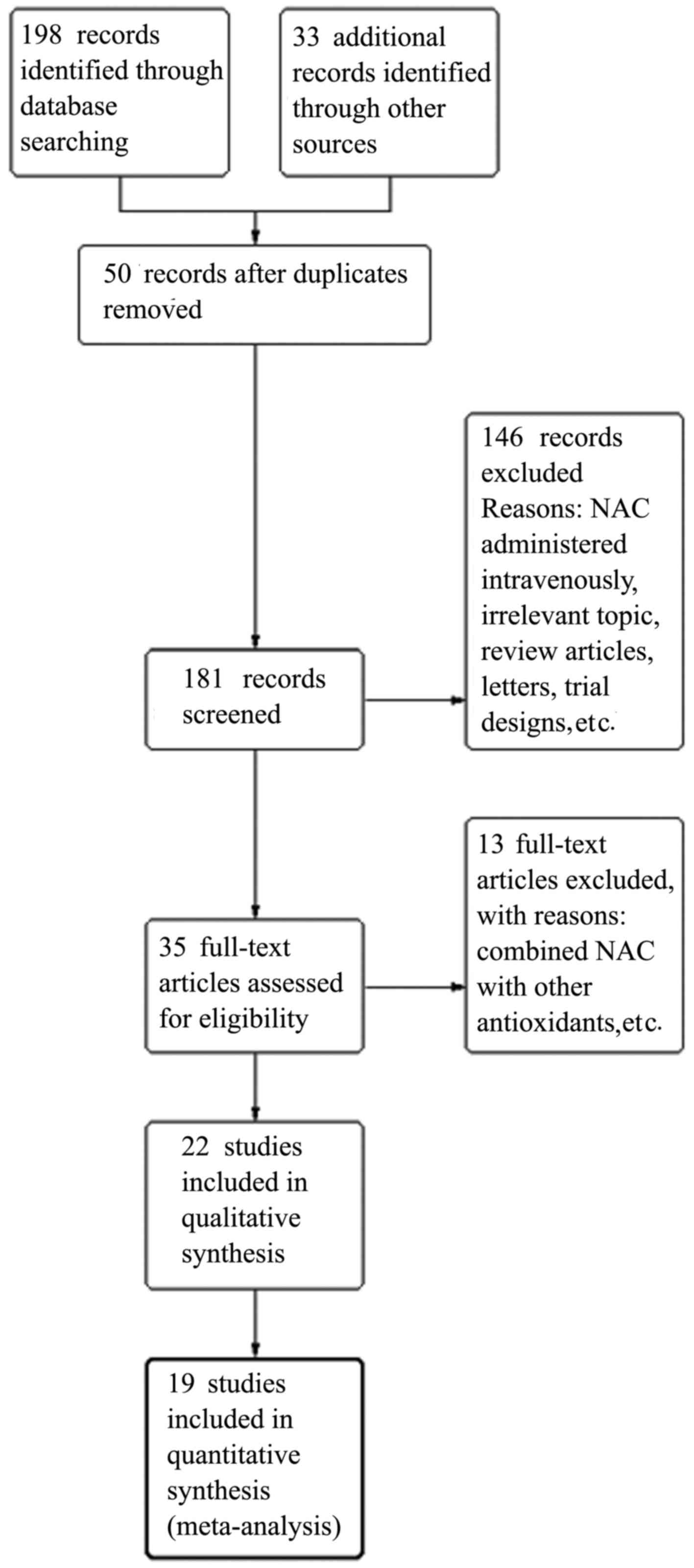
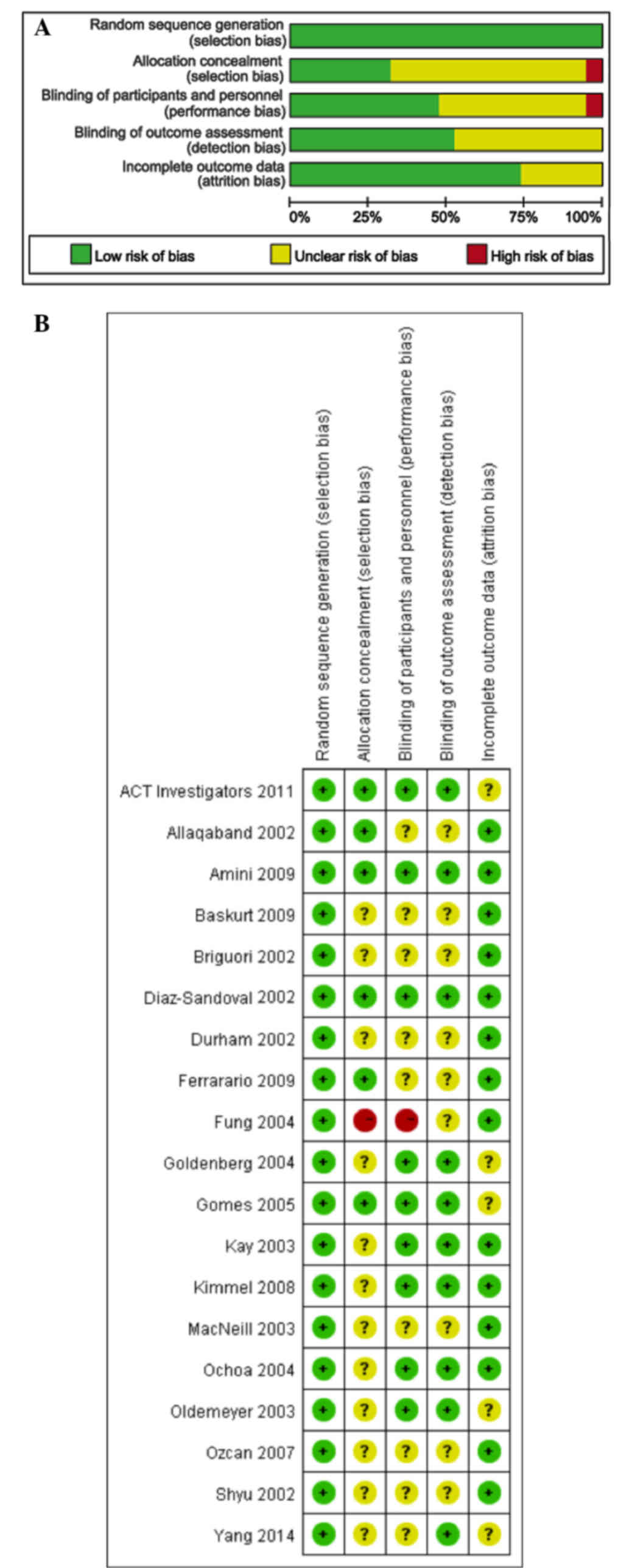
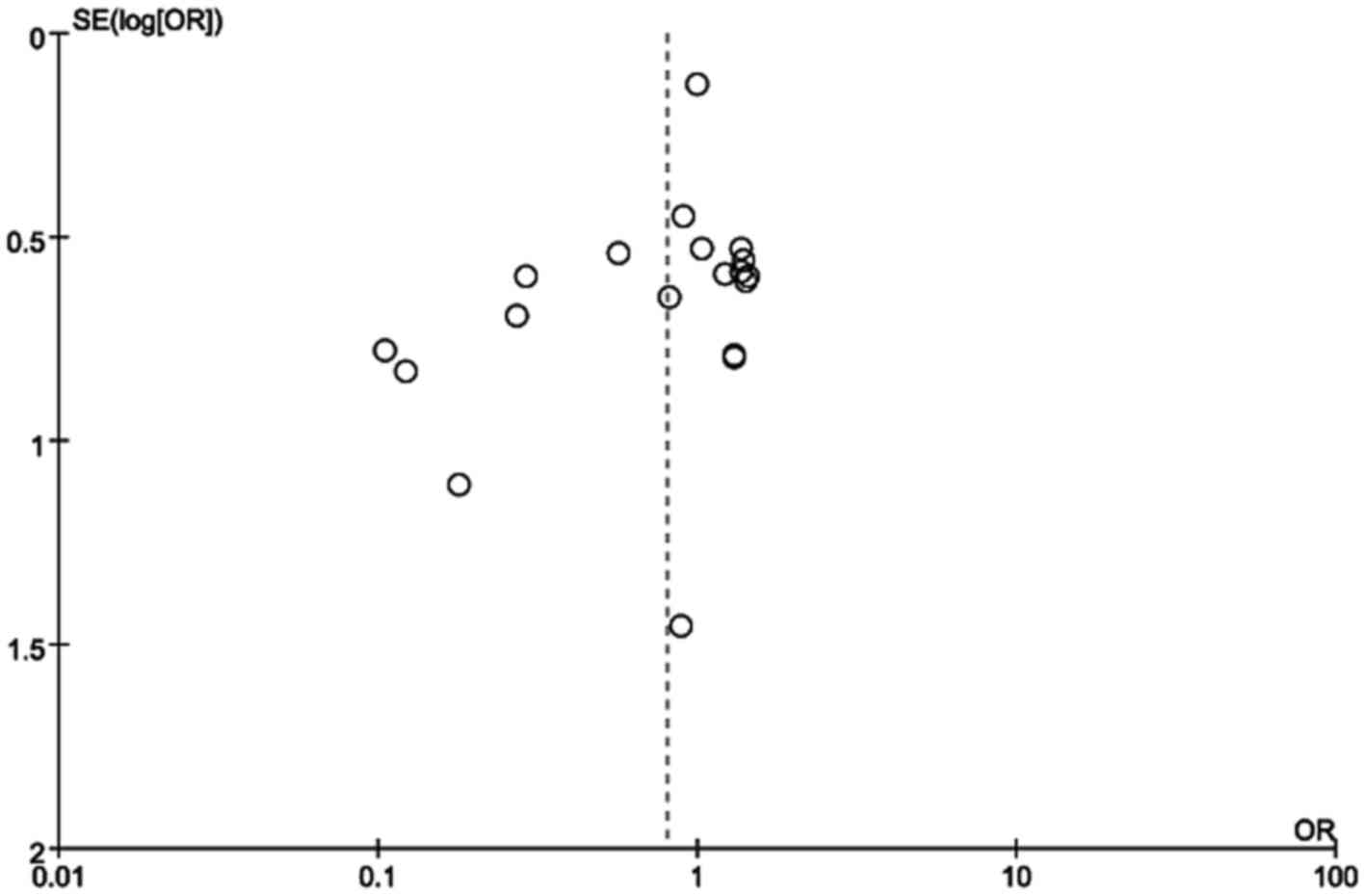
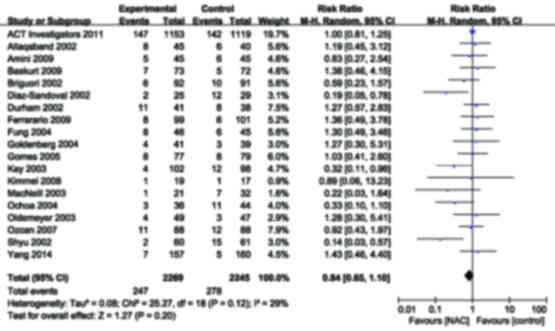
|
1
|
Tepel M, Aspelin P and Lameire N:
Contrast-induced nephropathy: A clinical and evidence-based
approach. Circulation. 113:1799–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomsen HS: European Society of Urogenital
Radiology (ESUR) guidelines on the safe use of iodinated contrast
media. Eur J Radiol. 60:307–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCullough PA, Wolyn R, Rocher LL, Levin
RN and O'Neill WW: Acute renal failure after coronary intervention:
Incidence, risk factors, and relationship to mortality. Am J Med.
103:368–375. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rihal CS, Textor SC, Grill DE, Berger PB,
Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, et al:
Incidence and prognostic importance of acute renal failure after
percutaneous coronary intervention. Circulation. 105:2259–2264.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikolsky E, Mehran R, Turcot D, Aymong ED,
Mintz GS, Lasic Z, Lansky AJ, Tsounias E, Moses JW, Stone GW, et
al: Impact of chronic kidney disease on prognosis of patients with
diabetes mellitus treated with percutaneous coronary intervention.
