Introduction
Pomegranate is a fruit that has been cultivated for
centuries and is considered of high nutritional and therapeutic
value (1). Its biological and
therapeutic properties are primarily attributed to the presence of
polyphenols (ellagitanins, flavonoids, phenolic acids, stilbenes,
tannins and anthocyanins) (2,3), which
are free radical scavenging compounds (4). Pomegranate is also rich in vitamins and
minerals (5). Generally, pomegranate
juice (PJ) is the greatest contributor to pomegranate ingestion in
daily nutrition. Notably, the in vitro antioxidant activity
of PJ has been determined to be threefold higher compared with red
wine and green tea and is typically more potent than that of other
natural juices (6,7).
Based on phytochemical compounds and their activity
in PJ, research on the effects of PJ supplementation on health
parameters has provided promising results regarding oxidative
stress, inflammation and metabolic diseases (8). These studies have been conducted in
vitro (9), in animals (10) and humans (11,12).
Research conducted on atherosclerotic mice indicated that PJ
supplementation reduced the size of atherosclerotic lesions and
inhibited disease progression (13–15).
Research on patients with cardiovascular disease demonstrated that
following PJ supplementation, low density lipoprotein (LDL) levels
were reduced (16,17), stress induced ischemia (18) and vascular thickness decreased
(19), while positive findings were
particularly evident in patients at higher risk of coronary heart
disease (20). In healthy
individuals, PJ consumption reduced the potential for LDL
aggregation, which represents an important step towards formation
of foam cells and thus vascular thrombi (13). Furthermore, it has been indicated
that PJ intake on a regular basis may affect cholesterol metabolism
in macrophages, leading to reduced cellular uptake of oxidized LDL
(12). PJ is also able to reduce
nitric oxide synthase expression in endothelial cells of coronary
vessels (15). In 2001, Das et
al (21) examined the effect of
pomegranate seed extract ingestion in diabetic rats. The results
indicated that there was a significant decrease in glucose levels
of ~50%, 12 h after taking the extract, regardless of the dose
given (300 and 600 mg/kg). These findings may be the result of an
increased absorption of glucose by peripheral tissues, which is
associated with the presence of polyphenols in pomegranate and
their function (22).
Studies in humans and mice demonstrated that even
moderate PJ consumption reduced susceptibility to free radical
lipid peroxidation, while it increased resistance to LDL and high
density lipoprotein (HDL)-cholesterol oxidation (23). Inhibition of lipid peroxidation with
PJ supplementation has been verified in carotid artery stenosis
patients (18), type II diabetics
(12) and healthy individuals
(3). In 2014, Matthaiou et al
(3) also determined that glutathione
(GSH) levels were increased following two weeks PJ supplementation,
indicating that PJ induced beneficial changes in erythrocyte
antioxidant concentration. In 2009, Boussetta et al
(24) reported that punicic acid, a
conjugated unsaturated fatty acid which is derived from the oil of
pomegranate seeds, has anti-inflammatory action due to its
potential to reduce the activity of neutrophils. Specific tannins
in pomegranate also exhibited anti-inflammatory action in mice
(25).
Although there have been numerous studies reporting
the benefits of the polyphenols contained in PJ on health
parameters in those with various diseases, research on healthy
humans is scarce. The antioxidant properties of polyphenolic
flavonoids may enhance cell resistance to oxidative stress,
including red blood cells; however, to the best of our knowledge,
the effects of PJ consumption on complete blood counts (CBC) have
not been investigated. Therefore, the purpose of the present study
was to examine the effects of PJ supplementation on CBC, in
addition to glucose, blood lipids and C-reactive protein (CRP)
levels in healthy individuals.
Material and methods
Participants
A total of 10 healthy and recreationally active
individuals (5 males and 5 females) aged 31.8±6.6 years and
weighing 66.2±12.9 kg were recruited for the current study through
flyers and word of mouth in the region of Thessaly, Greece. Power
analysis was run using G*Power Data Analysis
(Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany) to
estimate the required sample size. It was calculated that the
necessary sample size was n=10 for statistical power 0.8, P<0.05
and effect size 0.4. Although the majority of previous studies on
PJ supplementation have used 14–18 subjects, the sample size of the
present study is enough to support the findings. Inclusion criteria
included the absence of any clinical symptom or chronic disease as
determined by a health questionnaire. Exclusion criteria included
the presence of gastrointestinal or metabolic disease, the taking
of any medication (including nutritional supplements such as
vitamins, minerals and phytosterols), smoking, current pregnancy or
lactation in women or the following of a specific diet regime.
