
temperature for 120 min after washing with TBST. Protein bands were
visualized using enhanced chemiluminescence and the Fluor-S™ gel
imaging system (version no. 170-8195; Bio-Rad Laboratories, Inc.).
Images of every protein band were captured and the software was
used to calculate the density of every protein band. The quantity
of HIF-1α protein was expressed relative to β-actin expression.
P-p38 MAPK protein was expressed as a ratio to p38 MAPK
expression.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SAS software (version 6.12l; SAS Institute, Inc., Cary, NC, USA)
was used to analyze the data. The statistical significance of
differences in the mean between groups was determined by one-way
analysis of the variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
HMGB1 pretreatment decreases serum
levels of cTnI, TNF-α and IL-6 following I/R
As presented in Table
I, serum c-TnI, TNF-α and IL-6 levels in the I/R group were
significantly higher compared with those of the sham group (all
P<0.01). Serum c-TnI, TNF-α and IL-6 levels in the HMGB100 and
HMGB200 groups were significantly lower compared with those in the
I/R group in a dose-dependent manner (all P<0.05; Table I). Levels of c-TnI, TNF-α and IL-6 in
the HMGB50 group also decreased compared with the I/R group, but
statistical levels were not reached (Table I).
 | Table I.Effect of HMGB1 pretreatment of IL-6,
TNF-α, cTnI, SOD and MDA and IS after I/R. |
Table I.
Effect of HMGB1 pretreatment of IL-6,
TNF-α, cTnI, SOD and MDA and IS after I/R.
|
| Group |
|---|
|
|
|
|---|
| Variable | Sham | I/R | HMGB50 | HMGB100 | HMGB200 |
|---|
| IL-6 (pg/ml) | 149.39±14.02 |
398.23±17.15a |
302.48±24.22a |
276.68±19.05a–c |
219.78±20.53a–d |
| TNF-α (pg/ml) | 20.88±6.14 |
69.17±4.56a |
58.43±3.57a |
50.16±2.99a–c |
43.64±2.01a–d |
| cTnI (µg/l) |
0.13±0.12 |
75.87±7.71a |
69.43±5.17a |
60.19±5.71a–c |
49.36±5.08a–d |
| SOD (U/mg) |
138.9±29.70 |
68.91±21.90a |
87.32±31.60a |
98.01±6.37a–c |
123.89±8.33a–d |
| MDA (nmol/mg |
2.94±0.13 |
9.78±0.34a |
6.92±0.41a |
6.19±0.26a–c |
3.47±0.25a–d |
| IS (%) |
0.00±0.00 |
69.73±3.88a |
55.17±3.39a |
44.39±4.59a–c |
19.71±5.14a–d |
HMGB1 pretreatment increases MDA and
decreases SOD levels after I/R
After I/R, MDA levels were significantly higher and
SOD levels were significantly lower in the I/R group compared with
the sham group (all P<0.01; Table
I). The I/R-induced increase in MDA and reduction in SOD levels
were significantly inhibited by 100 and 200 ng/kg HMGB1
pretreatment (P<0.01 vs. the I/R group; Table I). Furthermore, this effect occurred
in a dose-dependent manner (HMGB100 vs. HMGB50, P<0.05; HMGB200
vs. HMGB100, P<0.01; Table
I).