Am J Cardiol. 94:300–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartholomew BA, Harjai KJ, Dukkipati S,
Boura JA, Yerkey MW, Glazier S, Grines CL and O'Neill WW: Impact of
nephropathy after percutaneous coronary intervention and a method
for risk stratification. Am J Cardiol. 93:1515–1519. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehran R, Aymong ED, Nikolsky E, Lasic Z,
Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al:
A simple risk score for prediction of contrast-induced nephropathy
after percutaneous coronary intervention: Development and initial
validation. J Am Coll Cardiol. 44:1393–1399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parfrey PS, Griffiths SM, Barrett BJ, Paul
MD, Genge M, Withers J, Farid N and McManamon PJ: Contrast
material-induced renal failure in patients with diabetes mellitus,
renal insufficiency, or both. A prospective controlled study. N Eng
J Med. 320:143–149. 1989. View Article : Google Scholar
|
|
9
|
Rudnick MR and Goldfarb S: Pathogenesis of
contrast-induced nephropathy: Experimental and clinical
observations with an emphasis on the role of osmolality. Rev
Cardiovasc Med. 4 Suppl 5:S28–S33. 2003.PubMed/NCBI
|
|
10
|
Murphy SW, Barrett BJ and Parfrey PS:
Contrast nephropathy. J Am Soc Nephrol. 11:177–182. 2000.PubMed/NCBI
|
|
11
|
Wright RS, Anderson JL, Adams CD, Bridges
CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H,
Lincoff AM, et al: 2011 ACCF/AHA focused update of the Guidelines
for the Management of Patients With Unstable
Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007
Guideline): A report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines developed in collaboration with the American College of
Emergency Physicians, Society for Cardiovascular Angiography and
Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol.
59:1920–1959. 2011. View Article : Google Scholar
|
|
12
|
Tepel M, van der Giet M, Schwarzfeld C,
Laufer U, Liermann D and Zidek W: Prevention of
radiographic-contrast-agent-induced reductions in renal function by
acetylcysteine. N Engl J Med. 343:180–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shalansky SJ, Vu T, Pate GE, Levin A,
Humphries KH and Webb JG: N-acetylcysteine for prevention of
radiographic contrast material-induced nephropathy: Is the
intravenous route best? Pharmacotherapy. 25:1095–1103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karimzadeh I, Khalili H, Sagheb MM and
Farsaei S: A double-blinded, placebo-controlled, multicenter
clinical trial of N-acetylcysteine for preventing amphotericin
B-induced nephrotoxicity. Expert Opin Drug Metab Toxicol.
11:1345–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker CS, Wragg A, Kumar S, De Palma R,
Baker LR and Knight CJ: A rapid protocol for the prevention of
contrast induced renal dysfunction: The RAPPID study. J Am Coll
Cardiol. 41:2114–2118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ochoa A, Pellizzon G, Addala S, Grines C,
Isayenko Y, Boura J, Rempinski D, O'Neill W and Kahn J: Abbreviated
dosing of N-acetylcysteine prevents contrast-induced nephropathy
after elective and urgent coronary angiography and intervention. J
Interv Cardiol. 17:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MacNeill BD, Harding SA, Bazaril H, Patton
KK, Colon-Hernadez P, DeJoseph D and Jang IK: Prophylaxis of
contrast-induced nephropathy in patients undergoing coronary
angiography. Catheter Cardiovasc Interv. 60:458–461. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Briguori C, Manganelli F, Scarpato P, Elia
PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A
and Ricciardelli B: Acetylcysteine and contrast agent associated
nephrotoxicity. J Am Coll Cardiol. 40:298–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diaz-Sandoval LJ, Kosowsky BD and Losordo
DW: Acetylcysteine to prevent angiography-related renal tissue
injury (the APART trial). Am J Cardiol. 89:356–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kay J, Chow WH, Chan TM, Lo SK, Kwok OH,
Yip A, Fan K, Lee CH and Lam WF: Acetylcysteine for prevention of
acute deterioration of renal function following elective coronary
angiography and intervention: A randomized controlled trial. JAMA.
289:553–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shyu KG, Cheng JJ and Kuan P:
Acetylcysteine protects against acute renal damage in patients with
abnormal renal function undergoing a coronary procedure. J Am Coll
Cardiol. 40:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
ACT Investigators, . Acetylcysteine for
prevention of renal outcomes in patients undergoing coronary and
peripheral vascularangiography: Main results from the randomized
Acetylcysteine for Contrast-induced nephropathy Trial (ACT).
Circulation. 124:1250–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allaqaband S, Tumuluri R, Malik AM, Gupta
A, Volkert P, Shalev Y and Bajwa TK: Prospective randomized study
of N-acetylcysteine, fenoldopam and saline for prevention of
radiocontrast-induced nephropathy. Catheter Cardiovasc Interv.
