Introduction
Sepsis is a systemic inflammatory reaction induced
by severe infections (1). This can
result in detrimental immunological responses characterized by the
production of inflammatory cytokines, arachidonic acid-derived
eicosanoids and reactive oxygen species, and lymphocyte apoptosis
(2). These factors contribute to a
range of acute and chronic diseases and may lead to high mortality
when they present with sepsis complicated with multiple organ
dysfunction syndrome (MODS) (3).
Currently, there are agents that are capable of blocking
inflammatory and immunological cascades, but their efficacy and
safety among septic patients have not been fully established. For
example, while thymic peptide is commonly used for modulating
immunological functions in septic patients, a recent systematic
review that included five randomized control studies showed that
thymic peptide-α1 did not reduce mortality rate (4). Large doses of immunoglobulin may be
able to regulate immunological responses, but its effect on septic
patients remains unclear (5).
There is an increasing interest in the
supplementation of clinical nutrition for improving clinical
outcomes (2). Omega-3 fatty acids
are essential fatty acids that are commonly found in fish such as
salmon and tuna. They cannot be made by the human body and must
instead be supplied through food intake. Omega-3 fatty acids have
been shown to prevent hyper-inflammatory processes by inhibiting
both the pro-inflammatory arachidonic acid metabolites and the
release of platelet-activating factor (6). Omega-3 fatty acids also reduce the
production of prostaglandin E2 and thromboxane B2 from inflammatory
cells (7,8). However, the immunomodulatory effects of
omega-3 fatty acid are less well-documented.
The aim of the current study was to determine if
Omega-3 fatty acids could have a significant effect on the length
of intensive care unit (ICU) stays and mortality rates among septic
patients with intestinal dysfunction, and if Omega-3 fatty acids
could modulate ratios of T-helper to inducer cells and CD4 to CD8
cells, which are the key immunity parameters associated with
disease severity and mortality. Septic patients with intestinal
dysfunction were chosen as subjects due to their high mortality
rate and the relative ease of Omega-3 fatty acid administration via
total parenteral nutrition (TPN).
Materials and methods
Study designs
Septic patients were prospectively enrolled in the
ICU of Shenzhen People's Hospital (Shenzhen, China), from August
2011 to April 2012. The study was approved by the Medical Ethic
Committee of Shenzhen People's Hospital. The study was conducted
according to The Declaration of Helsinki. Written informed consent
was obtained from all patients.
Study population
The eligibility of all participants admitted to the
ICU was assessed using the following inclusion criteria: Aged 18
years or above; systemic inflammatory reaction syndrome caused by
severe infection or trauma; Marshall score >3; intestinal
dysfunction, abdominal infection and/or post-operation of abdominal
or intestinal surgery and unable to intake nutrition via stomach
and intestine; and severe infection treated with carbapenems after
admission to the ICU. The exclusion criteria were: Marshall score
≥20; life expectancy <28 days due to a chronic or incurable
disease such as uncontrolled cancer; life expectancy <24 h; ICU
stay <7 days after meeting inclusion criteria; or the patient
having signed a ‘do-not-resuscitate’ form.
The definition of sepsis in the current study met
the sepsis diagnosis criteria published previously (9). The diagnosis criteria of intestinal
dysfunction were in accordance with the Chinese criteria of stage
and severity of MODS (10).
Intestinal dysfunction was classified into three severity
categories: Score 1, abdominal distension and hypoactive bowel
sounds; score 2, severe abdominal distention and absence of bowel
sounds; and score 3, paralytic ileus and stress ulcer.
Treatment protocols and
intervention
All patients received early goal-directed fluid
resuscitation, mechanical ventilation and carbapenems medication,
and additional antibiotics were administered according to the
results of microbiological cultivation. Blood glucose control and
prophylactic treatments of potential complications were also
conducted. In addition, thymic peptide-α1 (1.6 mg via subcutaneous
injection twice per day) and ulinastatin (20 million units, venous
injection three times per day) were administered, and the
physicians were asked to treat the patients based on the Sepsis
Survival Campaign guidelines (9).
All patients received a calorie intake of 20 kcal/kg per day in the
first 7 days after their admission to ICU. The study participants
were randomized into two groups. Patients in group B received
standard TPN with 100 ml Omega-3 fatty acids (containing 10 g
refined fish oil) per day (Fresenius SE & Co. KGaA, Bad
Homburg, Germany). Laboratory results were obtained by taking blood
samples at day 7 that were then analyzed using flow cytometry.
Briefly, a Beckman Coulter EPICS ALTRA flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA) was used. A total of 2 ml blood was
anticoagulated using heparin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). A 50 µl sample was extracted and placed in a flow
cytometer measuring probe with 20 µl fluorescence marked monoclonal
antibody (ab206509; 1:1,000; Abcam, Cambridge, MA, USA). The
mixture was agitated and incubated at room temperature for 20 min.