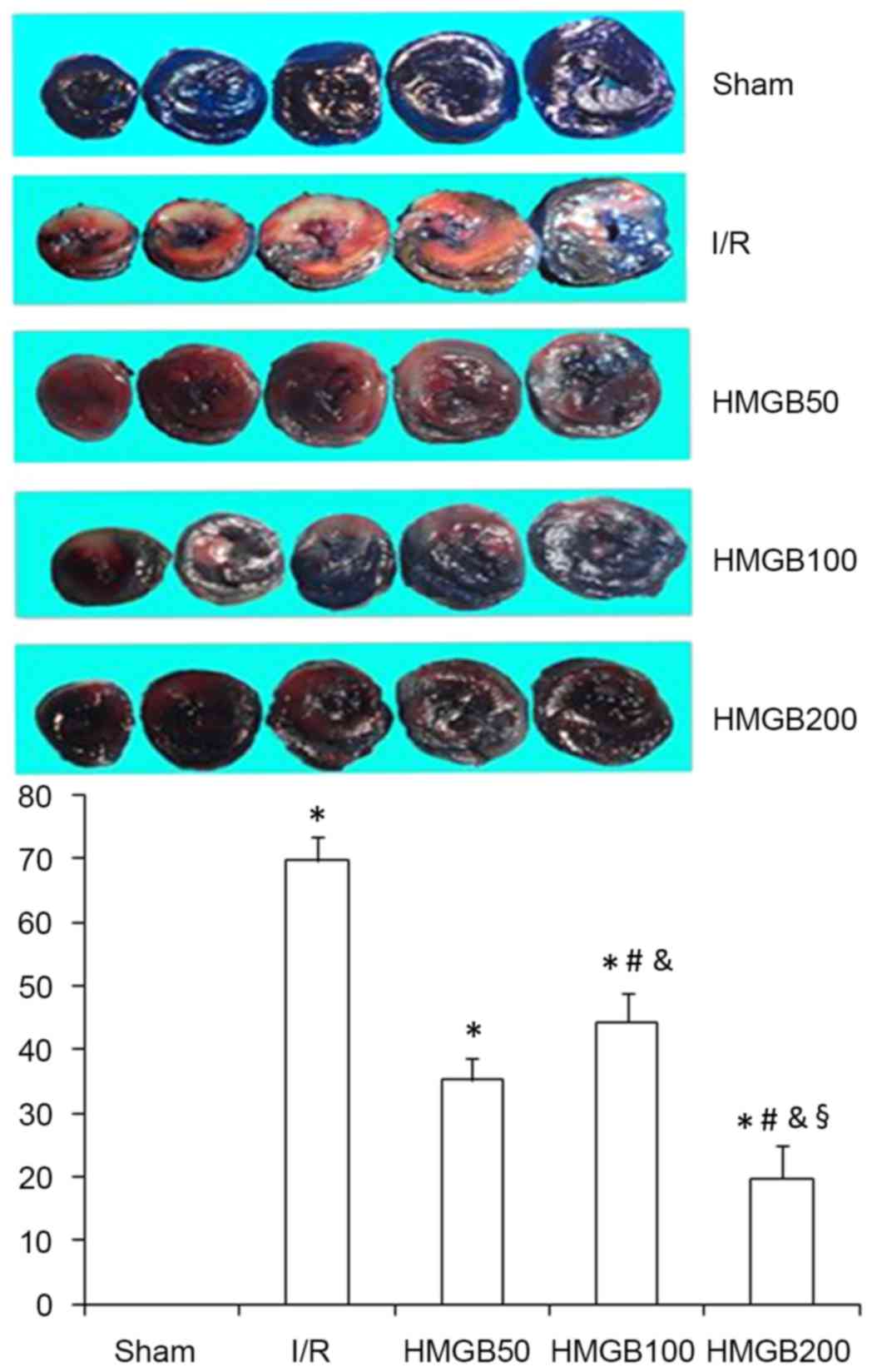
IS after I/R is decreased by HMGB1
pretreatment
TTC staining was used to detect the infarcted area.
IS was significantly higher in the I/R group compared with that in
the sham group (P<0.01; Table I
and Fig. 1). IS in the three HMGB1
treated groups were significantly decreased compared the I/R group
(all P<0.05; Table I and Fig. 1). Furthermore, ISs in the HMGB200
group was significantly decreased compared with the HMGB100 and
HMGB50 groups (both P<0.05; Table
I and Fig. 1), indicating that
the reduction in IS caused by HMGB1 was dose-dependent.
HMGB1 pretreatment increases LVEF and
LVFS, and decreases LVEDD after I/R
As presented in Table
II, LVEF and LVFS decreased significantly while LVEDD increased
significantly in the I/R group compared with the sham group (all
P<0.01). Compared with the I/R group, LVEF and LVFS in the
HMGB100 and HMGB200 groups were increased significantly, while
LVEDD was significantly reduced (all P<0.05; Table II).
 | Table II.Effect of HMGB1 on cardiac function
after I/R. |
Table II.
