Introduction
Cleft palate is a common congenital malformation in
children. The American Cleft Palate-craniofacial Association
recommends that primary cleft palate surgery should ideally be
performed within 12–18 months after birth (1). However, this would increase the
potential risk of airway obstruction during the recovery period
after general anesthetization for cleft palate repair surgery due
to the younger age and association with potent stimulation of
oropharyngeal pain, tissue swelling, blockage of blood or
secretions and gauze oppression (2,3).
Simultaneously, these factors may lead to respiratory depression,
restlessness, nausea and vomiting, and increase the days of
hospitalization and hospital costs (4). Other factors, such as anticipation of
postoperative pain, separation from the family, incapacity, loss of
independence, fear of surgery and fear of death may lead to
emergence agitation (EA) (5).
Currently, sevoflurane has been widely used in
pediatric anesthesia, particularly for infants, predominantly
because it is less pungent and has a more rapid onset and offset
than other potent inhaled agents due to lower solubility in blood,
a relative lack of airway irritation and greater hemodynamic
stability (6,7). However, numerous studies have
demonstrated that sevoflurane anesthesia in patients had a higher
risk of postoperative agitation, particularly in children (8,9). The
incidence rate of EA varies depending on the type and severity of
EA. The incidence of EA is relatively high regardless of the way of
the definition; 33% of the time when defining a high threshold for
agitation, and 80% of the time when using a lower threshold in
children after sevoflurane anesthesia without surgery (10). The exact mechanisms of sevoflurane
leading to postoperative agitation in children are unclear.
Relevant factors include age, pain stimulation and surgical methods
(11). Several currently available
randomized controlled trials have illustrated the effectiveness of
dexmedetomidine (DEX) in the prevention of sevoflurane-related EA
incidence, as well as severe EA (12). Administration of 0.2 µg/kg sufentanil
after induction of anesthesia was able to reduce EA in children
receiving sevoflurane anesthesia for adenotonsillectomy compared
with 2 µg/kg fentanyl (13).
However, the views on the influence of the agents on EA remain
controversial.
Various studies have focused on the application of
DEX and sufentanil separately regarding sevoflurane anesthesia in
children; however, research on the application of DEX combined with
sufentanil is limited. In the present study, the clinical efficacy
of DEX combined with sufentanil in children receiving sevoflurane
anesthesia for cleft palate repair surgery was investigated.
Materials and methods
Ethics statement and patients
The present study was approved by the Bioethics
Committee at the Second Xiangya Hospital of Central South
University (no. 2012045; Changsha, China). Following internal
review board approval and parental informed consent, 100 ASA
physical status I–II (14) children
(male/female: 42/58; 1–5 years old) who were scheduled to undergo
sevoflurane anesthesia for cleft palate repair surgery were
included. Following approval by the Ethics Committee of the Second
Xiangya Hospital of Central South University, the study was
prospectively conducted between January 2013 and October 2015.
Informed consent was obtained from all individual participants
included in the study. Exclusion criteria included the following:
Lack of consent; disorders of the respiratory, circulatory or
nervous system; known adverse reactions to DEX and sufentanil; a
history of adverse reactions to general anesthesia, difficulties
with airways, breath-holding and/or postoperative bleeding; or
illness that would be associated with agitation, such as seizures
or schizophrenia. Children were randomly allocated into the DEX +
sufentanil group (group DS; n=50) or the normal saline + fentanyl
group (group SF; n=50). Subjects were randomized by the use of a
computer-generated table of random numbers to receive DEX or
placebo.
Anesthetic management
Both groups of children were fasted from solid foods
for 6 h prior to the procedure; clear liquids were permitted until
2 h prior to admission to the operating room (OR). A total of 0.01
mg/kg penehyclidine hydrochloride (Lisite Biopharmaceuticals Co.,
Ltd., Chengdu, China) was administered intravenously to all
children as soon as intravenous access was established prior to
admission into the OR. A total of 0.5 µg/kg DEX (diluted to 20 ml;
Hengrui Biopharmaceuticals Co., Ltd., Lianyungang, China) was
administered intravenously over a 10-min period prior to induction
of anesthesia in group DS, and the patients in group SF received an
equal volume of normal saline. Intravenous induction of anesthesia
using midazolam (0.05–0.1 mg/kg; Enhua Pharmaceutical Group Co.,
Ltd., Xuzhou, China), propofol (2.0 mg/kg; Fresenius Kabi AB
Pharmaceutical Group Co., Ltd., Beijing, China) and cisatracurium
(0.15 mg/kg; Dongying Pharmaceutical Group Co., Ltd., Jiangsu,
China) was performed. Sufentanil (0.2 µg/kg; Renfu Pharmaceutical
Group Co., Ltd., Yichang, China) was administered intravenously
after induction and 30 min before the end of cleft palate repair
for patients in group DS. Fentanyl (2 µg/kg; Renfu Pharmaceutical
Group Co., Ltd.) was administered intravenously at the same time
for patients in group SF. Tracheal intubation (Meinuo Medical
Appliances Co., Ltd., Suzhou, China) was performed by an attending
doctor after loss of consciousness and the observer assessment of
alertness and sedation (OAAS) score was ≤3 (15). Dexamethasone (0.1 mg/kg; Fengyuan
Pharmaceutical Group Co., Ltd., Anhui, China) was administered
after anesthesia induction to reduce post-operative nausea and
vomiting. Cisatracurium (0.1 mg/kg; Dongying Pharmaceutical Group
Co., Ltd.) was selectively administered according to maintenance
time of muscle relaxant drugs and surgical time. Anesthesia was
maintained with 2% volume inhalation of sevoflurane (Yapei
Pharmaceutical Group Co., Ltd., Shanghai, China) and remifentanil
(Rennfu Pharmaceutical Group Co., Ltd.) 1.5–2.5 ng/ml
target-controlled infusion (TCI). All anesthetic administrations
were terminated 10 min before the end of surgery. All
pharmacological agents used in the present study were prepared and
administrated by the anesthesiologists who were blinded to the
details of the study.