Written informed consent was obtained from all participants when
they had been informed of all risks, discomforts and benefits
involved with the current study. The procedures were in accordance
to the 1975 Helsinki declaration and were approved by the
Institutional Review Board of the University of Thessaly (Larissa,
Greece).
Study design
During the first visit, participants received
information regarding the trial, completed a health and a physical
activity questionnaire and signed the consent form. A randomized,
counterbalanced, within-subjects experimental design was applied
and participants were randomly assigned into two groups (each,
n=5). One group consumed 500 ml/day PJ for fourteen days (the
experimental group); the other group consumed no PJ for fourteen
days (the control group). The four experimental weeks were
separated by a one-week washout period. All participants reported
to the laboratory at the start and the end of each two-week phase.
In each test day at the two-week time point, anthropometric and
physiological measurements in addition to blood sampling took place
from 8–10 a.m., following an overnight fast. Alcohol consumption
and smoking were not allowed for 12 h prior to each test day. Water
consumption was ad libitum. All measurements were performed
under controlled environmental conditions (the room temperature was
22±2°C, with a relative humidity of 45±4%).
Anthropometric and physiological
measurements
Body mass, body fat and hydration level were
assessed using leg-to-leg bioelectrical impedance scale (Tanita BF
522 W; Tanita Europe BV, Amsterdam, The Netherlands) while
participants were lightly dressed and barefooted. Standing height
was measured to the nearest 0.1 cm (Stadiometer 208, Seca GmbH,
Hamburg, Germany). Body mass index (BMI) was calculated using the
equation BMI=weight (kg)/[height (m)]2. Waist and hip
circumferences were obtained with measuring tape. Waist to hip
ratio (WHR) was calculated using the equation WHR=waist
circumference/hip circumference. Blood pressure (BP) was measured
with a manual sphygmomanometer (FC-101 Aneroid Sphygmomanometer;
Focal Corporation, Tokyo, Japan); the lowest of three readings was
recorded. Baseline anthropometric and physiological characteristics
of all participants are presented in Table I.
 | Table I.Baseline anthropometric and
physiological characteristics of all participants before and after
the experimental period. |
Table I.
Baseline anthropometric and
physiological characteristics of all participants before and after
the experimental period.
|
| EG | CG |
|---|
|
|
|
|
|---|
| Characteristic | Pre | Post | Pre | Post |
|---|
| Weight, Kg |
66.5±13.0 |
66.3±12.6 |
66.3±12.9 |
65.8±12.6 |
| Body Fat, % | 21.5±3.3 | 21.3±3.1 | 20.4±4.0 | 20.0±4.4 |
| WHR | 0.80±0.1 | 0.79±0.1 | 0.80±0.1 | 0.79±0.1 |
| SBP, mmHg | 107.0±9.8 | 106.5±8.5 | 108.0±9.5 | 103.0±10.6 |
| DBP, mmHg | 70.5±6.4 | 71.0±6.1 | 69.5±8.3 | 67.5±8.6 |
| Heart rate,
bpm |
75.3±10.3 |
73.4±10.1 | 78.1±9.1 | 73.5±11.3 |
| Hydration state,
% | 54.3±1.3 | 54.3±1.8 | 54.6±2.0 | 54.4±2.0 |
Diet and physical activity
To ensure that diet and/or exercise preceding the
trials would not affect biochemical parameters measured in the
current study, participants were instructed to refrain from
excessive exercise, record meals and physical activity two days
prior to the first trial, and follow the same diet and activities
for two days prior to each subsequent trial. Each participant was
provided with a written set of guidelines and record sheets for
recording food intake and physical activity. Diet records were
subsequently analyzed using the computerized nutritional analysis
system Science Fit Diet 200A (Science Technologies, Athens, Greece;
Table II). Participants were
instructed to follow their usual habits during the experiment
otherwise.
 | Table II.Nutritional analysis of the 2-day
diet records of all participants, in the experimental and control
groups. |
Table II.