Effect of HMGB1 on cardiac function
after I/R.
|
| Group |
|---|
|
|
|
|---|
| Variable | Sham | I/R | HMGB50 | HMGB100 | HMGB200 |
|---|
| LVEDD (mm) |
4.04±0.28 |
6.61±0.85a |
6.21±1.03a |
5.68±1.25a–c |
5.07±0.64a–d |
| LVFS (%) |
37.9±2.28 |
18.23±1.85a |
22.13±1.43a |
27.47±2.35a–c |
32.07±2.64a–d |
| LVEF (%) | 79.90±7.13 |
46.68±3.92a |
45.87±6.22a |
65.49±5.54a–c |
72.39±4.98a–d |
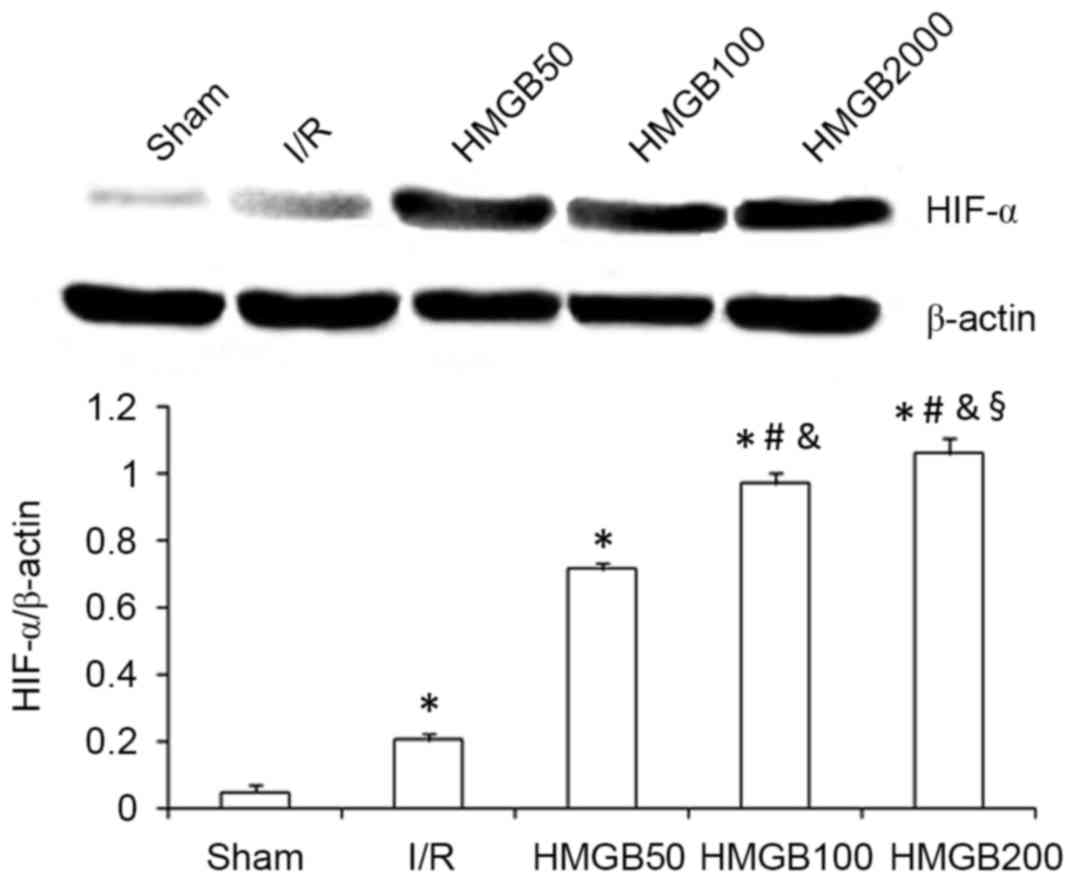
HMGB1 pretreatment increases HIF-1α
and decreases P-p38 MAPK myocardial protein expression after
I/R
HIF-1α protein expression in the I/R group was
significantly increased compared with that in the sham group
(P<0.01; Fig. 2). HMGB1
pretreatment (100 and 200 ng/kg) further significantly increased
the expression of HIF-1α protein compared with the I/R group (both
P<0.01; Fig. 2). Similarly to the
changes observed in IS, myocardial expression of HIF-1α protein in
the HMGB200 group was significantly increased compared with the
HMGB50 (P<0.01) and HMGB100 (P<0.05) groups (Fig. 2).