Anesthesia management
Pressure control ventilation mode was used to
maintain breathing, and set inspiratory pressure (10–15 mmHg),
oxygen flow (2 l/min), respiratory ratio (1:1.5), respiratory rate
(18–30 times/min) and the partial pressure of end-tidal carbon
dioxide (PETCO2; 35–45 mmHg). Anesthesia was maintained
with sevoflurane 2% volume inhalation and remifentanil 1.5–2.5
ng/ml TCI to control the change of mean arterial pressure (MAP) and
heart rate (HR) to within 25% of the baseline (baseline valued were
obtained from patients prior to any medication and surgery). MAP,
HR, peripheral capillary oxygen saturation (SpO2) and
PETCO2 during the surgery were monitored. The depth of
sedation was monitored using the A-2000 XP bispectral index monitor
(Aspect Medical Systems, Inc., Norwood, MA, USA). Children were
sent to the post-anesthesia care unit (PACU) after surgery, where
EA scores were observed and recorded. An emergence agitation scale
(EAS) was measured every 15 min in the PACU (16). Aono's scale (17) of 3 or 4 were classified as severe EA.
Tracheal extubation was performed with careful suction when
spontaneous breathing ventilation (indicated when PETCO2
reached 35 mmHg) was achieved and children regained gag or cough
reflex. Patients without pain that were calm and with a modified
Aldrete score >10 were transferred to the ward (18). The time at which these criteria were
met was also recorded. The Children and Infants Postoperative Pain
Scale (CHIPPS) was adopted to determine whether fentanyl at 1 µg/kg
was administered (CHIPPS pain score >5) as rescue medication or
not (19). Time to extubation was
defined from admission to PACU until the trachea was extubated. The
recovery time was defined as the time between the admission to and
discharge from PACU. EA, Pediatric Anesthesia Emergence Delirium
(PAED) (20) and CHIPPS scores were
documented every 15 min in PACU by a well-trained PACU nurse who
was blinded to the study.
Aono's scale and PAED scale
Aono's scale (17)
was used to evaluate post-operative behaviour (1=calm; 2=not calm
but could be easily consoled; 3=moderately agitated or restless and
not easily calmed; and 4=combative, excited or disoriented,
thrashing. around). Severe EA was defined as an Aono's score of 3
or 4. PAED scale was defined as follows: 1, the child makes eye
contact with the caregiver; 2, the child's actions are purposeful;
3, the child is aware of his/her surroundings; 4, the child is
restless; and 5, the child is inconsolable.
Observed parameters
MAP and HR at T1 (entering the OR), T2 (before
induction of anesthesia), T3 (immediately following tracheal
intubation), T4 (end of the surgery) and T5 (immediately following
tracheal extubation) were recorded. Duration of surgery and
anesthesia and the dosage of remifentanil was assessed. EA, PAED
and CHIPPS scores were documented every 15 min in the PACU. The
number of cases that required fentanyl (1 µg/kg) and the recovery
profile data were analyzed.
Statistical analysis
Data were presented as the mean ± standard
deviation. Statistical analyses were performed using the
statistical package, SPSS v. 16.0 for Windows (SPSS, Inc., Chicago,
IL, USA). Patient characteristics were compared using one-way
analysis of variance with Kruskal-Wallis post hoc tests and
χ2 test where appropriate. Differences in the incidence
of severe EA among the groups were analyzed using χ2
test with Fisher's exact test correction. Differences in PAED and
CHIPPS scores among groups were analyzed using the Kruskal-Wallis
test with Bonferroni's correction. P<0.05 was considered to
indicate a statistically significant difference.
Results
Excluded patients
A total of 100 patients were enrolled into the
present study, of which 93 fulfilled the inclusion criteria. A
total of 7 patients were excluded. The excluded patients included 4
patients from group DS and 3 patients from group SF, due to
breath-holding and postoperative bleeding (Fig. 1).
Patient demographics and duration of
surgery and anesthesia
Patient demographics and duration of surgery and
anesthesia are presented in Table I.
No significant differences were identified in patient demographics
or duration of surgery and anesthesia among the two groups.
 | Table I.Patient demographics and duration of
surgery and anesthesia. |
Table I.