A total of 2 ml hemolysin (Sigma-Aldrich; Merck KGaA) was added and
the mixture was incubated for 10 min at room temperature. The
sample was subsequently centrifuged for 50 min at 157 × g at room
temperature and the serum was discarded. The pellet was resuspended
in 2 ml PBS, and centrifuged for 5 min at 157 × g at room
temperature; this process was repeated twice. A fluorescent
monoclonal antibody (ab206511; 1:500; Abcam) was added with 500 µl
PBS resuspension. Finally, the cells were analyzed using a FACScan
system (BD Biosciences, Franklin Lakes, NJ, USA).
Randomization
The investigators referred to a manual of unique
numbers generated by an independent statistician prior to the study
activation to determine the study intervention for each patient.
Study investigators and research coordinators were blinded to
treatment allocation. The attending care nurses were aware of the
treatment allocation, while the patients (and patients' families)
remained unaware of the intervention throughout the trial.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM SPSS, Armonk, NY, USA). Data are presented as the mean ±
standard deviation. Student's t-test and Z-test were used to
analyze the difference between Group A and Group B. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic characteristics
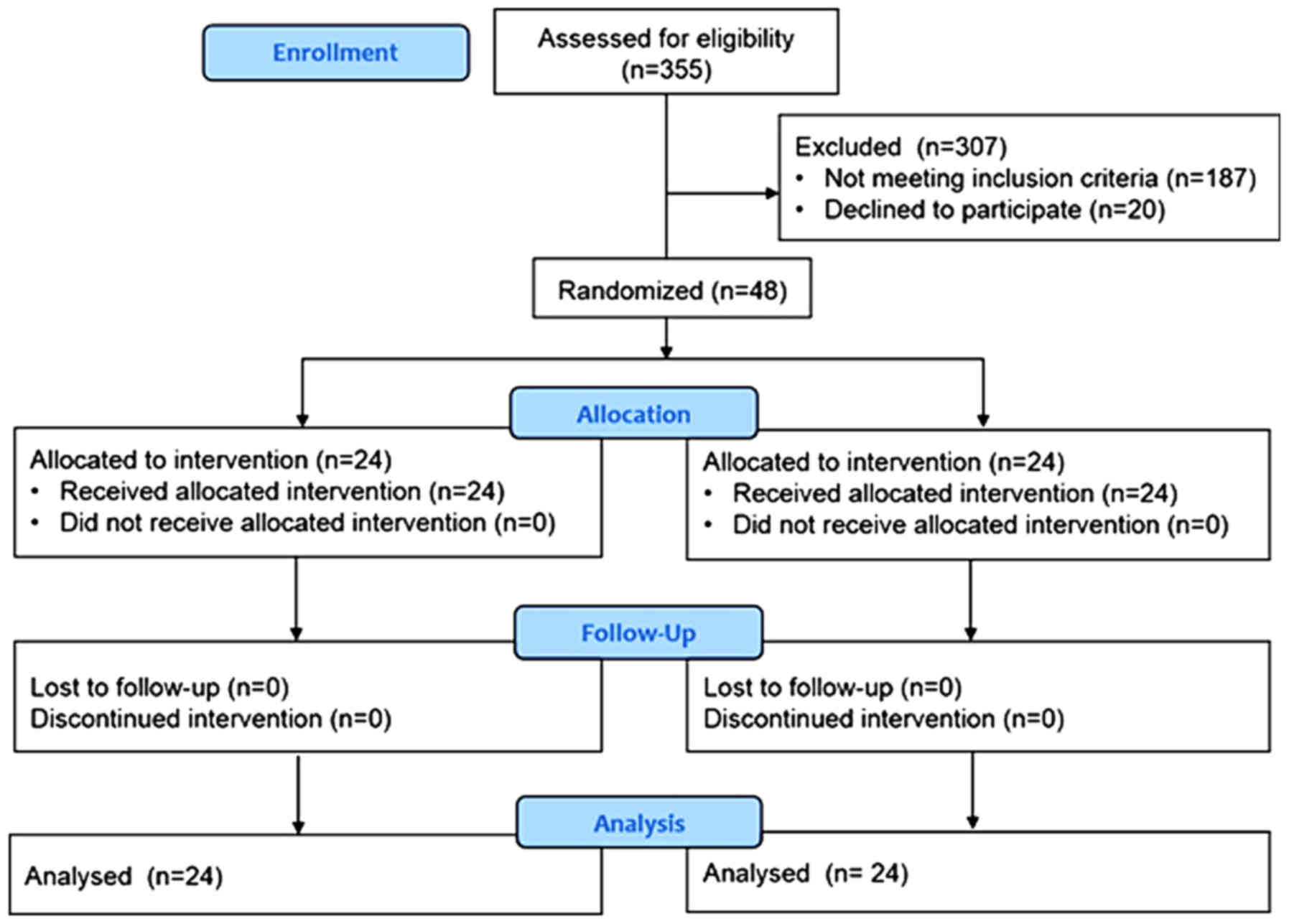
Between August 2011 and April 2012, 355 patients
were admitted to the ICU. Of these, 48 patients who met the
inclusion criteria were enrolled in the study and were randomized
into two groups, each consisting of 24 patients (Fig. 1). There were no significant
differences in terms of gender, abdominal infection severity or
standard treatments between groups A and B (Table I). Group A and B had similar
intestinal dysfunction scores (1.79±0.59 vs. 1.83±0.76; P=0.833).