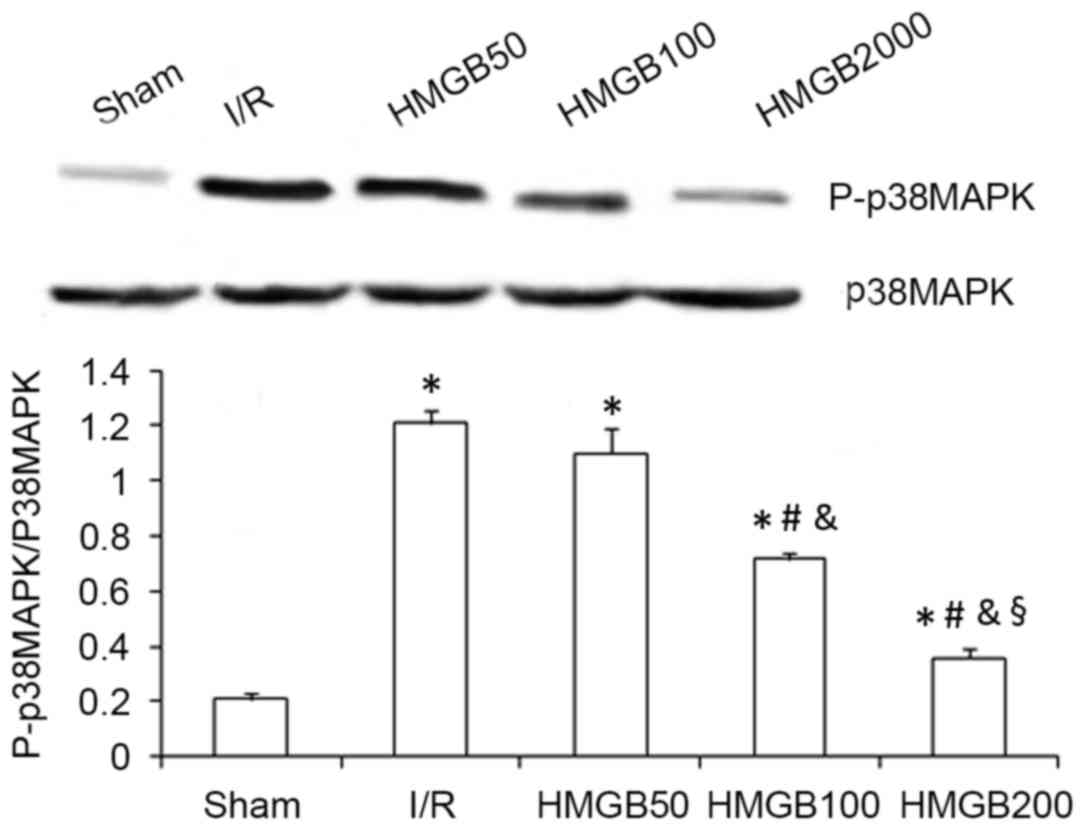
Expression of P-p38 MAPK protein in the I/R group
was significantly increased compared with the sham group
(P<0.01; Fig. 3). The HMGB100 and
HMGB200 groups had a significantly decreased expression of P-p38
MAPK protein compared with the I/R group (both P<0.05; Fig. 3). Furthermore, myocardial expression
of the P-p38 MAPK protein in the HMGB200 group was significantly
decreased compared with the HMGB50 (P<0.01) and HMGB100
(P<0.05) groups (Fig. 3).
Discussion
The present study identified the following: i)
Myocardial expression of HIF-1α was increased significantly in I/R
rats compared with the sham group; ii) HMGB1 could significantly
reduce IS, inhibit oxidative stress and increase myocardial
expression of HIF-1α; and iii) HMGB1 reduced the expression of
P-p38 MAPK. These results suggest that HMGB1 protects the
myocardium from I/R injury through increasing HIF-1α protein
expression, which may be contributed to its antioxidative and
anti-inflammatory properties, and through decreasing P-p38 MAPK
expression.