Patient demographics and duration of
surgery and anesthesia.
|
| Group |
|
|---|
|
|
|
|
|---|
| Parameter | DS (n=46) | SF (n=47) | P-value |
|---|
| Gender |
|
|
|
| Male, n
(%) | 18 (39.1) | 20 (42.6) | 0.21 |
| Female,
n (%) | 28 (61.9) | 27 (57.4) | 0.37 |
| Age, years |
2.9±1.2 |
3.0±1.0 | 0.65 |
| Weight, kg | 13.1±2.5 | 12.9±1.8 | 0.71 |
| Duration of
anesthesia, min | 105.4±7.5 | 106.5±5.8 | 0.79 |
| Duration of
surgery, min | 94.1±6.1 | 92.0±5.3 | 0.43 |
Anesthetic data
HR and MAP were maintained within 25% of the
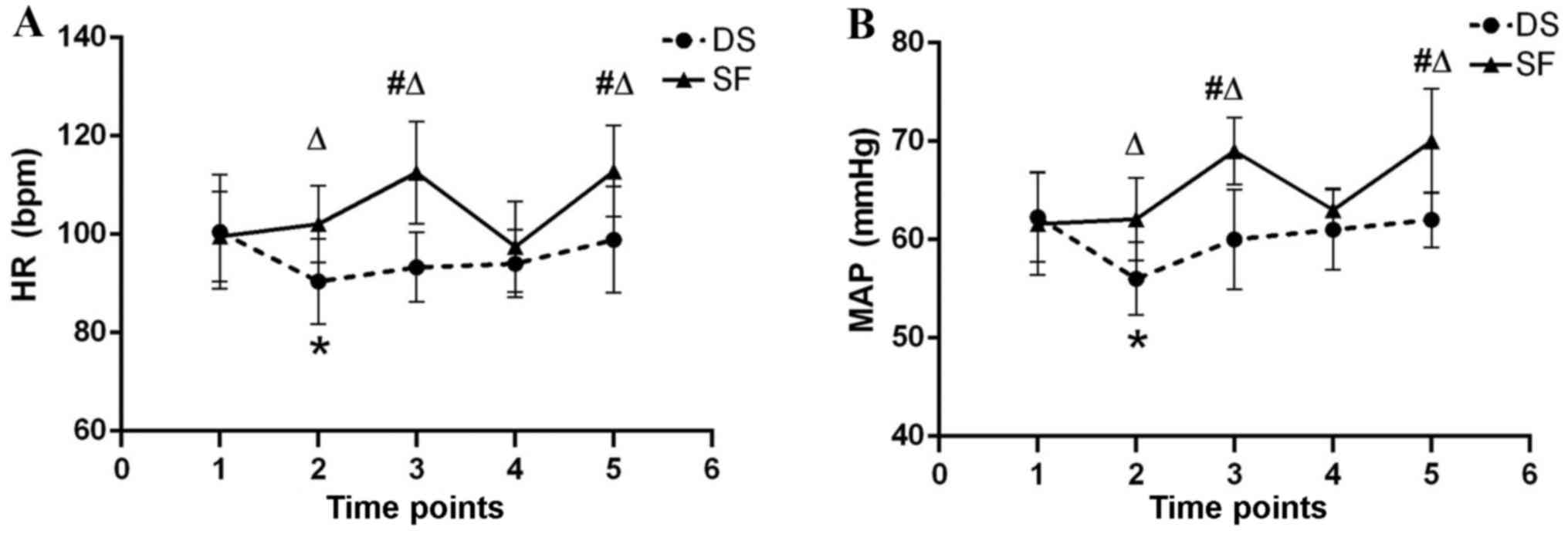
baseline in both groups throughout anesthesia (Fig. 2). HR and MAP were significantly
increased in group SF immediately following tracheal intubation and
extubation compared with group DS (P<0.05) and the SF baseline
(P<0.05). HR and MAP at T2 (before induction of anesthesia) were
significantly increased in group DS compared with the DS baseline
(P<0.05).
Postoperative pain and severe EA
The incidence of severe EA (Aono's scale 3 or 4) was
2/46 (4.3%) in the DS group and 26/47 (55.3%) in the SF group
(P<0.01). The incidence of EA was 4/46 (8.6%) in the group DS
and 36/47 (76.6%) in the SF group (P<0.01; Table II). The incidence of severe EA and
EA was significantly decreased in the DS group compared with the
group SF (P<0.01). The mean maximum PAED score was significantly
decreased in group DS compared with group SF (7.2±4.3 vs. 11.8±5.1,
respectively; P<0.01; Table II).
The mean maximum CHIPPS score was also significantly decreased in
Group DS compared with Group SF (3.0±2.5 vs. 6.2±3.1, respectively;
P<0.01; Table II). The dosage of
remifentanil required in group SF was significantly larger than
that required in group DS (279.6±32.8 vs. 191.25±28.5,
respectively; P<0.01; Table II).