Laboratory results were also compared between the two groups and no
significant differences were found (Table II).
 | Table I.Clinical characteristics of the study
participants. |
Table I.
Clinical characteristics of the study
participants.
| Variable (%) | Group A (n=24) | Group B (n=24) | χ2 | P-value |
|---|
| Male | 16 (66.7) | 18 (75) | 0.403 | 0.53 |
| Abdominal
infection | 20 (83.3) | 15 (62.5) | 0.075 | 0.78 |
| Abdominal
surgery | 6 (25.0) | 10 (41.7) | 1.500 | 0.18 |
| Use of teicoplanin
medication | 7 (29.2) | 9 (37.5) | 0.375 | 0.38 |
| Use of antifungal
medication | 8 (33.3) | 11 (45.8) | 0.784 | 0.28 |
| Hemofiltration | 3 (12.5) | 7 (29.2) | 2.021 | 0.14 |
 | Table II.Laboratory results of the study
participants at day 0. |
Table II.
Laboratory results of the study
participants at day 0.
| Variable | Group A (n=24) | Group B (n=24) | Z-value | P-value |
|---|
| Age, years | 65.6 (16.7) | 61.6 (16.2) | −1.888 | 0.059 |
| APACHE II score | 25.6 (5.2) | 23.4 (5.1) | −1.551 | 0.121 |
| Marshall score | 10.3 (2.1) | 9.8 (1.6) | −0.976 | 0.329 |
| Laboratory
results |
| White
blood cells (×109/l) | 15.7 (7.1) | 15.4 (7.4) | −0.392 | 0.695 |
|
Procalcitonin (ng/ml) | 5.4 (3.2) | 4.5 (3.1) | −0.072 | 0.942 |
|
Hypersensitive C-reactive
protein (mg/l) | 123.7 (83.5) | 159.9 (84.1) | −1.549 | 0.122 |
| CD3
lymphocyte (%) | 41.2 (14.1) | 44.3 (13.4) | −0.619 | 0.536 |
| T
helper/inducer (%) | 20.5 (10.2) | 21.5 (8.8) | −0.103 | 0.918 |
| T
suppressor (%) | 18.8 (13.0) | 18.1 (9.6) | −0.093 | 0.926 |
|
CD3/CD19 (%) | 9.9 (5.9) | 12.7 (6.2) | −0.711 | 0.477 |
| NK
cells (%) | 14.7 (9.5) | 13.2 (7.0) | −0.474 | 0.635 |
| CD4/CD8
(%) | 1.3 (0.7) | 1.5 (0.9) | −0.330 | 0.741 |
| IgG
(g/l) | 10.4 (3.7) | 9.0 (3.0) | −1.134 | 0.257 |
| IgA
(g/l) | 2.5 (1.6) | 1.9 (1.1) | −1.217 | 0.224 |
| IgM
(g/l) | 0.7 (0.5) | 0.7 (0.4) | −0.526 | 0.559 |
| C3
(g/l) | 0.9 (0.3) | 0.9 (0.6) | −0.258 | 0.797 |
| C4
(g/l) | 0.2 (0.1) | 0.2 (0.1) | −0.444 | 0.657 |
Primary outcomes
Ten patients (41.7%, 10/24) in group A succumbed to
the disease, while three (12.5%, 3/24) in group B succumbed during
the 28-day follow-up (P=0.023). For both groups, there was a
decline in both the Acute Physiology and Chronic Health Evaluation
II (APACHE II) and Marshall scores at 7 days after treatment began.
In addition, the APACHE II and Marshall scores in group B were
significantly lower than those in group A at day 7 (P<0.05;
Table III).
 | Table III.APACHE II score, Marshall score and
ICU stay of the study participants at day 7. |
Table III.
APACHE II score, Marshall score and
ICU stay of the study participants at day 7.