Nutritional analysis of the 2-day
diet records of all participants, in the experimental and control
groups.
|
| EG | CG |
|---|
|
|
|
|
|---|
| Component | Pre | Post | Pre | Post |
|---|
| Energy, kcal | 1771.5±425.1 | 1765.8±403.0 | 1737.8±398.2 | 1783.8±406.4 |
| Protein, g | 74.3±24.1 | 71.4±25.7 | 73.8±25.7 | 73.5±23.3 |
| Carbohydrate,
g | 194.2±59.6 | 193.3±40.4 | 199.1±68.4 | 200.7±69.9 |
| Fat, g | 73.3±22.7 | 75.8±18.1 | 71.7±21.0 | 75.1±24.1 |
| Cholesterol,
mg | 246.9±143.1 | 240.3±127.2 | 250.9±148.1 | 228.5±110.1 |
| Fiber, g | 15.8±8.4 | 16.3±8.4 | 15.5±9.9 | 15.8±10.2 |
| Vitamin A, IU |
4,264.5±2,193.9 |
4,443.4±2,895.5 |
4,513.9±2,917.1 |
4,527.7±2,854.3 |
| Vitamin B12,
µg | 3.5±1.5 | 3.6±1.7 | 3.4±2.1 | 3.6±1.5 |
| Vitamin B6, mg | 1.8±0.5 | 1.9±0.6 | 1.7±0.7 | 1.8±0.5 |
| Vitamin C, mg | 79.3±36.4 | 77.6±29.0 | 76.6±33.6 | 71.5±34.5 |
| Vitamin D, mg) | 114.1±81.3 | 116.3±75.0 | 105.9±69.7 | 100.9±56.1 |
| Vitamin E,
mg_RE | 8.7±10.5 | 9.0±9.9 | 8.6±11.0 | 8.2±8.9 |
| Thiamine, mg | 2.7±1.4 | 2.4±1.2 | 2.2±1.4 | 2.6±1.3 |
| Niacin, mg | 21.8±7.9 | 21.0±8.3 | 19.6±6.9 | 21.1±6.8 |
| Pantothenic Acid,
mg | 11.0±20.6 | 10.2±17.3 | 12.1±25.9 | 10.3±19.1 |
| Riboflavin, mg | 2.4±1.9 | 2.6±1.7 | 2.3±1.6 | 2.2±1.3 |
| Folate, µg | 351.6±106.4 | 345.5±103.7 | 346.8±138.8 | 335.9±87.5 |
| Iron, mg | 14.0±3.2 | 14.7±4.3 | 14.7±4.6 | 14.5±2.7 |
| Selenium, µg | 110.4±33.6 | 105.0±40.5 | 106.5±44.7 | 103.4±39.4 |
Test food
The juice tested was a commercially available
product (CBH-VITOM S.A., Athens, Greece). PJ was provided to the
participants in bottles containing 250 ml each and the participants
were instructed to consume one in the morning and one in the
afternoon (a total of 500 ml per day). Each 250 ml product was
prepared from 2.5 squeezed pomegranates. Nutritional values per 100
ml and as percentages of the Recommended Daily Allowance are
presented in Table III. The
polyphenolic composition of the PJ was measured by Matthaiou et
al (3), as the PJ was the same
as the one used in the present study. Briefly, the total
polyphenolic content was 405.00 mg/l of equivalent gallic acid and
the total amount of flavonoid was 12.67 mg/l of equivalent
quercetin. PJ also contained different classes of polyphenols as
flavonoids, phenolic acids and stilbenes. The concentrations of the
polyphenols contained in the PJ used in the current study were
comparable to those identified in previous studies (26). However, total polyphenols in PJ may
vary significantly between different pomegranate cultivars
(27).
 | Table III.Nutritional values of pomegranate
juice per 100 ml and as percentages of the RDA. |
Table III.