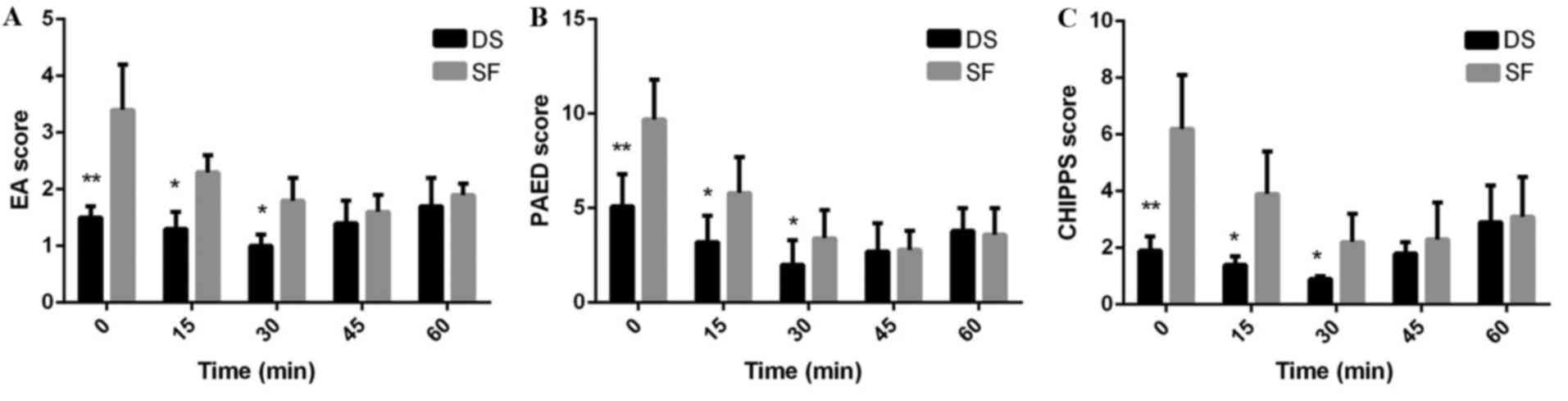
The mean maximum EA, PAED and CHIPPS scores were significantly
decreased in Group DS compared with Group SF at 0 (P<0.01), 15
(P<0.05) and 30 min (P<0.05) after arrival at PACU (Fig. 3).
 | Table II.Recovery profile of patients based on
EA, CHIPPS and PAED scores, and the dosage of remifentanil
required. |
Table II.
Recovery profile of patients based on
EA, CHIPPS and PAED scores, and the dosage of remifentanil
required.
|
| Group |
|
|---|
|
|
|
|
|---|
| Assessment
criteria | DS (n=46) | SF (n=47) | P-value |
|---|
| Severe EA, n
(%) | 2 (4.3) | 26 (55.3) | <0.01 |
| EA, n (%) | 4 (8.6) | 36 (76.6) | <0.01 |
| CHIPPS maximum
score | 3.0±2.5 | 6.2±3.1 | <0.01 |
| CHIPPS score >6,
n (%) | 6 (13.0) | 31 (66.0) | <0.01 |
| PAED maximum
score | 7.2±4.3 | 11.8±5.1 | <0.01 |
| PAED >11, n
(%) | 3 (6.5) | 38 (80.9) | <0.01 |
| Dosage of
remifentanil, µg | 191.5±28.5 | 279.6±32.8 | <0.01 |
Recovery profiles
The recovery profiles were listed in Table III. A total of 5 (10.8%) children
in group DS required rescue fentanyl doses of 1 µg/kg. This was
significantly higher than group SF, in which 29 (61.7%) children
required rescue fentanyl doses of 1 µg/kg (P<0.01; Table III). However, no significant
difference was indicated between the two groups in time to tracheal
extubation, recovery time and Aldrete score in PACU. There was no
significant difference in the incidence of side effects between the
two groups, such as coughing, chest wall rigidity, and
postoperative nausea and vomiting. Two cases of postoperative
bleeding and two cases of breath-holding occurred in the DS group,
whereas the SF group had two cases of postoperative bleeding and
one case of breath-holding.
 | Table III.Recovery profiles of patients. |
Table III.
Recovery profiles of patients.
|
| Group |
|
|---|
|
|
|
|
|---|
| Assessment
criteria | DS (n=46) | SF (n=47) | P-value |
|---|
| Time to extubation,
min | 10.6±4.1 | 9.7±3.9 | 0.45 |
| Recovery time,
min | 46.7±12.3 | 43.2±15.6 | 0.41 |
| Aldrete score | 7.8±1.1 (6–10) | 8.2±1.5 (6–10) | 0.51 |
| Patients requiring
1 µg/kg fentanyl rescue doses, n (%) | 5 (10.8) | 29 (61.7) | <0.01 |
| Side effects |
| Cough,
n (%) | 1 (2.2) | 2 (4.3) | 0.89 |
| Chest
wall rigidity, n (%) | 1 (2.2) | 0 (0) | 0.91 |
|
Postoperative nausea and
vomiting, n (%) | 3 (6.5) | 4 (8.5) | 0.94 |
Discussion
The present study was performed to evaluate whether
DEX combined with sufentanil was able to optimize the efficacy of
sevoflurane anesthesia in children receiving cleft palate repair
surgery. The present findings indicated that DEX combined with
sufentanil effectively inhibited intubation and extubation response
of children receiving sevoflurane anesthesia for cleft palate
repair surgery, maintained greater hemodynamic stability, reduced
the incidence of EA, reduced the dose of remifentanil and reduced
the requirement of fentanyl 1 µg/kg treatment. However, DEX
combined with sufentanil did not significantly alter the time to
extubation or the recovery time.