Previous studies have demonstrated that
intramyocardial injection of HMGB1 can reduce local myocardial
inflammation, reduce collagen volume fraction, reduce myocardial
remodeling and improve cardiac function in animal MI models
(22–25). However, the effect of intravenous
HMGB1 on myocardial I/R injury remains unclear. cTnI is a biomarker
of cardiac injury. In the present study, the effects of three
concentrations of intravenous HMGB1 on I/R injury were
investigated. Serum cTnI levels in the HMGB200 group were
significantly reduced compared with the HMGB50 and HMGB100 groups,
which suggests that the cardioprotective effects of intravenous
HMGB1 following I/R occur in a dose-dependent manner. In addition,
the results of the present study demonstrated that intravenous
HMGB1 increasesd LVEF and LVFS, and reduced LVEDD. Furthermore,
HMGB1 reduced myocardial IS. These results suggest that HMGB1
administered intravenously may protect the heart against I/R injury
and improve cardiac function. However, the underlying molecular
mechanism of this effect remains unclear.
TNF-α and IL-6 are proinflammatory cytokines are
associated with I/R injury. TNF-α enhances the inflammatory cascade
by increasing the expression of other proinflammatory cytokines,
including IL-6 (26). In addition,
TNF-α can cause cardiomyocyte apoptosis and is associated with
ventricular remodeling (27). The
results of the current study demonstrated that myocardial I/R
increased serum levels of TNF-α and IL-6, and that HMGB1
pretreatment reduced this increase. Thus, the inhibition of
proinflammatory cytokines, including TNF-α and IL-6, may contribute
to the cardioprotective effects of HMGB1.
Previous studies have demonstrated that myocardial
I/R injury is associated with the increased generation of reactive
oxygen species (ROS) and thus oxidative stress (28). ROS-mediated myocardiocyte apoptosis
and necrosis may be a determinant of IS (29). SOD and MDA levels are frequently used
to evaluate free radical metabolism, which is a component of the
process of oxidative stress. SOD levels reflect the cellular
capacity for scavenging/quenching free radicals. In the current
study, SOD levels were significantly decreased and MDA levels were
significantly increased in the heart tissue of the I/R group
compared with the sham group. HMGB1 increased SOD and decreased MDA
levels in the myocardium in a dose-dependent manner, which was
associated with a decrease in IS. These results indicate that HMGB1
also exerts its cardioprotective effects via acting as an
antioxidant.
HIF-1α can regulate the cellular response of
hypoxia. HIF-1α expression is increased in various organs and
tissues during ischemia, including the nervous system (30), and myocardium (4,31).
HIF-1α is required for the remote ischemic preconditioning of the
heart (32). Postconditioning can
decrease IS, reduce apoptosis and upregulate HIF-1α (33). The upregulation of HIF-1α can in turn
enhance cardioprotection from ischemic postconditioning (34). HIF-1α thus protects against
myocardial I/R injury (35). A
partial deficiency in HIF-1α can result in a complete loss of
cardioprotection against I/R injury (36). Our group previously reported that
basic fibroblast growth factor could enhance the myocardial
expression of HIF-1α mRNA, thus decreasing IS and improving left
ventricular function in rats following acute MI (4). Similarly, the current study
demonstrated that I/R significantly increased myocardial expression
of HIF-1α and that this expression was further elevated by HMGB1
pretreatment in a dose-dependent manner. This increase in HIF-1α
expression was associated with inhibition of I/R-induced myocardial
injury.
To the best of our knowledge, the present study is
the first report that HIF-1α may be associated with the
cardioprotective effects of intravenous HMGB1. However, the
underlying molecular mechanisms of how HIF-1α facilitates the
cardioprotective effects of HMGB1 following I/R are unclear. A
previous study revealed that the upregulation of HIF-1α was one of
the first responses to myocardial I/R injury at the molecular level
(37). HIF-1α activation confers
protective effects against I/R injury through enhancing the
expression of genes associated with glycolysis, cell survival,
apoptosis, mitochondrial function, glucose metabolism and
resistance to oxidative stress (38). HIF-1α activation also results in
activation of the inducible nitric oxide synthase signaling
pathway, which further stabilizes HIF-1α (39). The stabilization of HIF-1α under
normoxic conditions protects the heart from acute I/R injury via
triggering angiogenesis and preserving cardiac function (36,40). A
recent study suggested that the acute stabilization of HIF-1α
through pharmacological or genetic mechanisms protected the heart
from acute IR injury by increasing aerobic glycolysis, inhibiting
mitochondrial oxidative stress, activating hexokinase II and
inhibiting mitochondrial permeability (41).