Nutritional values of pomegranate
juice per 100 ml and as percentages of the RDA.
| Component | Per 100 ml | % RDA (250 ml) |
|---|
| Energy, kcal | 58 | 7 |
| Protein, g |
0.3 | 2 |
| Carbohydrate,
g | 14.1 | 12 |
| Sugars, g | 11.6 | – |
| Fat, g |
0.3 | 1 |
| Saturated Fat,
g |
0.0 | 0 |
| Fiber, g |
0.1 | 3 |
| Salt, mg |
0.01 | 1 |
Blood collection and analyses
Participants rested in the lab for 30 min prior to
blood sample being obtained. Participants were in a seated position
when 20 ml blood was drawn from the forearm vein. A portion (1–2
ml) of the blood collected was used to determine the parameters of
the CBC measured by an automatic hematology analyzer (Mythic™ 18;
Orphee Medical SA, Geneva, Switzerland). Each measurement was
performed twice. The remaining blood was centrifuged at 4°C, 1370 ×
g for 10 min to separate the plasma in a refrigerated
centrifuge (Heraeus™ Biogigure Primo; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Then the collected supernatant was
transferred to Eppendorf tubes (Sarstedt AG & Co., Numbrecht,
Germany). The samples were stored at −80°C and thawed only once
prior to analysis. Glucose, total cholesterol, HDL-cholesterol and
triglyceride concentrations in plasma were determined using a
biochemical analyzer (Clinical Chemistry Analyzer Z 1145;
Zafiropoulos Diagnostica S.A., Koropi, Greece). LDL-cholesterol was
calculated using the Friedewald equation (28). CRP was analyzed by a
semi-quantitative method using a commercial available kit (CRP
Latex Slide Test, Code 7300; Zafiropoulos Diagnostica S.A., Koropi,
Greece) according to the principal of the latex agglutination assay
described by Singer et al (29).
CBC test included: White blood cell (WBC),
lymphocytes (LYM), monocytes (MON), granulocytes (GRA), lymphocyte
percentage (LYM%), monocyte percentage (MON%), granulocyte
percentage (GRA%), red blood cell (RBC) count, hemoglobin (HGB),
hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular
hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC),
red blood cell distribution width (RDW), platelets (PLT), mean
platelet volume (MPV), plateletcrit (PCT) and platelet distribution
width (PDW).
Statistical analysis
Analysis of data was performed with two-way (group ×