57:279–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amini M, Salarifar M, Amirbaigloo A,
Masoudkabir F and Esfahani F: N-acetylcysteine does not prevent
contrast-induced nephropathy after cardiac catheterization in
patients with diabetes mellitus and chronic kidney disease: A
randomized clinical trial. Trials. 10:452009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baskurt M, Okcun B, Abaci O, Dogan GM,
Kilickesmez K, Ozkan AA, Ersanli M and Gurmen T: N-acetylcysteine
versus N-acetylcysteine+theophylline for the prevention of contrast
nephropathy. Eur J Clin Invest. 39:793–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oldemeyer JB, Biddle WP, Wurdeman RL,
Mooss AN, Cichowski E and Hilleman DE: Acetylcysteine in the
prevention of contrast induced nephropathy after coronary
angiography. Am Heart J. 146:E232003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Durham JD, Caputo C, Dokko J, Zaharakis T,
Pahlavan M, Keltz J, Dutka P, Marzo K, Maesaka JK and Fishbane S: A
randomized controlled trial of N-acetylcysteine to prevent contrast
nephropathy in cardiac angiography. Kidney Int. 62:2202–2207. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrario F, Barone MT, Landoni G,
Genderini A, Heidemperger M, Trezzi M, Piccaluga E, Danna P and
Scorza D: Acetylcysteine and non-ionic isosmolar contrast-induced
nephropathy - a randomized controlled study. Nephrol Dial
Transplant. 24:3103–3107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fung JW, Szeto CC, Chan WW, Kum LC, Chan
AK, Wong JT, Wu EB, Yip GW, Chan JY, Yu CM, et al: Effect of
N-acetylcysteine for prevention of contrast nephropathy in patients
with moderate to severe renal insufficiency: A randomized trial. Am
J Kidney Dis. 43:801–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldenberg I, Shechter M, Matetzky S,
Jonas M, Adam M, Pres H, Elian D, Agranat O, Schwammenthal E and
Guetta V: Oral acetylcysteine as an adjunct to saline hydration for
the prevention of contrast-induced nephropathy following coronary
angiography. A randomized controlled trial and review of the
current literature. Eur Heart J. 25:212–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomes VO, de Figueredo CE Poli, Caramori
P, Lasevitch R, Bodanese LC, Araújo A, Röedel AP, Caramori AP,
Brito FS Jr, Bezerra HG, et al: N-acetylcysteine does not prevent
contrast induced nephropathy after cardiac catheterisation with an
ionic low osmolality contrast medium: A multicentre clinical trial.
Heart. 91:774–778. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimmel M, Butscheid M, Brenner S, Kuhlmann
U, Klotz U and Alscher DM: Improved estimation of glomerular
filtration rate by serum cystatin C in preventing contrast induced
nephropathy by N-acetylcysteine or zinc - preliminary results.
Nephrol Dial Transplant. 23:1241–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozcan EE, Guneri S, Akdeniz B, Akyildiz
IZ, Senaslan O, Baris N, Aslan O and Badak O: Sodium Bicarbonate,
N-acetylcysteine and saline for prevention of radiocontrast-induced
nephropathy. A comparison of 3 regimens for protecting
contrast-induced nephropathy in patients undergoing coronary
procedures. A single-center prospective controlled trial. Am Heart
J. 154:539–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang K, Liu W, Ren W and Lv S: Different
interventions in preventing contrast-induced nephropathy after
percutaneous coronary intervention. Int Urol Nephrol. 46:1801–1807.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Task Force on Myocardial Revascularization
of the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS, European
Association for Percutaneous Cardiovascular Interventions (EAPCI),
; Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg
S, Huber K, et al: Guidelines on myocardial revascularization. Eur
Heart J. 31:2501–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stephan Windecker, Kolh P, Alfonso F,
Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P,
et al: 2014 ESC/EACTS guidelines on myocardial revascularization.
Rev Esp Cardiol (Engl Ed). 68:1442015.PubMed/NCBI
|
|
37
|
Nash K, Hafeez A and Hou S:
Hospital-acquired renal insufficiency. Am J Kidney Dis. 39:930–936.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morcos SK, Thomsen HS and Webb JA:
Contrast-media-induced nephrotoxicity: A consensus report. Contrast
Media Safety Committee, European Society of Urogenital Radiology
(ESUR). Eur Radiol. 9:1602–1613. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stacul F, van der Molen AJ, Reimer P, Webb
JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement
O, et al: Contrast induced nephropathy: Updated ESUR contrast media
safety committee guidelines. Eur Radiol. 21:2527–2541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waikar SS and Bonventre JV: Creatinine
kinetics and the definition of acute kidney injury. J Am Soc
Nephrol. 20:672–679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thomsen HS and Morcos SK: Risk of
contrast-medium-induced nephropathy in high-risk patients
undergoing MDCT - a pooled analysis of two randomized trials. Eur
Radiol. 19:891–897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reddan D, Laville M and Garovic VD:
Contrast-induced nephropathy and its prevention: What do we really
know from evidence-based findings? J Nephrol. 22:333–351.