| Variable | Group A (n=24) | Group B (n=24) | t value | P-value |
|---|
| APACHE II score
(SD) | 21.5 (8. 9) | 16.1 (6.1) | 2.435 | 0.019a |
| Marshall score
(SD) | 8.6 (4.3) | 6.2 (2.5) | 2.325 | 0.026a |
| ICU stay (days,
SD) | 24.4 (23.2) | 13.8 (9.9) | 2.055 | 0.046a |
Secondary outcomes
Levels of white blood cells, procalcitonin (PCT),
hypersensitive C-reactive protein (hsCRP) and other laboratory
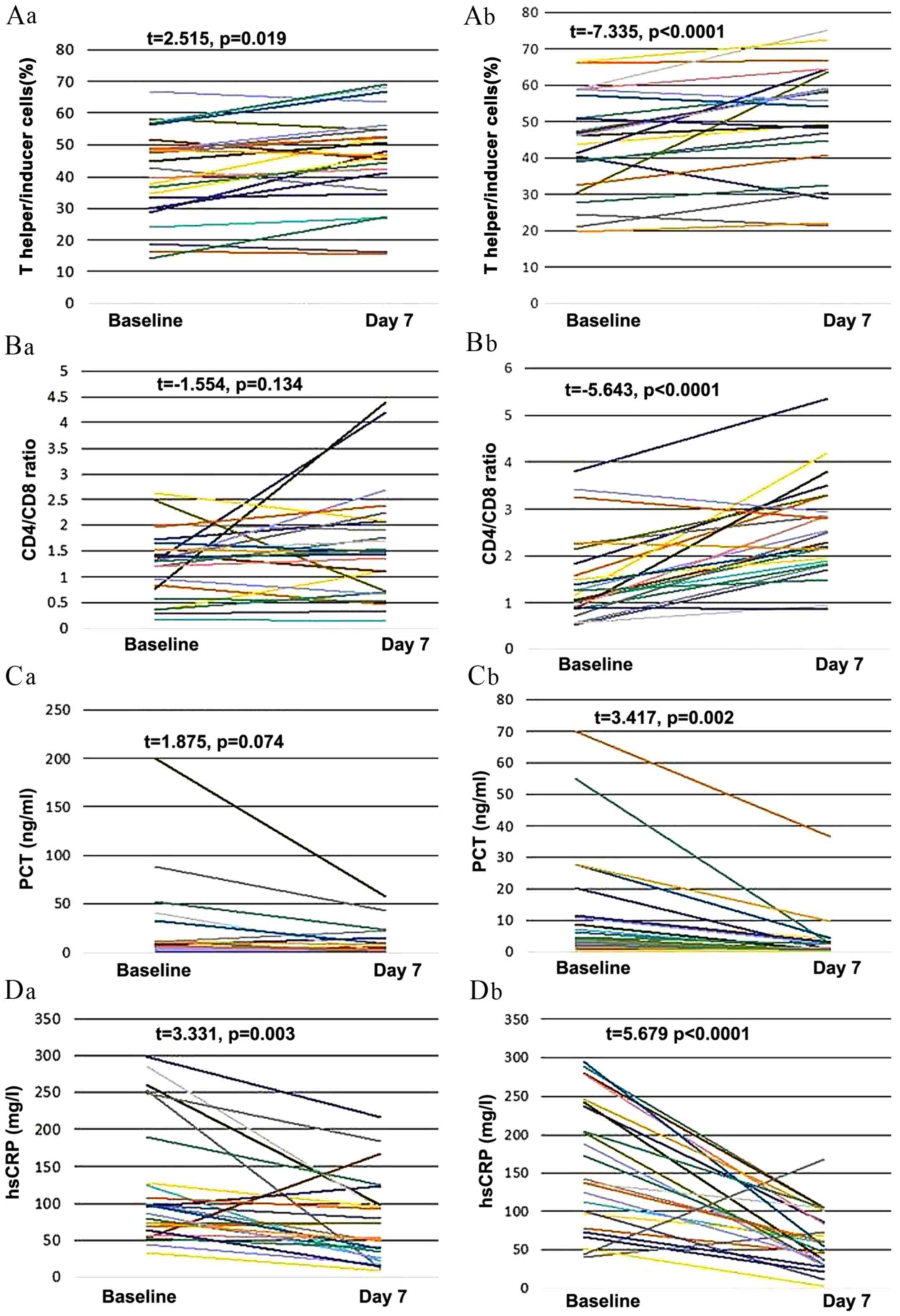
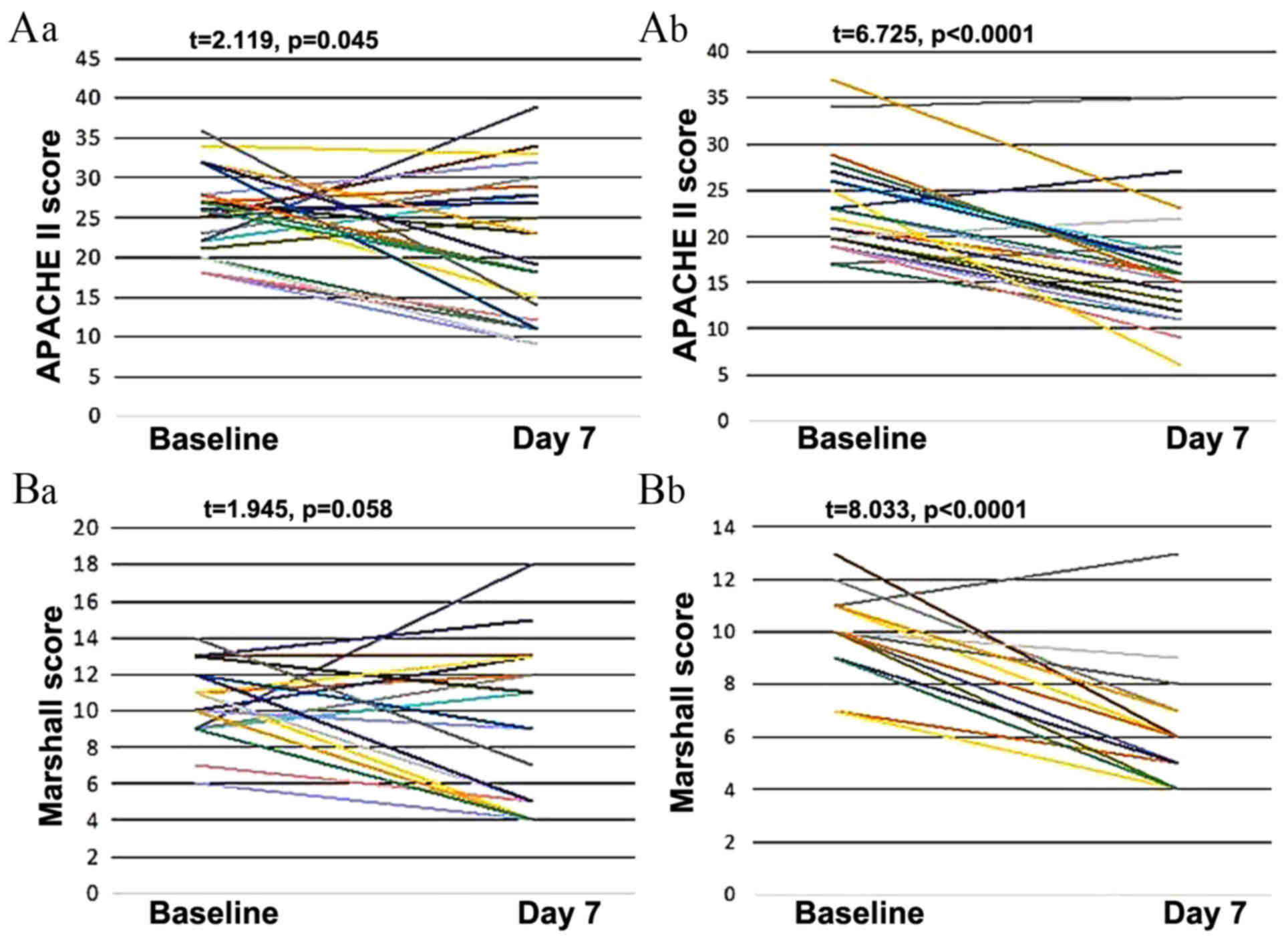
outcomes reflecting immunologic function are listed in Table IV. Changes in cells, protein levels,
and health scores are presented in Figs.
2 and 3. In group B, from day 1
to 7, there were significant increases in the ratio of T helper to
inducer cells (P<0.01; Fig. 2Ab)
and the ratio of CD4 to CD8 cells (P<0.01; Fig. 2Bb). There were also significant
decreases in PCT (P<0.01; Fig.
2Cb), hsCRP (P<0.01; Fig.
2Db), APACHE II scores (P<0.01; Fig. 3Ab) and Marshall scores (P<0.01;
Fig. 3Bb) between day 0 and 7 in
group B.
 | Table IV.Comparison of laboratory results at
day 7. |
Table IV.
Comparison of laboratory results at
day 7.
| Variable | Group A (n=24) | Group B (n=24) | Z value | P-value |
|---|
| White blood cells
(×109/L) | 13.9 (5.1) | 13.1 (3.5) | −0.381 | 0.703 |
| Procalcitonin
(ng/ml) | 2.6 | 0.6 | −1.794 | 0.073 |