time) repeated measures analysis of variance. If a significant
interaction was obtained, pairwise comparisons were performed
through simple main-effect analysis using the Bonferroni test
method. P<0.05 was considered to indicate a significant
difference. The statistical programme used was SPSS version 18
(SPSS Inc., Chicago, IL, USA). Results are presented as mean ±
standard deviation.
Results
General characteristics
Compliance with PJ consumption was 97.9%.
Anthropometric characteristics of participants did not change
markedly during the 5-week experimental period. Analysis of the
diet records did not reveal any significant difference in the macro
and micronutrients of the groups prior to each blood sampling
(Table II). No significant changes
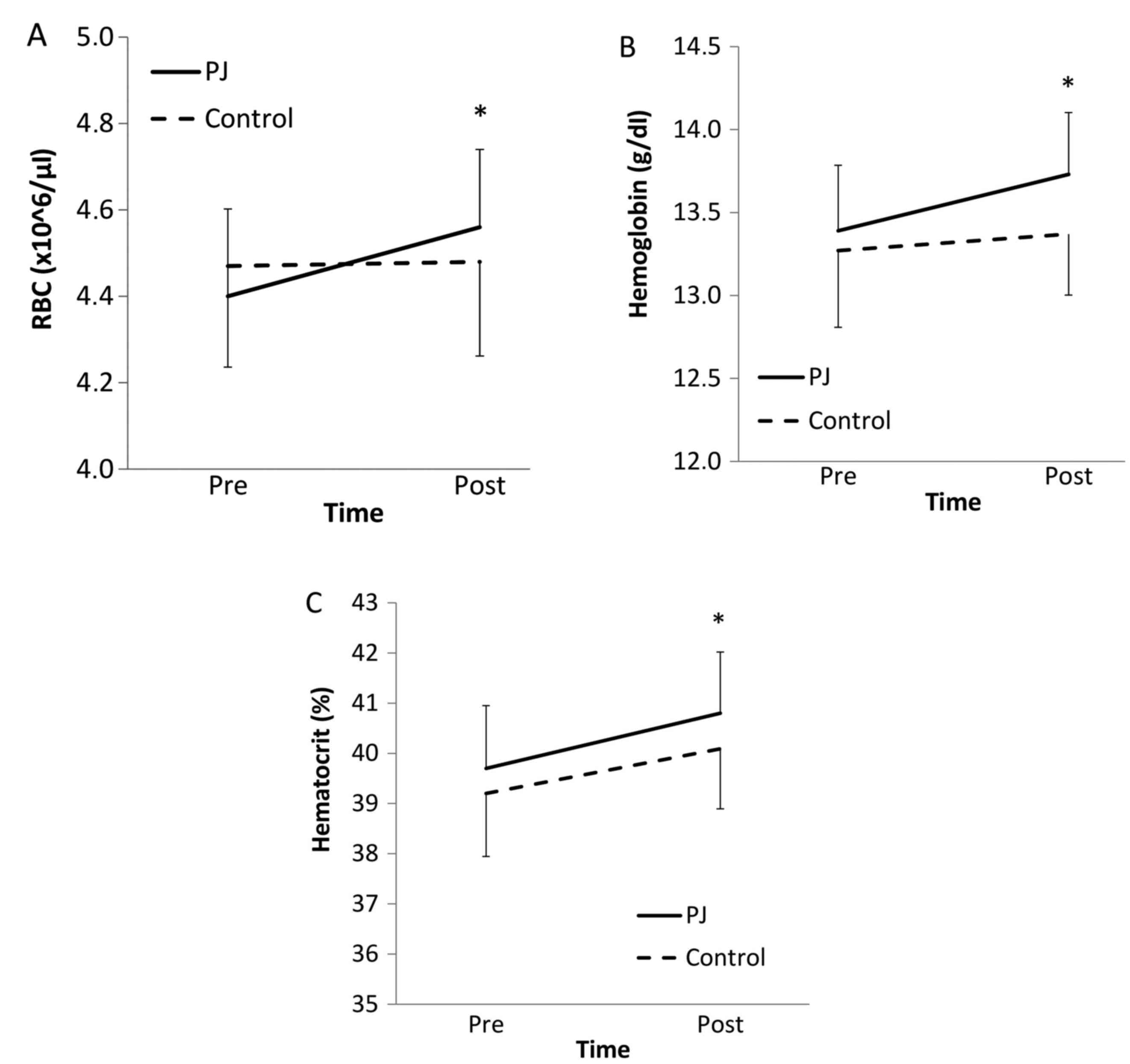
for group or group × time were identified for RBC count. However,
there were significant differences with respect to time
(F1,18=4.42; P<0.05). Pairwise comparison demonstrated a
significant increase (P<0.05) in the RBC count following PJ
supplementation, which did not occur in individuals in the control
group (Fig. 1A).
CBC parameters
No significant results for group or group × time
were identified for hemoglobin. However, there were significant
differences with respect to time (F1,18=5.12, P<0.05). Pairwise
comparison indicated a significant increase (P<0.001) in the
concentration of hemoglobin following PJ supplementation, which did
not occur in individuals in the control group (Fig. 1B).
No significant results for group or group × time
were identified for hematocrit. However, there were significant
differences with respect to time (F1,18=4.27, P<0.05). Pairwise
comparison indicated a significant increase (P<0.05) in
hematocrit following PJ supplementation (Fig. 1C).
The remaining CBC parameters prior to and following
completion of the two trials are presented in Table IV. In general, these parameters did
not change in either group. A significant difference was observed
for group × time (F1,17=3.98, P<0.05) in MCHC only. Pairwise
comparison demonstrated a significant increase (P<0.05) in MCHC
in the control group at the end of the experimental period
(Table IV).
 | Table IV.Complete blood count parameters of
all participants throughout the experimental period. |
Table IV.