p38 MAPK serves a role in numerous biological
process, including cell migration and death. A recent study
demonstrated that licochalcone D treatment enhanced cardiac
function, suppressed cardiac injury, reduced levels of
proinflammatory factors and enhanced the antioxidant capacity of
myocardial tissue following I/R (37). This effect was associated with
inhibition of the p38 MAPK signaling pathway (42). p38 MAPK inhibition also protects
mitochondria from I/R injury (43),
and improves cardiac function and ventricular remodeling following
I/R (44). In the present study, the
myocardial expression of P-p38 MAPK in the I/R group was
significantly higher compared with that in the sham group,
suggesting that myocardial ischemia increases P-p38 MAPK
expression. The expression of P-p38 MAPK was decreased by HMGB1
treatment in a dose-dependent manner. These results indicate that
intravenous HMGB1 protects the heart from I/R injury via inhibition
of P-p38 MAPK expression in ischemic myocardium.
A limitation of the current study was the lack of a
p38 MAPK inhibitor to confirm the role of p38 MAPK on the
cardioprotective effects of HMGB1 following I/R. Thus, further
studies using a p38 MAPK inhibitor are required to confirm the
underlying mechanism by which intravenous HMGB1 protects the heart
from I/R injury. In addition, HMGB1 was administered 30 min prior
to the ligation of the coronary arteries in the present study. In
clinical settings, drugs are typically administered several h after
the onset of ischemia. Therefore, the effect of HMGB1 given several
h after the ligation of the coronary arteries requires further
study in order to more closely emulate the clinical treatment of
ischemia.
In conclusion, the results of the present study on
an acute I/R rat model suggested that intravenous HMGB1 was
associated with a reduced IS, inhibition of oxidative stress,
improvement of cardiac function, increased HIF-1α expression and
reduced expression of P-p38 MAPK. These results suggest that
intravenous HMGB1 may exert its cardioprotective effect via
upregulating myocardial HIF-1α protein expression, at least in
part, via the inhibition of the P-p38 MAPK signaling pathway.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant nos. ZR2013HL017,
ZR2016HM49 and ZR2016HB73), the Natural Science Foundation of
Liaocheng City (grant no. 2012NS13), and the Science and Technology
Development Project of Liaocheng City (grant no. 2014GJH26).
References
|
1
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maes C, Carmeliet G and Schipani E:
Hypoxia-driven pathways in bone development, regeneration and
disease. Nat Rev Rheumatol. 8:358–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van de Sluis B, Groot AJ, Vermeulen J, van
der Wall E, van Diest PJ, Wijmenga C, Klomp LW and Vooijs M: COMMD1
Promotes pVHL and O2-Independent Proteolysis of HIF-1alpha via
HSP90/70. PLoS One. 4:e73322009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao HC, Liu T, Meng XY, Han QF, Zhang M
and Wang LX: Effect of basic fibroblast growth factor on the
myocardial expression of hypoxia-inducible factor-1α and vascular
endothelial growth factor following acute myocardial infarctio.
Heart Lung Circ. 22:946–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Kokkola R, Tabibzadeh S, Yang R,
Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, et al:
Structural basis for the proinflammatory cytokine activity of high
mobility group box 1. Mol Med. 9:37–45. 2003.PubMed/NCBI
|
|
6
|
Yan XX, Lu L, Peng WH, Wang LJ, Zhang Q,
Zhang RY, Chen QJ and Shen WF: Increased serum HMGB1 level is
associated with coronary artery disease in nondiabetic and type 2
diabetic patients. Atherosclerosis. 205:544–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohno T, Anzai T, Naito K, Miyasho T,
Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T,
et al: Role of high-mobility group box 1 protein in post-infarction
healing process and left ventricular remodelling. Cardiovasc Res.
81:565–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avalos AM, Kiefer K, Tian J, Christensen
S, Shlomchik M, Coyle AJ and Marshak-Rothstein A: RAGE-independent
autoreactive B cell activation in response to chromatin and
HMGB1/DNA immune complexes. Autoimmunity. 43:103–110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding HS and Yang J: High mobility group
box-1 and cardiovascular diseases. Saudi Med J. 31:486–489.
2010.PubMed/NCBI
|
|
10
|
Yao HC, Zhao AP, Han QF, Wu L, Yao DK and
Wang LX: Correlation between serum high-mobility group box-1 levels
and high-sensitivity C-reactive protein and troponin I in patients
with coronary artery disease. Exp Ther Med. 6:121–124.
