Introduction
Measurement of serum biomarkers including B-type
natriuretic peptide (BNP) and amino terminal pro-BNP (NT-proBNP)
has become important in evaluating the risk of congestive heart
failure (CHF) (1–6). The 32-amino acid polypeptide BNP is
secreted by cardiac myocytes in response to excessive distension
and stretching of the cardiac wall and it has a half-life of ~20
min (range, 18–22 min) (7,8). The biologically inactive 76-amino acid
N-terminal fragment NT-proBNP is co-secreted with BNP and it has a
half-life of ~90 min (range, 60–120 min) (9,10). The
diverse physiological effects of BNP include natriuresis and
peripheral vasodilation, as well as inhibition of the
renin-angiotensin-aldosterone and sympathetic nervous systems
(11,12).
Understanding the level of BNP or NT-proBNP may
provide an early diagnosis and guide for CHF therapy (1,5,13,14). The
level of BNP and NT-proBNP at the time of discharge from hospital
are important in the prediction of long-term outcomes among
patients with CHF (4,5). However, the significance of BNP and
NT-proBNP levels at the time of admission remains unknown. BNP and
NT-proBNP were initially regarded as interchangeable parameters in
CHF. However, this concept was challenged following the discovery
that BNP and NT-proBNP are cleared and degraded differently
(7,9,15,16).
Furthermore, BNP and NT-proBNP are affected by other factors
besides the severity of heart failure (HF), including obesity,
renal function and inflammation (17–20).
Differences between BNP and NT-proBNP in CHF assessment and the
value of the NT-proBNP/BNP ratio remain unknown.
The present study aimed to determine which of these
factors is the optimal marker of long-term CHF outcomes. The
effects of a number of factors on the NT-proBNP/BNP ratio were
analyzed and the predictive value of BNP was determined with regard
to NT-proBNP and their ratios for short- and long-term outcomes of
CHF.
Patients and methods
Patients
The present study is an observational study that
consecutively enrolled 195 patients with acute HF between January
and December 2009. All patients provided their informed consent and
approved the present study. Patients were aged 65–80 years with a
mean age of 73. All patients were hospitalized for acute HF and
admitted to the cardiac care unit (CCU) of Juntendo University
Hospital (Tokyo, Japan). Clinical and demographic information
including gender, age, left ventricular ejection fraction,
hypertension, New York Heart Association functional class (13,14),
history of ischemic heart disease, atrial fibrillation and
hemodialysis were obtained from a review of medical records. Height
and weight data was used to calculate the body mass index (BMI).
Cardiac structure and function was determined following a standard
echocardiography. The present study was performed according to the
ethics policies of Juntendo University Hospital and was approved by
the internal review board of the hospital.
End points
The end point for the short-term outcome was
in-hospital mortality and for long-term outcomes was all-cause
mortality and readmission. Follow-up ended in August 2010.
Laboratory measurements
Blood samples obtained from the patients immediately
following admission to the CCU were sent to the clinical chemistry
laboratory (Tokyo, Japan) and analyzed. Plasma BNP and NT-proBNP
were analyzed using an AIA360 enzyme immunoassay analyzer (Tosho
Corporation, Tokyo, Japan) and an electro-chemiluminescence
immunoassay (cobas e411; Roche Diagnostics, Basel, Switzerland),
respectively, according to the manufacturer's instructions.
Estimated glomerular filtration rate (eGFR) was calculated
according to the Modification of Diet in Renal Disease equation as
follows: eGFR (ml/min/1.73 m2) = 186 ×
(SCr)−1.154 × (age)−0.203 × (0.742 if
female), where SCr represents serum creatinine level (9,15).
Statistical analysis
All variables were analyzed using the normal
distribution test. Discrete variables are presented as frequency
counts and ratios (%). Continuous variables are expressed as mean ±
standard deviation when normally distributed, and otherwise as
medians (inter-quartile range). Proportions and means/medians were
compared using the χ2 test, Student's t-test, one-way
analysis of variance and the Mann-Whitney U test. Univariate
correlations were tested in the context of normality using
Pearson's correlation coefficient. Correlations among non-normally
distributed variables were assessed using Spearman's rank
correlation (ρ). Variables with a non-normal distribution were
log-transformed prior to entry into the regression model. The
results were statistically analyzed using JMP 8.0 software (SAS
Institute Inc., Cary, NC, USA). All probabilities were two-tailed,
and P<0.05 was considered to represent a statistically
significant difference.