Complete blood count parameters of
all participants throughout the experimental period.
|
| EG | CG |
|---|
|
|
|
|
|---|
| Parameter | Pre | Post | Pre | Post |
|---|
| WBC,
×103/µl | 5.9±0.8 | 5.8±1.0 | 6.0±0.7 | 5.6±0.7 |
| LYM, % | 38.0±7.6 | 39.6±2.6 | 38.4±6.0 | 39.2±6.2 |
| MONO, % | 9.6±2.0 | 9.8±2.1 | 11.3±4.3 | 8.8±1.9 |
| GRA, % | 52.4±7.9 | 50.6±2.3 | 50.3±5.9 | 51.9±5.6 |
| MCV, fl | 88.6±7.6 | 90.2±5.1 | 88.8±7.8 | 88.6±7.7 |
| MCH, pg | 30.2±3.2 | 30.7±2.3 | 29.8±3.0 | 30.5±3.0 |
| MCHC, g/dl | 33.9±0.9 | 34.1±0.9 | 33.5±0.9 |
34.3±0.7a |
| RDW, % | 11.5±0.5 | 11.5±0.9 | 11.8±0.7 | 11.3±0.6 |
| PLT,
×103/µl | 254.2±84.5 | 262.7±56.6 | 247.9±65.6 | 258.1±51.1 |
| MPV, fl | 8.2±0.9 | 8.4±0.7 | 8.6±0.9 | 8.2±0.8 |
| PCT, % | 0.20±0.06 | 0.22±0.03 | 0.21±0.04 | 0.21±0.03 |
| PDW, % | 15.1±1.2 | 14.8±1.7 | 14.2±1.9 | 15.1±1.2 |
Biochemical parameters
Furthermore, PJ supplementation did not
significantly alter the concentrations of total cholesterol, HDL,
LDL, glucose and triglycerides (Table
V). CRP values were not >6 mg/dl, indicating decreased
inflammation either at baseline or following the experimental
period.
 | Table V.Biochemical parameters of all
participants in the experimental and control groups. |
Table V.
Biochemical parameters of all
participants in the experimental and control groups.
|
| Control | Pomegranate |
|---|
|
|
|
|
|---|
| Parameter,
mmol/l | Pre | Post | Pre | Post |
|---|
| TG | 0.92±0.14 | 0.87±0.12 | 0.91±0.12 | 0.95±0.14 |
| TC | 4.9±0.3 | 5.1±0.3 | 5.0±0.3 | 5.1±0.3 |
| HDL | 1.51±0.09 | 1.55±0.12 | 1.53±0.11 | 1.57±0.12 |
| LDL | 2.98±0.31 | 3.15±0.30 | 3.07±0.31 | 3.05±0.29 |
| Glucose | 5.3±0.1 | 5.2±0.1 | 5.2±0.1 | 5.1±0.1 |
Discussion
The results of the present study demonstrate that PJ
consumption of 500 ml/day for two weeks increased the RBC count,
hemoglobin concentration and hematocrit in healthy individuals.
Other parameters concerning complete blood count, metabolic health
or inflammation were not altered in this cohort of individuals.
Therefore, PJ intake for a short period of time may result in
increased erythropoiesis or prevention of RBC degradation without
any significant alterations in factors associated with metabolic
health and inflammation in healthy individuals.
RBCs and hemoglobin constitute the transport system
of oxygen from the lungs to the tissues. It has been demonstrated
that a high concentration of polyphenols may increase the
resistance of RBC to oxidative stress (30). Therefore, a potential explanation for
the results of the current study is that the increased content of
polyphenols in PJ may have prevented RBC destruction due to reduced
oxidative stress. Indeed, this hypothesis is further supported by a
study reporting higher reduced GSH levels in erythrocytes following
500 ml PJ supplementation every day for two weeks (3). It was noted that this increase may be
due to the induction of expression or the catalytic activity of
enzymes involved in GSH biosynthesis that are known to be increased
by plant polyphenols (31).