| Hypersensitive
C-reactive protein (mg/l) | 74.2
(56.42) | 63.3
(38.3) | −0.278 | 0.781 |
| CD3 lymphocyte
(%) | 46.4
(14.73) | 51.1
(15.2) | −1.299 | 0.194 |
| T helper/inducer
(%) | 24.3
(10.50) | 35.3 (8.9) | −3.423 | 0.001a |
| T suppressor
(%) | 18.0
(9.12) | 15.8 (6.5) | −0.505 | 0.613 |
| CD3/CD19 (%) | 11.6
(7.93) | 13.7 (7.5) | −1.031 | 0.302 |
| NK cells (%) | 15.6
(9.05) | 12.3 (5.9) | −1.227 | 0.220 |
| CD4/CD8 (%) | 1.6
(1.08) | 2.6
(1.0) | −3.300 | 0.001a |
| IgG (g/l) | 10.5
(4.51) | 9.1
(4.2) | −1.227 | 0.220 |
| IgA (g/l) | 2.9
(1.94) | 2.0
(0.9) | −1.547 | 0.122 |
| IgM (g/l) | 0.6 | 0.7 | −1.011 | 0.312 |
| C3 (g/l) | 0.8
(0.32) | 0.9
(0.4) | −1.176 | 0.240 |
| C4 (g/l) | 0.2 | 0.3 | −1.383 | 0.167 |
In group A, however, the ratio of T helper to
inducer cells significantly decreased instead of increased
(P<0.05; Fig. 2Aa) and there was
no significant change in the ratio of CD4 to CD8 cells (Fig. 2Ba) or the level of PCT (Fig. 2Ca) between day 0 and day 7. There was
a significant reduction in hsCRP in group A, but this result was
less significant than in group B (P=0.003 and P<0.0001
respectively; Fig. 2Da). There was
also a significant reduction in APACHE II score in group A, but
this result was less significant than in group B (P=0.045 and
P<0.0001 respectively; Fig. 3Aa).