Results
Patient characteristics
The study population included 195 patients aged
65–80 years, with acute onset of HF. The patients' demographics,
primary disease, cardiac function evaluation, clinical presentation
and comorbidities are presented in Table
I.
 | Table I.Baseline clinical characteristics of
patients enrolled onto the present study (n=195). |
Table I.
Baseline clinical characteristics of
patients enrolled onto the present study (n=195).
| Characteristics | Values |
|---|
| Demographics |
|
| Age,
yearsa | 73 (65, 80) |
| Gender,
male/femaleb | 123 (63.1)/72
(36.9) |
| Primary disease |
|
| Ischemic
heart diseaseb | 71 (36.4) |
|
Hypertensive heart
diseaseb | 40 (20.5) |
| Valvular
heart disease and congenital heart disease b | 34 (17.4) |
| Dilated
cardiomyopathyb | 18 (11.3) |
| Cardiac function |
|
|
LVEFc, % | 43.7±18.5 |
| NYHAb,
II, III, IV | 81 (41.5), 58 (29.7),
56 (28.7) |
| BNP,
pg/mla | 757.8 (348.9,
1,554) |
|
NT-proBNP, pg/mla | 6,258 (1,873.5,
18,410.3) |
|
NT-proBNP/BNP
ratioa | 8.7 (5.3,
14.3) |
| Clinical
presentation |
|
|
Hemodialysisb | 10 (5.1) |
| eGFR,
ml/(min·1.73 m2)a | 48.1 (25.7,
65.5) |
|
BMI3,
kg/m2 | 20.3±3.7 |
|
Hgb3, mg/dl | 11.7±2.6 |
|
LDL-Ca, mg/dl | 96 (74, 119) |
| HbA1c,
mmol/mola | 5.6 (5.1, 6.2) |
| CRP,
mg/la | 0.8 (0.2, 3.5) |
| Comorbidity |
|
|
Hypertensionb | 61 (31.3) |
|
DMb | 19 (9.7) |
|
Hyperlipidemiab | 10 (5.1) |
|
Afb | 38 (19.5) |
Factors affecting NT-proBNP/BNP
ratio
Multiple linear regression analysis demonstrated
that BMI, low density lipoprotein cholesterol (LDL-C), hemoglobin
(Hgb), eGFR and C-reactive protein (CRP) were independent
predictors of the NT-proBNP/BNP ratio. Further univariate
correlation analysis indicated that BNP, NT-proBNP and their ratios
were significantly and negatively associated with BMI, Hgb and
eGFR, and positively associated with CRP. These findings indicated
that BMI, Hgb, eGFR and CPR affect NT-proBNP more than BNP. A
significant and negative association was identified between LDL-C
and NT-proBNP/BNP, but not with either NT-proBNP or BNP (Table II).
 | Table II.Multiple linear regression and
univariate Spearman correlation analysis to assess influence of
variables on BNP, NT-proBNP and NT-proBNP/BNP. |
Table II.
Multiple linear regression and
univariate Spearman correlation analysis to assess influence of
variables on BNP, NT-proBNP and NT-proBNP/BNP.
|
| Multiple linear
regression analysis (r2=0.62) | Univariate Spearman
correlation analysis |
|---|
|
|
|
|
|---|
|
| NT-proBNP/BNP | NT-proBNP/BNP | BNP | NT-proBNP |
|---|
|
|
|
|
|
|
|---|
| Variable | Estimated
coefficient | P-value | ρ | P-value | ρ | P-value | ρ | P-value |
|---|
| BMI | −0.25 |
0.04 | −0.19 |
0.02 | −0.22 | <0.01 | −0.16 |
0.05 |
| LDL-C (log) | −12.90 | <0.01 | −0.36 | <0.01 | −0.01 |
0.89 | −0.13 |
0.12 |
| Hgb | −0.35 |
0.04 | −0.29 | <0.01 | −0.16 |
0.03 | −0.22 | <0.01 |
| eGFR (log) | −18.60 | <0.01 | −0.58 | <0.01 | −0.46 | <0.01 | −0.52 | <0.01 |
| CRP (log) |
3.21 | <0.01 |
0.33 | <0.01 |
0.15 |
0.05 |
0.19 |
0.02 |
| Age (log) | −13.20 |
0.09 |
0.04 |
0.62 |
0.02 |
0.81 |
0.02 |
0.80 |
| Gender (male) |
2.18 |
0.21 | −0.02 |
0.83 |
0.01 |
0.91 |
0.03 |
0.68 |
| LVEF |
0.05 |
0.25 |
0.04 |
0.68 | −0.28 |
0.01 | −0.16 |
0.07 |
| HbA1c (log) |
0.28 |
0.98 | −0.07 |
0.40 | −0.07 |
0.34 | −0.10 |
0.22 |
| CK (log) |
1.04 |
0.59 |
0.11 |
0.17 |
0.05 |
0.52 |
0.05 |
0.52 |
Predictive value of in-hospital
mortality and long-term outcome
A total of 16 (8.2%) patients succumbed to HF in
hospital and 124 (69.3%) had an endpoint of mortality or
readmission following a median follow-up of 14 months (range, 8–20
months).