The exact causes of postoperative agitation in
children following general anesthesia are not clear. However,
several associated risk factors associated with EA have been
indicated, including preschool age, pain, preoperative anxiety,
personal character of the patient, too-rapid awakening,
otolaryngologic procedures (cleft palate repair surgery) and the
use of sevoflurane (21–24). Although sevoflurane is associated
with a high incidence of EA, it has been widely used as a sole
agent for pediatric anesthesia (25). Numerous studies have shown that
benzodiazepines, barbiturates and opioids contribute to behavioral
disturbances after sevoflurane general anesthesia (26,27).
The predominant outcome of the present study was
that DEX combined with sufentanil significantly reduced the
incidence of EA by 68% compared with saline and fentanyl treatment
(8.6 vs. 76.6%, respectively). When severe EA was defined as an
Aono's scale of 3 or 4, DEX combined with sufentanil significantly
reduced the incidence of severe EA by 51% compared with saline and
fentanyl treatment (4.3 vs. 55.3%, respectively). A previous study
by Özcengiz et al (28)
demonstrated that oral melatonin (0.1 mg/kg), DEX (2.5 µg/kg) and
midazolam (0.5 mg/kg) reduced the incidence of EA in children after
sevoflurane anesthesia and that the incidence of EA among the three
groups was similar. Furthermore, intranasal DEX (1 µg/kg) and
midazolam (0.2 mg/kg) were demonstrated to be equally effective in
decreasing anxiety at parental separation; however, midazolam was
superior in terms of providing satisfactory conditions during mask
induction (29). A study by Tan
et al (30) revealed that
intraoperative continuous infusion of low-dose DEX (0.2 µg/kg/h)
was able to reduce EA following desflurane anesthesia without
hemodynamic compromise or delayed awakening in pediatric patients
undergoing strabismus surgery.
Opioids have been demonstrated to reduce the
incidence of EA effectively after sevoflurane anesthesia (31). Two previous studies have shown that a
single bolus dose of fentanyl (either 1 or 2 µg/kg) reduced the
incidence of severe EA following sevoflurane anesthesia (32,33). The
intravenous administration of a single dose of 0.15 µg/kg
sufentanil just before skin incision significantly decreased the
incidence of EA in children undergoing inguinal hernia repair under
sevoflurane anesthesia compared with a single dose of 1.5 µg/kg
fentanyl (34). Although the dosage
of opioids is different (0.2 µg/kg sufentanil vs. 1.5 µg/kg
fentanyl), Bedirli et al (8)
arrived at the same conclusion for adenotonsillectomy. In the
present study, sufentanil (0.2 µg/kg) and fentanyl (2 µg/kg) were
administered intravenously 30 min prior to the end of surgery. The
present study aimed to exclude the influence of post-operative pain
on EA and decrease the incidence of EA. Due to regional block,
opioids and nonsteroidal anti-inflammatory agents have been
reported to decrease the incidence of EA (35,36).
However, EA still occurs, even after adequate pain treatment or
procedures that are not associated with pain (37). Furthermore, findings from the present
study indicated that the mean maximum PAED and CHIPPS scores were
significantly lower in group DS than in group SF. The above results
demonstrate that DEX combined with sufentanil may alleviate the
pain following cleft palate surgery in children and reduce the EA
following sevoflurane anesthesia more effectively than saline
combined with fentanyl.
In the present study, a significantly increased
number of children in group DS required rescue fentanyl doses of 1
µg/kg compared with children in group SF [5 (10.8%) vs. 29 (61.7%),
respectively]. This was likely due to the fact that the analgesic
effectiveness of sufentanil is 10 times than that of fentanyl,
which has a shorter duration of action (38). Furthermore, compared with saline, DEX
provided the effect of analgesia and sedation. Sufentanil provides
a fast onset due to its high lipid solubility (39). The clearance of sufentanil in
children was twice as rapid as described in adults and adolescence
(40). In the present study,
remifentanil TCI was utilized for the maintenance of anesthesia, as
remifentanil TCI reduces the emergence of coughing from general
anesthesia more effectively than a single-dose of DEX (41). However, a single-dose of DEX is able
to maintain respiratory and hemodynamic stability during emergence
(42).
DEX, a selective α-2 adrenoceptor agonist, has
sedative, analgesic and anxiolytic effects after intravenous
administration (43). DEX also
produces dose-dependent HR and blood pressure reductions (44,45). In
the present study, compared with group DS and the DS baseline, HR
and MAP were significantly increased in group SF immediately
following tracheal intubation and extubation. HR and MAP of T2
(before induction) significantly increased in group DS compared
with the DS baseline. A study by Kim et al (31) concluded that a 1 µg/kg-dose of
intravenous DEX reduced EA following sevoflurane anesthesia in
children undergoing magnetic resonance imaging. The bolus
administration of DEX in this dose was safe and did not lead to an
increased incidence of side effects (46). An intraoperative infusion of DEX
combined with inhalation anesthetics provided satisfactory
intraoperative conditions for tonsillectomy and adenoidectomy
without adverse hemodynamic effects (47). The study also indicated that
postoperative opioid requirements were significantly reduced, and
the incidence and duration of severe EA was lower, with fewer
patients having desaturation episodes. Furthermore, the number of
rescue fentanyl doses of 1 µg/kg in the present study was
significantly lower in the DS group than that of the SF group in
the present study. A study by Kim et al (48) demonstrated that DEX appeared to be a
safe and effective alternative to reduce the occurrence of early EA
in children after tonsillectomy and that the mechanism would be
associated with the sedation and analgesia effects provided by DEX.