2013.PubMed/NCBI
|
|
11
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin
CM and Rui T: Endogenous HMGB1 contributes to
ischemia-reperfusion-induced myocardial apoptosis by potentiating
the effect of TNF-α/JNK. Am J Physiol Heart Circ Physiol.
300:H913–H921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ran K, Gong ZX, Yang DL, Chang YT, Duan KM
and Ou YW: Effect of morphine preconditioning in the delayed phase
on the expression of p38 mitogen-activated protein kinase in a
rabbit model of myocardial ischemia-reperfusion injury. Genet Mol
Res. 14:6642–6648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Limana F, Germani A, Zacheo A, Kajstura J,
Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L,
Rastaldo R, et al: Exogenous high-mobility group box 1 protein
induces myocardial regeneration after infarction via enhanced
cardiac C-kit+ cell proliferation and differentiation. Circ Res.
97:e73–e83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Limana F, Esposito G, Fasanaro P, Foglio
E, Arcelli D, Voellenkle C, Di Carlo A, Avitabile D, Martelli F,
Russo M Antonio, et al: Transcriptional profiling of hmgb1-induced
myocardial repair identifies a key role for notch signaling. Mol
Ther. 21:1841–1851. 2013. View Article : Google Scholar
|
|
16
|
Abarbanell AM, Hartley JA, Herrmann JL,
Weil BR, Wang Y, Manukyan MC, Poynter JA and Meldrum DR: Exogenous
high-mobility group box 1 improves myocardial recovery after acute
global ischemia/reperfusion injury. Surgery. 149:329–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang DY, Zhang AX, Zhou YH, Wang LH and
Yao HC: Protection of intravenous HMGB1 on myocardial ischemia
reperfusion injury. Int J Cardiol. 184:280–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biscetti F, Ghirlanda G and Flex A:
Therapeutic potential of high mobility group box-1 in ischemic
injury and tissue regeneration. Curr Vasc Pharmacol. 9:677–681.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8th. The National Academies
Press; Washington, DC: 2011, PubMed/NCBI
|
|
20
|
Takahashi K, Fukushima S, Yamahara K,
Yashiro K, Shintani Y, Coppen SR, Salem HK, Brouilette SW, Yacoub
MH and Suzuki K: Modulated inflammation by injection of
high-mobility group box 1 recovers post-infarction chronically
failing heart. Circulation. 118 14 Suppl:S106–S114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Limana F, Esposito G, D'Arcangelo D, Di
Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G,
Germani A and Capogrossi MC: HMGB1 attenuates cardiac remodelling
in the failing heart via enhanced cardiac regeneration and
miR-206-mediated inhibition of TIMP-3. PLoS One. 6:e198452011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao HC, Yang LJ, Han QF, Wang LH, Wu L,
Zhang CY, Tian KL and Zhang M: Postconditioning with simvastatin
decreases myocardial injury in rats following acute myocardial
ischemia. Exp Ther Med. 9:1166–1170. 2015.PubMed/NCBI
|
|
23
|
Chen M, Huang W, Wang C, Nie H, Li G, Sun
T, Yang F, Zhang Y, Shu K, Wang C and Gong Q: High-mobility group
box 1 exacerbates CCl4-induced acute liver injury in mice. Clin
Immunol. 153:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Hu X, Xie J, Xu C, Xu W and Jiang
H: Exogenous high-mobility group box 1 protein injection improves
cardiac function after myocardial infarction: Involvement of Wnt
signaling activation. J Biomed Biotechnol. 2012:7438792012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Zhou X, Zheng X and Jiang X:
Exogenous high-mobility group box 1 protein prevents postinfarction
adverse myocardial remodeling through TGF-β/Smad signaling pathway.
J Cell Biochem. 114:1634–1641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khimenko PL, Bagby GJ, Fuseler J and
Taylor AE: Tumor necrosis factor-alpha in ischemia and reperfusion
injury in rat lungs. J Appl Physiol (1985). 85:2005–2011.
1998.PubMed/NCBI
|
|
27
|
Zhu J, Liu M, Kennedy RH and Liu SJ:
TNF-alpha-induced impairment of mitochondrial integrity and
apoptosis mediated by caspase-8 in adult ventricular myocytes.