All BNP [700.0 (331.2–1,465.9) vs. 1613.7
(997.6–1,981.4), P<0.05], NT-proBNP [5,358 (1,525–14,169] vs.
18,449 (9,068–41,093), P<0.01] and the ratio of NT-proBNP to BNP
[8.4 (5.0–12.3) vs. 17.6 (7.3–28.3), P<0.01] were significantly
increased in the patients who succumbed to HF, compared with those
who remained alive while in hospital (Fig. 1). Logistic regression analysis
including NT-proBNP/BNP, NT-proBNP and BNP indicated that the
NT-proBNP/BNP ratio was the only independent predictor of
in-hospital mortality (Table III).
The Kaplan-Meier survival curves for all-cause mortality and
readmission are presented in Fig. 2
for BNP, NT-proBNP and the NT-proBNP/BNP ratio, respectively.
Quartiles of NT-proBNP and the NT-proBNP/BNP ratio significantly
differed (log-rank test, P=0.018 and P=0.0035, respectively),
whereas those of BNP (log-rank test, P=0.21) did not. Cox
proportional-hazard models of long-term outcomes including
NT-proBNP/BNP, NT-proBNP and BNP indicated that the NT-proBNP/BNP
ratio remained the only independent predictor of long-term outcomes
(Table IV).
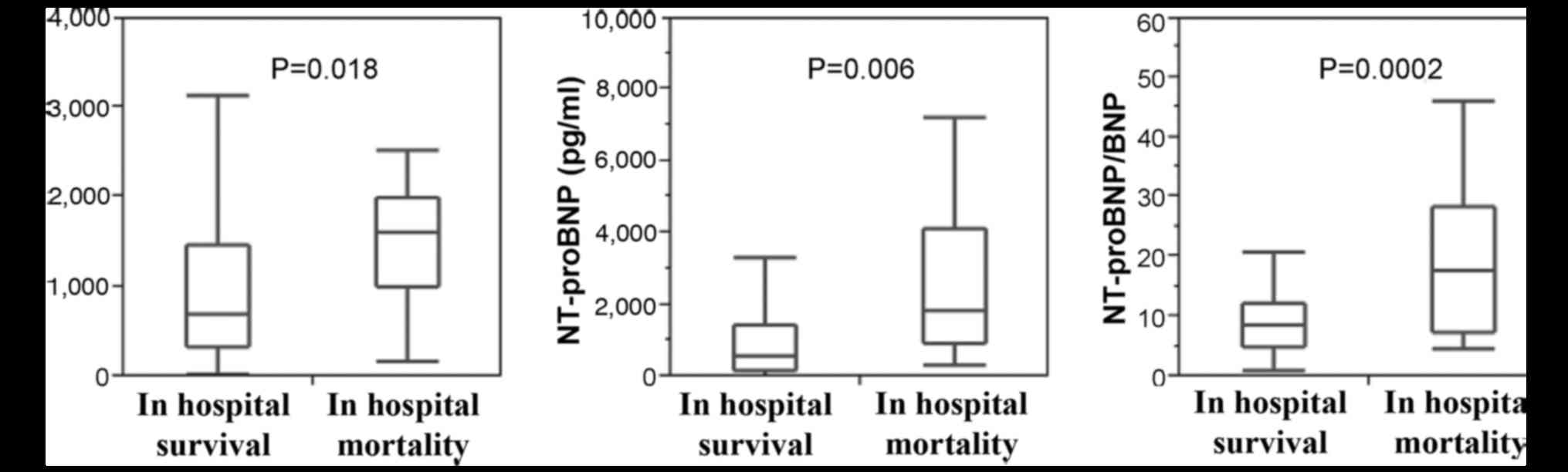 | Figure 1.BNP, NT-proBNP and ratio of NT-proBNP