For prevention of EA after desflurane anesthesia for 50 and 95% of
children undergoing tonsillectomies or adenoidectomies,
administration of 0.25 or 0.38 µg/kg DEX has been suggested
(31). DEX produces an analgesic
effect; therefore, it may reduce postoperative opioid use and the
incidence of opioid-related complications, such as respiratory
depression, nausea and pruritus (49,50).
The present study had some limitations. Firstly, for
the consideration of safety and shortage of personnel, there were
only two groups in the present study. Four groups would have
improved the study design and may have achieved a superior result
to demonstrate that DEX combined with sufentanil is able to
optimize sevoflurane anesthesia in children. Experiment design will
be optimized in our further research. Secondly, the type of surgery
was limited. The reason why palate repair surgery was studied
instead of alternative surgeries, such as adenotonsillectomy or
cleft lip repair, is due to the increased irritation associated
with cleft palate repair surgery (51). Thirdly, the dose of DEX (0.5 µg/kg)
used in the present study was selected based on doses cited in
previous studies (52,53). A higher dose of DEX may be more
effective to decrease EA; however, it may also cause other
problems, such as dose-dependent HR and blood pressure reductions
or a delay in the restoration of consciousness (54).
In conclusion, we believe that DEX (0.5 µg/kg)
administered intravenously 10 min prior to induction of anesthesia
combined with sufentanil (0.2 µg/kg) administered intravenously
prior to incision and 30 min before the end of surgery may
effectively inhibit intubation and extubation response of children
receiving sevoflurane anesthesia for cleft palate repair surgery.
This combined DEX and sufentanil administration may also maintain
greater hemodynamic stability, reduce the incidence of EA, reduce
the dose of remifentanil required and reduce the number of 1 µg/kg
fentanyl administrations. Additionally, the present study
demonstrated that this treatment did not increase the time to
extubation or the recovery time.
Acknowledgements
The present study was funded by the National Natural
Science Fund, China (grant nos. 81571936, 81401626 and
81500947).
References
|
1
|
Raj S: The timing of primary cleft lip and
palate surgery-the experience of a craniofacial center in South
India. Brit J Oral Max Surg. 49:S91–S92. 2011. View Article : Google Scholar
|
|
2
|
Klingmann A: Anesthetic management of
pediatric cleft lip and cleft palate repair. Anaesthesist.
55:93–94. 2006.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milić M, Goranović T and Knezević P:
Complications of sevoflurane-fentanyl versus midazolam-fentanyl
anesthesia in pediatric cleft lip and palate surgery: A randomized
comparison study. Int J Oral Maxillofac Surg. 39:5–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen C, Hernandez-Boussard T, Davies SM,
Bhattacharya J, Khosla RK and Curtin CM: Cleft palate surgery: An
evaluation of length of stay, complications and costs by hospital
type. Cleft Palate Craniofac J. 51:412–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silva LM, Braz LG and Módolo NS: Emergence
agitation in pediatric anesthesia: Current features. J Pediatr (Rio
J). 84:107–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goa KL, Noble S and Spencer CM:
Sevoflurane in paediatric anesthesia: A review. Paediatr drugs.
1:127–153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatch DJ: New inhalation agents in
paediatric anesthesia. Br J Anaesth. 83:42–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bedirli N, Akçabay M and Emik U: Tramadol
vs dexmedetomidine for emergence agitation control in pediatric
patients undergoing adenotonsillectomy with sevoflurane anesthesia:
Prospective randomized controlled clinical study. Bmc
Anesthesiology. 17:412017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Use T, Nakahara H, Kimoto A, Beppu Y,
Yoshimura M, Kojima T and Fukano T: Barbiturate induction for the
prevention of emergence agitation after pediatric sevoflurane
anesthesia. J Pediatr Pharmacol Ther. 20:385–392. 2015.PubMed/NCBI
|
|
10
|
Cravero J, Surgenor S and Whalen K:
Emergence agitation in paediatric patients after sevoflurane
anesthesia and no surgery: A comparison with halothane. Paediatr
Anaesth. 10:419–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beskow A and Westrin P: Sevoflurane causes
more postoperative agitation in children than does halothane. Acta
Anesthesiol Scand. 43:536–541. 1999. View Article : Google Scholar
|
|
12
|
Sun L, Guo R and Sun L: Dexmedetomidine
for preventing sevoflurane-related emergence agitation in children:
A meta-analysis of randomized controlled trials. Acta Anesthesiol
Scand. 58:642–650. 2014. View Article : Google Scholar
|
|
13
|
Li J, Huang ZL, Zhang XT, Luo K, Zhang ZQ,
Mao Y, Zhuang XB, Lian QQ and Cao H: Sufentanil reduces emergence
agitation in children receiving sevoflurane anesthesia for
adenotonsillectomy compared with fentanyl. Chin Med J (Engl).