2009.PubMed/NCBI
|
|
43
|
Toprak O: What is the best definition of
contrast-induced nephropathy? Ren Fail. 29:387–388. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bräutigam M and Persson PB: Do iodinated
contrast media interfere with renal tubular creatinine secretion?
Radiology. 240:6152006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Z, Fu Q, Cao L, Jin W, Cheng L and Li
Z: Intravenous N-acetylcysteine for prevention of contrast-induced
nephropathy: A meta-analysis of randomized, controlled trials. PLoS
One. 8:e551242013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim BJ, Sung KC, Kim BS, Kang JH, Lee KB,
Kim H and Lee MH: Effect of N-acetylcysteine on cystatin C-based
renal function after elective coronary angiography (ENABLE Study):
A prospective, randomized trial. Int J Cardiol. 138:239–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Molitoris BA, Levin A, Warnock DG,
Joannidis M, Mehta RL, Kellum JA, Ronco C and Shah SV; Acute Kidney
Injury Network working group, : Improving outcomes of acute kidney
injury: report of an initiative. Nat Clin Pract Nephrol. 3:439–442.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, Hu S, Liu Y, Zhao R, Wang L, Fu G,
He Q, Su X, Zheng Y, Qi X, et al: Renal tolerability of iopromide
and iodixanol in 562 renally impaired patients undergoing cardiac
catheterisation: The DIRECT study. EuroIntervention. 8:830–838.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stacul F, Adam A, Becker CR, Davidson C,
Lameire N, McCullough PA and Tumlin J; CIN Consensus Working Panel,
: Strategies to reduce the risk of contrast-induced nephropathy. Am
J Cardiol. 98:59K–77K. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mueller C, Buerkle G, Buettner HJ,
Petersen J, Perruchoud AP, Eriksson U, Marsch S and Roskamm H:
Prevention of contrast media-associated nephropathy: randomized
comparison of 2 hydration regimens in 1620 patients undergoing
coronary angioplasty. Arch Intern Med. 162:329–336. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Erley CM, Duda SH, Rehfuss D, Scholtes B,
Bock J, Müller C, Osswald H and Risler T: Prevention of
radiocontrast-media-induced nephropathy in patients with
pre-existing renal insufficiency by hydration in combination with
the adenosine antagonist theophylline. Nephrol Dial Transplant.
14:1146–1149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sendeski M, Patzak A and Persson PB:
Constriction of the vasa recta, the vessels supplying the area at
risk for acute kidney injury, by four different iodinated contrast
media, evaluating ionic, nonionic, monomeric and dimeric agents.
Invest Radiol. 45:453–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Seeliger E, Sendeski M, Rihal CS and
Persson PB: Contrast-induced kidney injury: Mechanisms, risk
factors, and prevention. Eur Heart J. 33:2007–2015. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Seeliger E, Lenhard DC and Persson PB:
Contrast media viscosity versus osmolality in kidney injury:
Lessons from animal studies. Biomed Res Int. 2014:3581362014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Parfrey P: The clinical epidemiology of
contrast-induced nephropathy. Cardiovasc Intervent Radiol. 28 Suppl
2:S3–S11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kiefer P, Vogt J and Radermacher P: From
mucolytic to antioxidant and liver protection: New aspects in the
intensive care unit career of N-acetylcysteine. Crit Care Med.
28:3935–3936. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Efrati S, Dishy V, Averbukh M, Blatt A,
Krakover R, Weisgarten J, Morrow JD, Stein MC and Golik A: The
effect of N-acetylcysteine on renal function, nitric oxide, and
oxidative stress after angiography. Kidney Int. 64:2182–2187. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Quintavalle C, Brenca M, De Micco F, Fiore
D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G,
et al: In vivo and in vitro assessment of pathways involved in
contrast media-induced renal cells apoptosis. Cell Death Dis.
2:e1552011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li JX, Shen YQ, Cai BZ, Zhao J, Bai X, Lu
YJ and Li XQ: Arsenic trioxide induces the apoptosis in vascular
smooth muscle cells via increasing intracellular calcium and ROS
formation. Mol Biol Rep. 37:1569–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|