to BNP levels of patients in hospital. A total of 16 patients
succumbed to heart failure and 179 survived in hospital. Values of
BNP [700.0 (331.2–1,465.9) vs. 1,613.7 (997.6–1,981.4), P=0.018],
NT-proBNP [5,358 (1,525–14,169) vs. 18,449 (9,068–41,093), P=0.006]
and NT-proBNP/BNP ratio [8.4 (5.0–12.3) vs. 17.6 (7.3–28.3),
P=0.0002] are significantly higher in the group who succumbed to
heart failure in hospital than those who survived. BNP, B-type
natriuretic peptide; NT-proBNP, amino-terminal pro-BNP. |
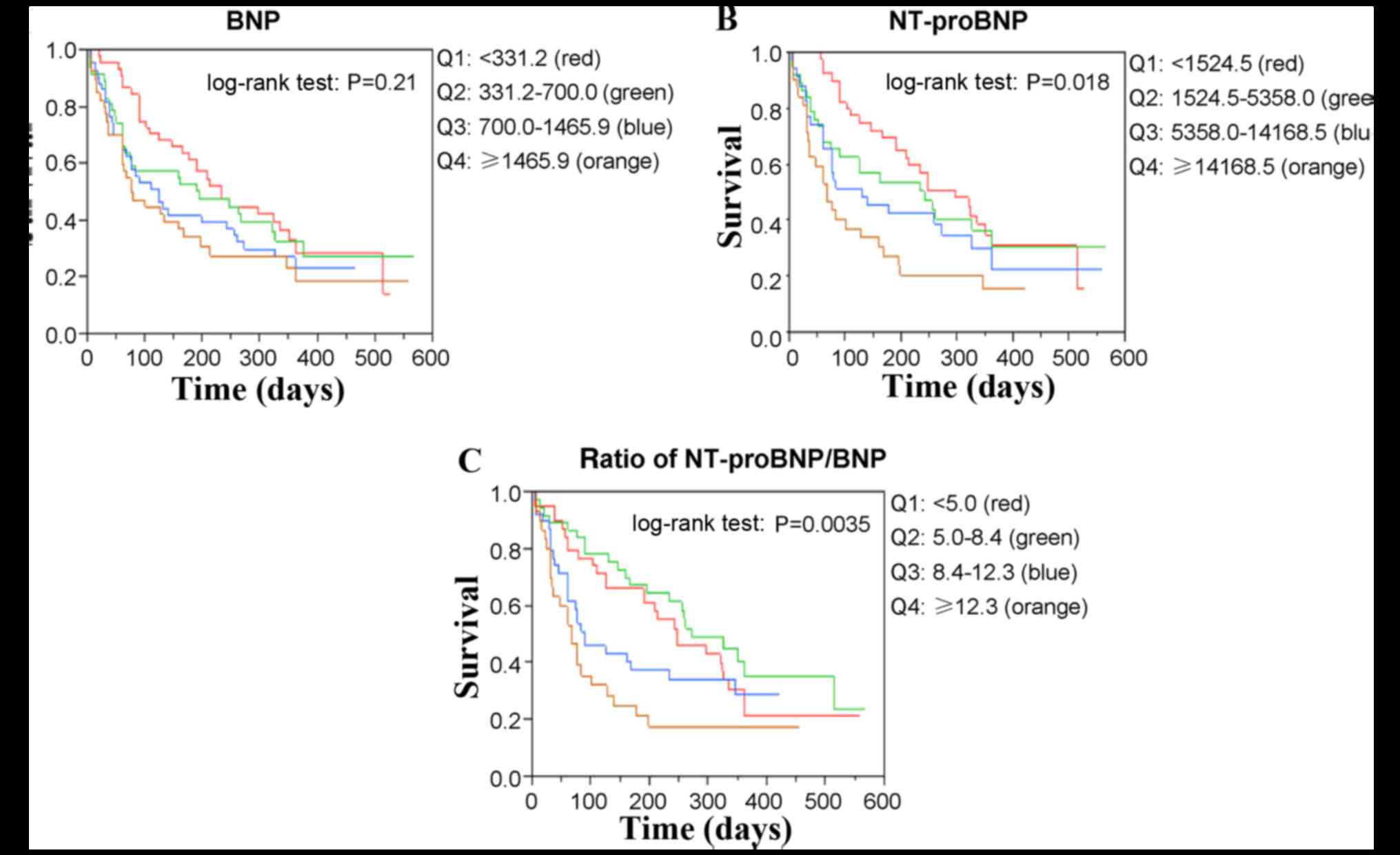 | Figure 2.Kaplan-Meier survival curves for
all-cause mortality and readmission stratified according to Q of
(A) BNP, (B) NT-proBNP and (C) ratio of NT-proBNP to BNP. Red,
green, blue and orange lines represent Q1, Q2, Q3 and Q4,
respectively. Quartiles of NT-proBNP and NT-proBNP/BNP ratio
(log-rank test, P=0.018 and P=0.0035, respectively) significantly
differed, whereas those of BNP did not (log-rank test, P=0.21). Q,
quartiles; BNP, B-type natriuretic peptide; NT-proBNP,
amino-terminal pro-B-type natriuretic peptide. |
 | Table III.Logistic regression analysis of
in-hospital mortality of 16 patients. |
Table III.