The Marshall score did not change significantly in group A
(Fig. 3Ba).
When comparing the measurements between groups A and
B at day 7, group B had a significantly higher ratio of T helper to
inducer lymphocytes and a significantly higher ratio of CD4 to CD8
lymphocytes than Group A (P<0.01 for both; Table IV).
Discussion
Sepsis is a systemic inflammatory reaction induced
by severe infection and is a common cause of MODS (11). Previous studies have shown that
Omega-3 fatty acids could reduce inflammation and lower the risk of
chronic diseases such as heart disease and arthritis (12,13). The
current study provides new evidence in support of the beneficial
effect of Omega-3 fatty acids. The results suggest they could help
modulate immunological responses and potentially reduce mortality
rate among septic patients with intestinal dysfunction.
The average mortality rate during the 28-day
follow-up for all the participants in the present study was 27.1%,
which was similar to that reported in a previous study (14). The mortality rate of the control
group was 41.7% (10/24), despite the fact that all patients
received sepsis resuscitation bundle treatment. This was probably
due to the small sample size and/or different healthcare conditions
to those in the previous study. Compared with the control group,
patients who received Omega-3 fatty acids had a significantly lower
mortality rate (12.5%, 3/24) and a significantly shorter ICU stay
by day 28, suggesting a therapeutic function of Omega-3 fatty acids
for patients with sepsis. In addition, the APACHE II and Marshall
scores for the experimental group at day 7 were also significantly
lower compared with the control group.
The target organ of Omega-3 fatty acid treatment is
still unclear thus far. Previous studies investigating the effects
of Omega-3 fatty acids on inflammatory bowel disease symptoms have
produced mixed results (15).
Previous results have suggested that Omega-3 fatty acids have
beneficial effects on renal function and could reduce the demand
for renal replacement treatment (16). In addition, Omega-3 fatty acids
appear to improve liver and pancreas function in post-operative
cancer patients (17).
Intestinal dysfunction in septic patients is a
common condition that is associated with mortality, but a validated
definition of intestinal dysfunction has not been established
(18,19). The long-held notion that sepsis is
not a distinct inflammatory disorder has been challenged (20). Rather, an understanding of sepsis
will require the immunological mechanisms of cellular and organ
disorders to be identified.
In the current study, the ratio of T helper to
inducer cells at day 7 was significantly higher in the treatment
group compared with the control. Consistent with this, previous
animal experiments found that CD4+ T lymphocytes and the
ratio of CD4 to CD8 cells were significantly lower in septic rats
compared with the controls (21).
Furthermore, immunomodulation therapy has been found to increase
CD4+ T lymphocytes for animals with sepsis (22).
In the present study, Omega-3 fatty acids appeared
to have no effect on levels of immunoglobulin and complements (C3
and C4). However, an animal study showed an increasing level of
immunoglobulin G in chickens fed with polyunsaturated fatty acids
(23). Thus, further studies are
needed to investigate whether Omega-3 fatty acids could improve
immunoglobulin levels among patients with sepsis. Other
immunomodulatory functions of Omega-3 fatty acids include reducing
interleukin-1, −2 and −6, reducing secretion and release of
cytokines such as tumor necrosis factor (24,25), and
promoting phagocytosis of macrophages and leukocytes (26). Therefore, Omega-3 fatty acids may
serve as an immune supportive agent for patients with sepsis and
MODS, resulting in improved immune function and better clinical
outcomes.
Sepsis is known to be caused by an uncontrolled
inflammatory response. Excessive activation of the inflammatory
response results in impairment of CD4 T lymphocytes in patients
with sepsis (27). CD4 T lymphocytes
include two major populations, Th1 and Th2 cells. Th1 cells can
inhibit the secretion of cytokine, and Th2 cells can evoke an
anti-inflammatory response (22).
Omega-3 fatty acids in fish oil have beneficial effects on septic
patients by inhibiting the expression of inflammatory cytokines
(26), and by blocking the pathways
of nuclear factor κB and Toll-like receptor 4 (28). In a previous study, septic rat models
that were pre-treated with Omega-3 fatty acids in fish oil showed
an increased percentage of peripheral blood lymphocyte CD4 cells
and an increased ratio of CD4 to CD8 cells (29). HsCRP is an acute phase response
protein and its level inversely correlates with the prognosis of
patients with sepsis (30). In the
current study, hsCRP levels were slightly lower in the treatment
group compared with the control at day 7 (Table IV). Omega-3 fatty acids may also
modulate inflammation by reducing the synthesis of prostaglandin E2
through the arachidonic acid metabolism pathway (31). In summary, there is evidence to
suggest that Omega-3 fatty acids serve a function in inhibiting
inflammation (26). However, their
effect may have been overridden by other anti-inflammatory
medicines (e.g., ulinastatin) and has not been conclusively
demonstrated.