Similarly, the participants that consumed PJ
exhibited a ~3% increase in hemoglobin by at the end of the two
week intervention. This finding is particularly important
considering the short period during which participants consumed the
juice. It has been determined that flavonoids serve an important
role in preventing oxidation of hemoglobin by various factors, such
as hypochlorous acid (32).
Flavonoids bind to hemoglobin and are able to inhibit oxidation of
the hemoglobin molecule by oxidizing agents. In addition to
flavonoids, PJ exhibits antioxidant activity that is much higher
than that of red wine, green tea and other natural juices (4). Antioxidant capacity in erythrocytes has
been identified to increase following two weeks of PJ
supplementation in healthy individuals (3). Therefore, a potential explanation for
the increased hemoglobin levels observed in the current study may
be that the phytochemicals contained in PJ juice may have protected
hemoglobin from oxidizing agents.
Hematocrit also exhibited a significant increase
following two weeks of PJ supplementation. Increasing levels of
hematocrit may be due to reduced RBC destruction. The high
concentration of antioxidants in PJ is likely to have protected RBC
and resulted in their subsequent reduced destruction. However, the
measurements completed in the present study cannot confirm which
factors caused this change. Therefore, further studies are required
to highlight the contribution and importance of each factor, in
addition to any potential synergistic effect.
Other parameters of the CBC did not change
significantly over the course of PJ supplementation, with the
exception of MCHC levels, which were significantly increased in the
control group at the end of the experimental period. MCHC is an
indicator of the average density of hemoglobin per RBC medium and
usually increases in dehydration and hereditary spherocytosis,
sickle cell disease and homozygous hemoglobin C disease (33). The change in MCHC was significant
only for individuals in the control group. It may be suggested that
the increase in MCHC levels is due to dehydration in the control
group. However, assessment of hydration status performed through
bioelectrical impedance (Table I)
did not identify any significant differences over time or between
groups. Indeed, this is something that requires further
investigation.
Lipid profile was not altered following
supplementation of PJ. Previous studies have reported that
pomegranate may reduce blood lipid levels and lipid peroxidation
(3,34). In diabetic subjects with
hyperlipidemia, consumption of PJ concentrate for 8 weeks
significantly reduced levels of total cholesterol and LDL (16,17). The
absence of any significant changes in the present study may be due
to the short duration of supplementation. Also, participants in the
current study were individuals with normal lipid levels compared
with previous studies where participants had elevated lipid levels
(15,16). A previous study has demonstrated that
the higher the cardiovascular risk, the greater the changes
occurring in risk factors such as cholesterol (20). Individuals in the current study were
also normoglycaemic and CRP was within normal levels at baseline.
Therefore, this may determine why changes in glucose and CRP were
not evident in either group, although a decrease in both parameters
has previously been recorded in rats following PJ supplementation
(21,24). Finally, studies investigating a
greater number of participants would provide more reliable results
on the effects of PJ supplementation in various health
parameters.
PJ supplementation may result in an increased RBC
count, hemoglobin concentration and hematocrit in healthy
individuals. Therefore, PJ supplementation may affect factors
involved either in RBC formation and erythropoiesis, or in
prevention of RBC degradation. It has been postulated that
increased generation of ROS may contribute to the shorter lifespan
of RBCs associated with iron-deficiency anemia (35). Therefore, antioxidants such as
polyphenols may help counterbalance increased oxidative stress in
RBCs, thus preventing their degradation. PJ is a rich source of
polyphenols and nutrients and may therefore aid RBCs and other
cells to counterbalance oxidative stress. Further research on this
topic may reveal if PJ may be used as an adjunctive strategy for
the prevention or treatment of certain types of anemia. In
conclusion, two weeks of PJ supplementation resulted in an
increased RBC count, hemoglobin concentration and hematocrit in
healthy individuals. Due to the confirmed functional properties of
PJ, further research is required to determine the long-term effects
of PJ supplementation on healthy individuals in addition to the
effect of different amounts of PJ on various biomedical parameters.
Furthermore, it is necessary to investigate the effect of
supplementation of PJ on factors affecting RBC formation and
erythropoiesis, or prevention of RBC degradation.