Logistic regression analysis of
in-hospital mortality of 16 patients.
|
| Model includes
NT-proBNP/BNP, adjusted | Model includes
NT-proBNP and BNP, adjusted |
|---|
|
|
|
|
|---|
| Variable | Estimated
coefficient | P-value | Estimated
coefficient | P-value |
|---|
| NT-proBNP/BNP
(log) | −10.200 | 0.038 | – | – |
| BNP (log) | – | – | 11.900 | 0.170 |
| NT-proBNP
(log) | – | – | −14.800 | 0.090 |
| Age (log) | −15.400 | 0.100 | 12.500 | 0.640 |
| Gender (male) | 1.740 | 0.150 | 1.620 | 0.510 |
| BMI | 0.349 | 0.200 | 0.250 | 0.420 |
| fEF | 0.080 | 0.130 | 0.071 | 0.480 |
| Hgb | −0.812 | 0.130 | −0.742 | 0.510 |
| eGFR (log) | −2.490 | 0.370 | −2.730 | 0.620 |
| LDL-C (log) | 1.770 | 0.670 | 6.100 | 0.540 |
| HbA1c (log) | −17.100 | 0.180 | 7.960 | 0.670 |
| CK (log) | −3.620 | 0.090 | −5.760 | 0.230 |
| CRP (log) | −1.860 | 0.120 | −0.506 | 0.730 |
 | Table IV.Cox proportional-hazard models of
long-term outcomes. |
Table IV.
Cox proportional-hazard models of
long-term outcomes.
|
| Model includes
NT-proBNP/BNP, adjusted | Model includes
NT-proBNP and BNP, adjusted |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
|
NT-proBNP/BNPa | 1.40
(1.01–1.93) | 0.040 | – | – |
| BNPa | – | – | 0.90
(0.55–1.46) | 0.670 |
|
NT-proBNPa | – | – | 1.39
(0.84–2.30) | 0.200 |
| Gender
(female) | 0.48
(0.26–0.84) | 0.010 | 0.42
(0.22–0.78) | 0.005 |
| eGFRa | 0.63
(0.44–0.89) | 0.009 | 0.63
(0.43–0.90) | 0.012 |
| Hemodialysis | 2.35
(1.52–4.38) | 0.009 | 2.42
(1.54–4.32) | 0.015 |
| Agea | 1.11
(0.86–1.45) | 0.420 | 1.12
(0.85–1.47) | 0.420 |
| NYHA | 0.82
(0.54–1.24) | 0.350 | 0.91
(0.61–1.37) | 0.660 |
| BMIa | 1.13
(0.88–1.46) | 0.320 | 1.19
(0.90–1.57) | 0.220 |
| LDL-Ca | 0.91
(0.72–1.15) | 0.410 | 0.88
(0.69–1.12) | 0.290 |
| Hgba | 0.93
(0.68–1.26) | 0.640 | 0.88
(0.63–1.22) | 0.440 |
| HbA1ca | 0.93
(0.72–1.20) | 0.580 | 0.89
(0.69–1.16) | 0.400 |
| CRPa | 0.95
(0.73–1.23) | 0.700 | 0.98
(0.75–1.27) | 0.860 |
Discussion
The present study demonstrated that the
NT-proBNP/BNP ratio predicts the in-hospital and long-term outcomes
of decompensated CHF more accurately than does the level of either
BNP or NT-proBNP at the time of admission. The majority of previous
studies (9,15,16) that
have aimed to assess the predictive value of BNP and NT-proBNP were
based on data at the time of discharge, when these levels may be
more stable than at the time of admission and reflect the basic and
long-term ventricular status. However, an increase at the time of
discharge provides more current information about exacerbation in
acute HF. Therefore, the current study predicted in-hospital and
long-term outcomes of CHF on the basis of the level of BNP and
NT-proBNP upon admission.