Serum PCT is a useful diagnostic indicator for
various infections. For instance, serum PCT levels increase
significantly in patients with bacteremia (32–36). In
the current study, PCT levels in both the treatment and control
groups decreased at day 7. However, they were lower in the
treatment group than the control group, suggesting that Omega-3
fatty acids may help to increase the efficacy of antibiotics.
The major limitation of the current study is that it
was not possible to monitor the adverse effects of Omega-3 fatty
acid treatment, given the current sample size. No significant
harmful effects were noted in the current study, but there is some
evidence that Omega-3 fatty acid treatment may be harmful for
patients with acute lung injury (37).
In summary, septic patients with intestinal
dysfunction may have immunodeficiencies. Omega-3 fatty acids may be
able to modulate immune function in order to counter these
deficiencies and reduce mortality. Large-scale randomized
controlled trials will need to be conducted in order to confirm the
current findings.
Acknowledgements
The authors would like to acknowledge Ms. Xiu-li Wen
and Ms. Ya-ying Zhou for their technical support in flow cytometry.
We would also like to thank Dr Yu-Cheng Chen (West China Hospital,
Sichuan University) and Dr Yuan-yang Wang (Emergency Intensive Care
Unit of Shenzhen People's Hospital) for editing the manuscript.
References
|
1
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hosny M, Nahas R, Ali S, Elshafei SA and
Khaled H: Impact of oral omega-3 fatty acids supplementation in
early sepsis on clinical outcome and immunomodulation. Egyptian J
Crit Care Med. 1:119–126. 2013. View Article : Google Scholar
|
|
3
|
Tinsley KW, Grayson MH, Swanson PE, Drewry
AM, Chang KC, Karl IE and Hotchkiss RS: Sepsis induces apoptosis
and profound depletion of splenic interdigitating and follicular
dendritic cells. J Immunol. 171:909–914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Y, Tian JH, Yang KH and Zhang P:
Evaluation of efficacy of thymosin alpha1 in the treatment of
sepsis: A systematic review. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
21:21–24. 2009.(In Chinese). PubMed/NCBI
|
|
5
|
Tugrul S, Ozcan PE, Akinci O, Seyhun Y,
Cagatay A, Cakar N and Esen F: The effects of Igm-enriched
immunoglobulin preparations in patients with severe sepsis
[ISRCTN28863830]. Crit Care. 6:357–362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calder PC: Omega-3 fatty acids and
inflammatory processes. Nutrients. 2:355–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andersson A, Fenhammar J, Weitzberg E,
Sollevi A, Hjelmqvist H and Frithiof R: Endothelin-mediated gut
microcirculatory dysfunction during porcine endotoxaemia. Br J
Anaesth. 105:640–647. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin JM and Stapleton RD: Omega-3 fatty
acids in critical illness. Nutr Rev. 68:531–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levy MM, Dellinger RP, Townsend SR,
Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay
G, Beale R, et al: The surviving sepsis campaign: Results of an
international guideline-based performance improvement program
targeting severe sepsis. Crit Care Med. 38:367–374. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong J, Zhang SW and Wang BE: Intestinal
dysfunction and multiple organ dysfunction syndrome. Chin Crit Care
Med. (issue 12): 764–767. 2005.(In Chinese).
|
|
11
|
Gillejohnson P, Hansson KE and Gårdlund B:
Severe sepsis and systemic inflammatory response syndrome in
emergency department patients with suspected severe infection.
Scand J Infect Dis. 45:186–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori TA: Omega-3 fatty acids and
cardiovascular disease: Epidemiology and effects on cardiometabolic
risk factors. Food Funct. 5:2004–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simopoulos AP: The importance of the
omega-6/omega-3 fatty acid ratio in cardiovascular disease and
other chronic diseases. Exp Biol Med (Maywood). 233:674–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiramizo SC, Marra AR, Durão MS, Paes ÂT,
Edmond MB and Pavão dos Santos OF: Decreasing mortality in severe
sepsis and septic shock patients by implementing a sepsis bundle in
a hospital setting. Plos One. 6:e267902011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barbalho SM, Rde A Goulart, Quesada K,
Bechara MD and de Carvalho Ade C: Inflammatory bowel disease: Can
omega-3 fatty acids really help? Ann Gastroenterol. 29:37–43.
2016.PubMed/NCBI
|
|
16
|
Fassett RG, Gobe GC, Peake JM and Coombes
JS: Omega-3 polyunsaturated fatty acids in the treatment of kidney
disease. Am J Kidney Dis. 56:728–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heller AR, Rössel T, Gottschlich B, Tiebel
O, Menschikowski M, Litz RJ, Zimmermann T and Koch T: Omega-3 fatty
acids improve liver and pancreas function in postoperative cancer
patients. Int J Cancer. 111:611–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wohlmuth C, Dünser MW, Wurzinger B,
Deutinger M, Ulmer H, Torgersen C, Schmittinger CA, Grander W and
Hasibeder WR: Early fish oil supplementation and organ failure in
patients with septic shock from abdominal infections: A
propensity-matched cohort study. JPEN J Parenter Enteral Nutr.