124:3682–3685. 2011.PubMed/NCBI
|
|
14
|
Theivanayagam S, Lopez KT and Asombang AW,
Bechtold ML and Asombang AW: ASA classification prior to endoscopic
procedures: A retrospective analysis on accuracy of
gastroenterologists. South Med J. 110:79–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barakat AR, Sutcliffe N and Schwab M:
Effect site concentration during propofol TCI sedation: A
comparison of sedation score with two pharmacokinetic models.
Anesthesia. 62:661–666. 2007. View Article : Google Scholar
|
|
16
|
Sikich N and Lerman J: Development and
psychometric evaluation of the pediatric anesthesia emergence
delirium scale. Anesthesiology. 100:1138–1145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aono J, Ueda W, Mamiya K, Takimoto E and
Manabe M: Greater incidence of delirium during recovery from
sevoflurane anesthesia in preschool boys. Anesthesiology.
87:1298–1300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aldrete JA: The post-anesthesia recovery
score revisited. J Clin Anesth. 7:89–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Büttner W and Finke W: Analysis of
behavioural and physiological parameters for the assessment of
postoperative analgesic demand in newborns, infants and young
children: A comprehensive report on seven consecutive studies.
Paediatr Anaesth. 10:303–318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jürgens S: Sevoflurane conscious sedation
for MRI scanning. Anesthesia. 58:296–297. 2003. View Article : Google Scholar
|
|
21
|
Malviya S, Voepel-Lewis T, Ramamurthi RJ,
Burke C and Tait AR: Clonidine for the prevention of emergence
agitation in young children: Efficacy and recovery profile.
Paediatr Anaesth. 16:554–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keaney A, Diviney D, Harte S and Lyons B:
Postoperative behavioral changes following anesthesia with
sevoflurane. Pediatr Anesth. 14:866–870. 2004. View Article : Google Scholar
|
|
23
|
Cohen IT, Drewsen S and Hannallah RS:
Propofol or midazolam do not reduce the incidence of emergence
agitation associated with desflurane anesthesia in children
undergoing adenotonsillectomy. Paediatr Anaesth. 12:604–609. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KM, Lee KH, Kim YH, Ko MJ, Jung JW and
Kang E: Comparison of effects of intravenous midazolam and ketamine
on emergence agitation in children: Randomized controlled trial. J
Int Med Res. 44:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Voepel-Lewis T, Malviya S and Tait AR: A
prospective cohort study of emergence agitation in the pediatric
postanesthesia care unit. Anesth Analg. 96:1625–1630. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demirbilek S, Togal T, Cicek M, Aslan U,
Sizanli E and Ersoy MO: Effects of fentanyl on the incidence of
emergence agitation in children receiving desflurane or
sevoflurane. anaesthesia. Eur J Anaesthesiol. 21:538–542. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maeda S, Tomoyasu Y, Higuchi H,
Ishii-Maruhama M, Egusa M and Miyawaki T: Independent predictors of
delay in emergence from general anesthesia. Anesth Prog. 62:8–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Özcengiz D, Gunes Y and Ozmete O: Oral
melatonin, dexmedetomidine, and midazolam for prevention of
postoperative agitation in children. J Anesth. 25:184–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akin A, Bayram A, Esmaoglu A, Tosun Z,
Aksu R, Altuntas R and Boyaci A: Dexmedetomidine vs midazolam for
premedication of pediatric patients undergoing anesthesia. Paediatr
Anaesth. 22:871–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan Y, Shi Y, Ding H, Kong X, Zhou H and
Tian J: µ-Opioid agonists for preventing emergence agitation under
sevoflurane anesthesia in children: A meta-analysis of randomized
controlled trials. Paediatr Anaesth. 26:139–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim J, Kim SY, Lee JH, Kang YR and Koo BN:
Low-dose dexmedetomidine reduces emergence agitation after
desflurane anesthesia in children undergoing strabismus surgery.
Yonsei Med J. 55:508–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cravero JP, Beach M, Thyr B and Whalen K:
The effect of small dose fentanyl on the emergence characteristics
of pediatric patients after sevoflurane anesthesia without surgery.
Anesth Analg. 97:364–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galinkin JL, Fazi LM, Cuy RM, Chiavacci
RM, Kurth CD, Shah UK, Jacobs IN and Watcha MF: Use of intranasal
fentanyl in children undergoing myringotomy and tube placement
during halothane and sevoflurane anesthesia. Anesthesiology.
93:1378–1383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Zhang Y, Zhou M, Xia Q, Li W and Lu
Q: The effect of small dose sufentanil on emergence agitation in
preschool children following sevoflurane anesthesia for elective
repair of unilateral inguinal hernia. Saudi Med J. 34:40–45.