Cardiac myocytes synthesize proBNP that is then
transformed into hormonally active BNP and inactive NT-proBNP.
Although it is suggested that they are released from the heart in
equimolar amounts, their half-lives and clearance pathways differ
(7–9). Plasma clearance of BNP is achieved via
binding to natriuretic peptide receptor type C (NPR-C) and
proteolysis by neutral endopeptidases, whereas NT-proBNP is
primarily cleared by renal excretion (15,16).
Exogenous atrial natriuretic peptide (ANP) has been applied as part
of a treatment strategy for CHF (15). Both ANP and BNP competitively bind to
natriuretic peptide receptors but NT-proBNP does not. Nishiyama
et al (20) reported that
infused exogenous ANP (carperitide) influenced plasma BNP but not
NT-proBNP. Therefore, plasma BNP and NT-proBNP are of similar
relevance to the diagnosis and prognosis of HF but they are not
equal and each has a different role in the assessment of HF due to
different biological activities and sensitivities to
pharmacological therapy.
According to the method of Jensen et al
(21), the current study assumed
that BNP and NT-proBNP are produced at the same constant rate with
a similar distribution volume in a one-compartment model, and that
the production and elimination rates are equal. The calculated
NT-proBNP/BNP ratio is 10.9, when the supposed half-lives of BNP
and NT-proBNP are 20 and 90 min, respectively. However, the median
value of the eNT-proBNP/BNP ratio in the present study was 8.7
(5.3–14.3), which was potentially due to differences in the
influence of renal dysfunction on the elimination rate of NT-proBNP
and BNP.
The mammalian natriuretic peptide system consists of
the neurohormones, ANP, BNP, C-type natriuretic peptide (CNP) and
NT-proBNP. ANP, BNP and CNP have a common 17-amino acid ring
structure in which the majority of the amino acid residues are
conserved. Atrial myocytes release stored 28-amino acid ANP in
response to atrial distension and stretching (15,16). The
physiological actions of ANP are similar to those of BNP, including
a reduction in systemic vascular resistance and central venous
pressure and an increase in natriuresis (22). The ANP level has been demonstrated to
be significant in the prognosis of patients with atrial
fibrillation and CHF (23–25). However, BNP and NT-BNP appear
superior to ANP in the diagnosis of HF due to a closer association
with echocardiographic evidence of left ventricular dysfunction and
a longer half-life. Thus, the present study primarily focused on
BNP and NT-proBNP to reflect ventricular status.
The present study demonstrated that the major
factors affecting the NT-proBNP/BNP ratio included dystrophic
status (BMI, LDL-C), anemia (Hgb), renal dysfunction (eGFR) and
inflammation (CRP). Renal function (17,18,26,27),
obesity (28–30) and inflammation (19,21) are
non-cardiac factors that should be considered during BNP and
NT-proBNP evaluations.
The levels of BNP and NT-proBNP increase in patients
with renal failure (16). The
clearance of NT-proBNP may be more reliant upon renal filtration
and the levels may thus increase in response to renal failure to a
greater extent than those of BNP, since BNP is also cleared by
receptor-mediated uptake and protease action; therefore, the
NT-proBNP/BNP ratio would increase (31–33).
Jensen et al (21) suggested
that the increased NT-proBNP/BNP ratio is due to inflammation
increasing the level of NT-proBNP more than that of BNP.
Inflammation may increase BNP clearance mediated by protease or
receptors, thus balancing the increase in BNP production. As
NT-proBNP is not affected by these clearance mechanisms, NT-proBNP
is elevated to a larger extent by inflammation, leading to an
increased NT-proBNP/BNP ratio. The mechanism of different effects
of dystrophic status and anemia on NT-proBNP and BNP remains
unknown. However, changes in the level of NPR-C, receptor-mediated
uptake and protease action in dystrophic and anemia status may be
one explanation.