Cytokine. 34:96–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jahangiri A, Leifert WR, Kind KL and
McMurchie EJ: Dietary fish oil alters cardiomyocyte Ca2+ dynamics
and antioxidant status. Free Radic Bio Med. 40:1592–1602. 2006.
View Article : Google Scholar
|
|
29
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rapino C, Bianchi G, Di Giulio C,
Centurione L, Cacchio M, Antonucci A and Cataldi A: HIF-1alpha
cytoplasmic accumulation is associated with cell death in old rat
cerebral cortex exposed to intermittent hypoxia. Aging Cell.
4:177–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
AI-Salam S and Hashmi S: Galectin-1 in
early acute myocardial infarction. PLoS One. 9:e869942014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai Z, Luo W, Zhan H and Semenza GL:
Hypoxia-inducible factor 1 is required for remote ischemic
preconditioning of the heart. Proc Natl Acad Sci USA. 110:pp.
17462–17467. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q Fang, Xu H, Sun Y, Hu R and Jiang H:
Induction of inducible nitric oxide synthase by isoflurane
post-conditioning via hypoxia inducible factor-1α during tolerance
against ischemic neuronal injury. Brain Res. 1451:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Zhao H, Wu Y, Zhang S, Zhao X, Zhang
Y, Wang J, Wang J and Liu H: Up-regulation of hypoxia-inducible
factor-1α enhanced the cardioprotective effects of ischemic
postconditioning in hyperlipidemic rats. Acta Biochim Biophys Sin
(Shanghai). 46:112–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Poynter JA, Manukyan MC, Wang Y, Brewster
BD, Herrmann JL, Weil BR, Abarbanell AM and Meldrum DR: Systemic
pretreatment with dimethyloxalylglycine increases myocardial HIF-1α
and VEGF production and improves functional recovery after acute
ischemia/reperfusion. Surgery. 150:278–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot
K, Wang L, Wei C, Trush MA and Semenza GL: Complete loss of
ischaemic preconditioning-induced cardioprotection in mice with
partial deficiency of HIF-1 alpha. Cardiovasc Res. 77:463–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adluri RS, Thirunavukkarasu M, Dunna NR,
Zhan L, Oriowo B, Takeda K, Sanchez JA, Otani H, Maulik G, Fong GH
and Maulik N: Disruption of hypoxia-inducible transcription
factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo
myocardial ischemia/reperfusion injury through hypoxia-inducible
factor-1α transcription factor and its target genes in mice.
Antioxid Redox Signal. 15:1789–1797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Si J, Wang N, Wang H, Xie J, Yang J, Yi H,
Shi Z, Ma J, Wang W, Yang L, et al: HIF-1α signaling activation by
post-ischemia treatment with astragaloside IV attenuates myocardial
ischemia-reperfusion injury. PLoS One. 9:e1078322014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oriowo B, Thirunavukkarasu M, Selvaraju V,
Adluri RS, Zhan L, Takeda K, Fong GH, Sanchez JA and Maulik N:
Targeted gene deletion of prolyl hydroxylase domain protein 3
triggers angiogenesis and preserves cardiac function by stabilizing
hypoxia inducible factor 1 alpha following myocardial infarction.
Curr Pharm Des. 20:1305–1310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ong SG, Lee WH, Theodorou L, Kodo K, Lim
SY, Shukla DH, Briston T, Kiriakidis S, Ashcroft M, Davidson SM, et
al: HIF-1 reduces ischaemia-reperfusion injury in the heart by
targeting the mitochondrial permeability transition pore.
Cardiovasc Res. 104:24–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan X, Niu HT, Wang PL, Lu J, Zhao H, Liu
SH, Zheng QS and Li CG: Cardioprotective effect of licochalcone d
against myocardial ischemia/reperfusion injury in
langendorff-perfused rat hearts. PLoS One. 10:e01283752015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumphune S, Surinkaew S, Chattipakorn SC
and Chattipakorn N: Inhibition of p38 MAPK activation protects
cardiac mitochondria from ischemia/reperfusion injury. Pharm Biol.
53:1831–1841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Lv H, Gu Y, Wang X, Cao H, Tang Y,
Chen H and Huang C: Protective effect of lycopene on cardiac
function and myocardial fibrosis after acute myocardial infarction
in rats via the modulation of p38 and MMP-9. J Mol Histol.
45:113–120. 2014. View Article : Google Scholar : PubMed/NCBI
|