2013.PubMed/NCBI
|
|
35
|
Weldon BC, Bell M and Craddock T: The
effect of caudal analgesia on emergence agitation in children after
sevoflurane versus halothane anesthesia. Anesth Analg. 98:321–326.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aouad MT, Kanazi GE, Siddik-Sayyid SM,
Gerges FJ, Rizk LB and Baraka AS: Preoperative caudal block
prevents emergence agitation in children following sevoflurane
anesthesia. Acta Anaesthesiol Scand. 49:300–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen IT, Hannallah RS and Hummer KA: The
incidence of emergence agitation associated with desflurane
anesthesia in children is reduced by fentanyl. Anesth Analg.
93:88–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Panchgar V, Shetti AN, Sunitha HB,
Dhulkhed VK and Nadkarni AV: The effectiveness of intravenous
dexmedetomidine on perioperative hemodynamics, analgesic
requirement, and side effects profile in patients undergoing
laparoscopic surgery under general anesthesia. Anesth Essays Res.
11:72–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lundeberg S and Roelofse JA: Aspects of
pharmacokinetics and pharmacodynamics of sufentanil in pediatric
practice. Pediatr Anesth. 21:274–279. 2011. View Article : Google Scholar
|
|
40
|
Anderson BJ and Meakin GH: Scaling for
size: Some implications for paediatric anaesthesia dosing. Paediatr
Anaesth. 12:205–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murat G, Yasemin G, Hayri O, Dervis Y and
Geylan I: Comparison of dexmedetomidine or remifentanil infusion
combined with sevoflurane anesthesia in craniotomy: Hemodynamic
variables and recovery. Neurosurg Quart. 19:116–119. 2009.
View Article : Google Scholar
|
|
42
|
Park JS, Kim KJ, Lee JH, Jeong WY and Lee
JR: A randomized comparison of remifentanil target-controlled
infusion versus dexmedetomidine single-dose administration: A
better method for smooth recovery from general sevoflurane
anesthesia. Am J Ther. 23:e690–e696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Talke PO, Caldwell JE, Richardson CA,
Kirkegaard-Nielsen H and Stafford M: The effects of dexmedetomidine
on neuromuscular blockade in human volunteers. Anesth Analg.
88:633–639. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Unlugenc H, Gunduz M, Guler T, Yagmur O
and Isik G: The effect of preanaesthetic administration of
intravenous dexmedetomidine on postoperative pain in patients
receiving patient-controlled morphine. Eur J Anaesthesiol.
22:386–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arain SR, Ruehlow RM, Uhrich TD and Ebert
TJ: The efficacy of dexmedetomidine versus morphine for
postoperative analgesia after major inpatient surgery. Anesth
Analg. 98:153–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Isik B, Arslan M, Tunga AD and Kurtipek O:
Dexmedetomidine decreases emergence agitation in pediatric patients
after sevoflurane anesthesia without surgery. Pediatr Anesth.
16:748–753. 2006. View Article : Google Scholar
|
|
47
|
Patel A, Davidson M, Tran MC, Quraishi H,
Schoenberg C, Sant M, Lin A and Sun X: Dexmedetomidine infusion for
analgesia and prevention of emergence agitation in children with
obstructive sleep apnea syndrome undergoing tonsillectomy and
adenoidectomy. Anesth Analg. 111:1004–1110. 2010.PubMed/NCBI
|
|
48
|
Kim HS, Byon HJ, Kim JE, Park YH, Lee JH
and Kim JT: Appropriate dose of dexmedetomidine for the prevention
of emergence agitation after desflurane anesthesia for
tonsillectomy or adenoidectomy in children: Up and down sequential
allocation. BMC Anesthesiol. 15:792015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Messerer B, Gutmann A, Weinberg A and
Sandner-Kiesling A: Implementation of a standardized pain
management in a pediatric surgery unit. Pediatr Surg Int.
26:879–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tufanogullari B, White PF, Peixoto MP,
Kianpour D, Lacour T, Griffin J, Skrivanek G, Macaluso A, Shah M
and Provost DA: Dexmedetomidine infusion during laparoscopic
bariatric surgery: The effect on recovery outcome variables. Anesth
Analg. 106:1741–1748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Swennen G, Berten JL, Schliephake H,
Treutlein C, Dempf R, Malevez C and De MA: Midfacial morphology in
children with unilateral cleft lip and palate treated by different
surgical protocols. Int J Oral Maxillofac Surg. 31:13–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guler G, Akin A, Tosun Z, Eskitascoglu E,
Mizrak A and Boyaci A: Single-dose dexmedetomidine attenuates
airway and circulatory reflexes during extubation. Acta
Anaesthesiol Scand. 49:1088–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Turan G, Ozgultekin A, Turan C, Dincer E
and Yuksel G: Advantageous effects of dexmedetomidine on
haemodynamic and recovery responses during extubation for
intracranial surgery. Eur J Anaesthesiol. 25:816–820. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kato J, Ogawa Y, Kojima W, Aoki K, Ogawa S
and Iwasaki K: Cardiovascular reflex responses to temporal
reduction in arterial pressure during dexmedetomidine infusion: A
double-blind, randomized and placebo-controlled study. Br J
Anaesth. 103:561–565. 2009. View Article : Google Scholar : PubMed/NCBI
|