Previous studies have primarily focused on the
predictive value of NT-proBNP and BNP and both have been
demonstrated to be predictors of CHF (9,16).
However, each has advantages and disadvantages in predicting the
outcomes of CHF: BNP has a shorter half life and may theoretically
reflect more recent ventricular status, whereas NT-proBNP has
greater stability and provides more information, but not regarding
ventricular tension and function. Thus, the NT-proBNP/BNP ratio
provides considerable information about parameters including renal
dysfunction, nutrition status, anemia and inflammation, and may
therefore serve as a new index in combination with other
biomarkers. The NT-proBNP/BNP ratio may be a more useful and
accurate predictor of CHF than single biomarkers.
Although the present study indicated some
interesting results, there were also a few limitations. Firstly,
the present study involved relatively small sample amounts. We
would involve a larger patient sample in a further study. Secondly,
the predictive function of the NT-proBNP to the BNP combining
method has not been compared with the classical methods fully in a
clinical setting. We will compare the present method with the
classical methods in a further study.
In conclusion, the NT-proBNP/BNP ratio is an
improved predictor of in-hospital and long-term outcomes of CHF,
compared with BNP or NT-proBNP alone. The NT-proBNP/BNP ratio is
negatively associated with Hgb, eGFR, BMI and positively with CRP,
as each variable exerts different effects on NT-proBNP and BNP.
Anemia, renal dysfunction, inflammation and dystrophic status
affect NT-proBNP more than they affect BNP. Thus, the NT-proBNP/BNP
ratio may be useful to predict the short- and long-term outcomes of
patients with acute exacerbation of HF.
References
|
1
|
Bozkurt B and Mann DL: Use of biomarkers
in the management of heart failure: Are we there yet? Circulation.
107:1231–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jortani SA, Prabhu SD and Valdes R Jr:
Strategies for developing biomarkers of heart failure. Clin Chem.
50:265–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison A and Amundson S: Evaluation and
management of the acutely dyspneic patient: The role of biomarkers.
Am J Emerg Med. 23:371–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Costello-Boerrigter LC and Burnett JC Jr:
The prognostic value of N-terminal proB-type natriuretic peptide.
Nat Clin Pract Cardiovasc Med. 2:194–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarolim P: Serum biomarkers for heart
failure. Cardiovasc Pathol. 15:144–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aviles JM and Aviles RJ: Advances in
cardiac biomarkers. Emerg Med Clin North Am. 23:959–975. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moe GW: BNP in the diagnosis and risk
stratification of heart failure. Heart Fail Monit. 4:116–122.
2005.PubMed/NCBI
|
|
8
|
Bettencourt P: NT-proBNP and BNP:
Biomarkers for heart failure management. Eur J Heart Fail.
6:359–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richards M and Troughton RW: NT-proBNP in
heart failure: Therapy decisions and monitoring. Eur J Heart Fail.
6:351–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuat A, Murphy JJ, Hungin AP, Curry J,
Mehrzad AA, Hetherington A, Johnston JI, Smellie WS, Duffy V and
Cawley P: The diagnostic accuracy and utility of a B-type
natriuretic peptide test in a community population of patients with
suspected heart failure. Br J Gen Pract. 56:327–333.
2006.PubMed/NCBI
|
|
11
|
Moe GW: B-type natriuretic peptide in
heart failure. Curr Opin Cardiol. 21:208–214. 2006.PubMed/NCBI
|
|
12
|
Palazzuoli A, Gallotta M, Quatrini I and
Nuti R: Natriuretic peptides (BNP and NT-proBNP): Measurement and
relevance in heart failure. Vasc Health Risk Manag. 6:411–418.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McKie PM, Rodeheffer RJ, Cataliotti A,
Martin FL, Urban LH, Mahoney DW, Jacobsen SJ, Redfield MM and
Burnett JC Jr: Amino-terminal pro-B-type natriuretic peptide and
B-type natriuretic peptide: Biomarkers for mortality in a large
community-based cohort free of heart failure. Hypertension.