34:431–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li G, Li YS and Li JS: Acute bowel injury
and acute intestinal distress syndrome: New concept of intestinal
dysfunction. Chang Wai Yu Chang Nei Ying Yang. 17:302–305.
2010.
|
|
20
|
Carré JE and Singer M: Cellular energetic
metabolism in sepsis: The need for a systems approach. Biochim
Biophys Acta. 1777:763–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang S, Guan X, Chen J, Ou-yang B, Yang C
and Chen M: The role of immunomodulation on immune function and
prognosis in sepsis. Zhongguo Pu Tong Wai Ke Za Zhi. 1–931.
2009.
|
|
22
|
Huang S, Guan X, Chen J, Ou-yang B, Yang C
and Liu Y: Immunomodulation effect on neutrophil leukocyte and
lymphocyte subsets in septic rats. Zhonghua Pu Tong Wai Ke Xue Wen
Xian. 3:362–365. 2009.
|
|
23
|
Wang YW, Field CJ and Sim JS: Dietary
polyunsaturated fatty acids alter lymphocyte subset proportion and
proliferation, serum immunoglobulin g concentration, and immune
tissue development in chicks. Poult Sci. 79:1741–1748. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grimble RF and Tappia PS: Modulation of
pro-inflammatory cytokine biology by unsaturated fatty acids. Z
Ernahrungswiss. 37 Suppl 1:S57–S65. 1998.
|
|
25
|
Hsu CS, Chiu WC, Yeh CL, Hou YC, Chou SY
and Yeh SL: Dietary fish oil enhances adhesion molecule and
interleukin-6 expression in mice with polymicrobial sepsis. Br J
Nutr. 96:854–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lopez-Garcia E, Schulze MB, Manson JE,
Meigs JB, Albert CM, Rifai N, Willett WC and Hu FB: Consumption of
(n-3) fatty acids is related to plasma biomarkers of inflammation
and endothelial activation in women. J Nutr. 134:1806–1811.
2004.PubMed/NCBI
|
|
27
|
Guo L, Song Z, Li M, Wu Q, Wang D, Feng H,
Bernard P, Daugherty A, Huang B and Li XA: Scavenger receptor bi
protects against septic death through its role in modulating
inflammatory response. J Biol Chem. 284:19826–19834. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singer P, Shapiro H, Theilla M, Anbar R,
Singer J and Cohen J: Anti-inflammatory properties of omega-3 fatty
acids in critical illness: Novel mechanisms and an integrative
perspective. Intensive Care Med. 34:1580–1592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calder PC: The relationship between the
fatty acid composition of immune cells and their function.
Prostaglandins Leukot Essent Fatty Acids. 79:101–368. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen B, Ni D and Zhang G: The role of
early monitoring of CRP on evaluating severe sepsis. Shandong Yi
Yao. 1–95. 2010.
|
|
31
|
Barton RG, Wells CL, Carlson A, Singh R,
Sullivan JJ and Cerra FB: Dietary omega-3 fatty acids decrease
mortality and kupffer cell prostaglandin E2 production in a rat
model of chronic sepsis. J Trauma. 31:768–774. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castelli GP, Pognani C, Cita M and
Paladini R: Procalcitonin as a prognostic and diagnostic tool for
septic complications after major trauma. Crit Care Med.
37:1845–1849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jongwutiwes U, Suitharak K, Tiengrim S and
Thamlikitkul V: Serum procalcitonin in diagnosis of bacteremia. J
Med Assoc Thai. 92 Suppl 2:S79–S87. 2009.PubMed/NCBI
|
|
34
|
Ko YC, Wu WP, Hsu CS, Dai MP, Ou CC and
Kao CH: Serum and pleural fluid procalcitonin in predicting
bacterial infection in patients with parapneumonic effusion. J
Korean Med Sci. 24:398–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mofidi R, Suttie SA, Patil PV, Ogston S
and Parks RW: The value of procalcitonin at predicting the severity
of acute pancreatitis and development of infected pancreatic
necrosis: Systematic review. Surgery. 146:72–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viñas Trullen X, Rodríguez López R, Pi S
Porta, Terceros D Salazar, Sanz E Macarulla, Canal P Besora, Tellez
F Alvarez, Castro C Iglesias and Palá X Feliu: Prospective study of
procalcitonin as a diagnostic marker of the severity of secondary
peritonitis. Cir Esp. 86:24–28. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rice TW, Wheeler AP, Thompson BT, de
Boisblanc BP, Steingrub J and Rock P; NIH NHLBI Acute Respiratory
Distress Syndrome Network of Investigators, : Enteral omega-3 fatty
acid, gamma-linolenic acid, and antioxidant supplementation in
acute lung injury. JAMA. 306:1574–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|