47:874–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maisel AS, Krishnaswamy P, Nowak RM,
McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu
AH, et al: Rapid measurement of B-type natriuretic peptide in the
emergency diagnosis of heart failure. N Engl J Med. 347:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayek S and Nemer M: Cardiac natriuretic
peptides: From basic discovery to clinical practice. Cardiovasc
Ther. 29:362–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valle R and Aspromonte N: Use of brain
natriuretic peptide and bioimpedance to guide therapy in heart
failure patients. Contrib Nephrol. 164:209–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Kimmenade RR, Januzzi JL Jr, Bakker
JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van
Dieijen-Visser MP, de Leeuw PW and Pinto YM: Renal clearance of
B-type natriuretic peptide and amino terminal pro-B-type
natriuretic peptide a mechanistic study in hypertensive subjects. J
Am Coll Cardiol. 53:884–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tagore R, Ling LH, Yang H, Daw HY, Chan YH
and Sethi SK: Natriuretic peptides in chronic kidney disease. Clin
J Am Soc Nephrol. 3:1644–1651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bednarek-Skublewska A, Zaluska W and
Ksiazek A: The relationship between serum level of N-terminal
pro-B-type natriuretic peptide and nutritional status, and
inflammation in chronic hemodialysis patients. Clin Nephrol.
73:14–20. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishiyama K, Tsutamoto T, Tanaka T, Fujii
M, Yamamoto T, Yamaji M and Horie M: Plasma NT-proBNP as a more
reliable biomarker of endogenous cardiac natriuretic peptides than
BNP during carperitide infusion. Int Heart J. 50:183–190. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jensen J, Ma LP, Fu ML, Svaninger D,
Lundberg PA and Hammarsten O: Inflammation increases NT-proBNP and
the NT-proBNP/BNP ratio. Clin Res Cardiol. 99:445–452. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Bold AJ: Atrial natriuretic factor: A
hormone produced by the heart. Science. 230:767–770. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clerico A, Iervasi G, Del Chicca MG, Emdin
M, Maffei S, Nannipieri M, Sabatino L, Forini F, Manfredi C and
Donato L: Circulating levels of cardiac natriuretic peptides (ANP
and BNP) measured by highly sensitive and specific
immunoradiometric assays in normal subjects and in patients with
different degrees of heart failure. J Endocrinol Invest.
21:170–179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rienstra M, Van Gelder IC, Van den Berg
MP, Boomsma F and Van Veldhuisen DJ: Natriuretic peptides in
patients with atrial fibrillation and advanced chronic heart
failure: Determinants and prognostic value of (NT-)ANP and
(NT-pro)BNP. Europace. 8:482–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Falcão LM, Pinto F, Ravara L and van
Zwieten PA: BNP and ANP as diagnostic and predictive markers in
heart failure with left ventricular systolic dysfunction. J Renin
Angiotensin Aldosterone Syst. 5:121–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vanderheyden M, Bartunek J, Filippatos G,
Goethals M, Vlem BV and Maisel A: Cardiovascular disease in
patients with chronic renal impairment: Role of natriuretic
peptides. Congest Heart Fail. 14 4 Suppl 1:1–42. 2008. View Article : Google Scholar
|
|
27
|
Bayes-Genis A, DeFilippi C and Januzzi JL
Jr: Understanding amino-terminal pro-B-type natriuretic peptide in
obesity. Am J Cardiol. 101:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugisawa T, Kishimoto I, Kokubo Y, Makino
H, Miyamoto Y and Yoshimasa Y: Association of plasma B-type
natriuretic peptide levels with obesity in a general urban Japanese
population: The Suita Study. Endocr J. 57:727–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srisawasdi P, Vanavanan S,
Charoenpanichkit C and Kroll MH: The effect of renal dysfunction on
BNP, NT-proBNP, and their ratio. Am J Clin Pathol. 133:14–23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kälsch H, Neumann T and Erbel R: Less
increase of BNP and NT-proBNP levels in obese patient with
decompensated heart failure: Interpretation of natriuretic peptides
in obesity. Int J Cardiol. 133:e22–e24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
deFilippi CR, Seliger SL, Maynard S and
Christenson RH: Impact of renal disease on natriuretic peptide
testing for diagnosing decompensated heart failure and predicting
mortality. Clin Chem. 53:1511–1519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DeFilippi C, van Kimmenade RR and Pinto
YM: Amino-terminal pro-B-type natriuretic peptide testing in renal
disease. Am J Cardiol. 101:82–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCullough PA and Sandberg KR: B-type
natriuretic peptide and renal disease. Heart Fail Rev. 8:355–358.
2003. View Article : Google Scholar : PubMed/NCBI
|
