Acknowledgements
The authors of the present study wish to thank
CBH-VITOM S.A., Athens, Greece, for the provision of the commercial
product of pomegranate juice used in the current study.
References
|
1
|
Vidal A, Fallarero A, Peña BR, Medina ME,
Gra B, Rivera F, Gutierrez Y and Vuorela PM: Studies on the
toxicity of Punica granatum L. (Punicaceae) whole fruit extracts. J
Ethnopharmacol. 89:295–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Guo C, Yang J, Wei J, Xu J and Cheng
S: Evaluation of antioxidant properties of pomegranate peel extract
in comparison with pomegranate pulp extract. Food Chem. 96:254–260.
2006. View Article : Google Scholar
|
|
3
|
Matthaiou CM, Goutzourelas N, Stagos D,
Sarafoglou E, Jamurtas A, Koulocheri SD, Haroutounian SA, Tsatsakis
AM and Kouretas D: Pomegranate juice consumption increases GSH
levels and reduces lipid and protein oxidation in human blood. Food
Chem Toxicol. 73:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gil MI, Tomás-Barberán FA, Hess-Pierce B,
Holcroft DM and Kader AA: Antioxidant activity of pomegranate juice
and its relationship with phenolic composition and processing. J
Agric Food Chem. 48:4581–4589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirdehghan SH and Rahemi M: Seasonal
changes of mineral nutrients and phenolics in pomegranate (Punica
granatum L.) fruit. Sci Hort. 111:120–127. 2007. View Article : Google Scholar
|
|
6
|
Castilla P, Dávalos A, Teruel JL, Cerrato
F, Fernández-Lucas M, Merino JL, Sánchez-Martín CC, Ortuño J and
Lasunción MA: Comparative effects of dietary supplementation with
red grape juice and vitamin E on production of superoxide by
circulating neutrophil NADPH oxidase in hemodialysis patients. Am J
Clin Nutr. 87:1053–1061. 2008.PubMed/NCBI
|
|
7
|
Seeram NP, Aviram M, Zhang Y, Henning SM,
Feng L, Dreher M and Heber D: Comparison of antioxidant potency of
commonly consumed polyphenol-rich beverages in the United States. J
Agric Food Chem. 56:1415–1422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viuda-Martos M, Fernandez-López J and
Pérez-Álvarez JA: Pomegranate and its many functional components as
related to human health: A review. Compr Rev Food Sci Food Saf.
9:635–654. 2010. View Article : Google Scholar
|
|
9
|
Aharoni S, Lati Y, Aviram M and Fuhrman B:
Pomegranate juice polyphenols induce a phenotypic switch in
macrophage polarization favoring a M2 anti-inflammatory state.
Biofactors. 41:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faria A, Monteiro R, Mateus N, Azevedo I
and Calhau C: Effect of pomegranate (Punica granatum) juice intake
on hepatic oxidative stress. Eur J Nutr. 46:271–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo C, Wei J, Yang JJ, Xu J, Pang W and
Jiang YG: Pomegranate juice is potentially better than apple juice
in improving antioxidant function in elderly subjects. Nutr Res.
28:72–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenblat M, Hayek T and Aviram M:
Anti-oxidative effects of pomegranate juice (PJ) consumption by
diabetic patients on serum and on macrophages. Atherosclerosis.
187:363–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaplan M, Hayek T, Raz A, Coleman R,
Dornfeld L, Vaya J and Aviram M: Pomegranate juice supplementation
to atherosclerotic mice reduces macrophage lipid peroxidation,
cellular cholesterol accumulation and development of
atherosclerosis. J Nutr. 131:2082–2089. 2001.PubMed/NCBI
|
|
14
|
de Nigris F, Balestrieri ML,
Williams-Ignarro S, D'Armiento FP, Fiorito C, Ignarro LJ and Napoli
C: The influence of pomegranate fruit extract in comparison to
regular pomegranate juice and seed oil on nitric oxide and arterial
function in obese Zucker rats. Nitric Oxide. 17:50–54. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esmaillzadeh A, Tahbaz F, Gaieni I,
Alavi-Majd H and Azadbakht L: Concentrated pomegranate juice
improves lipid profiles in diabetic patients with hyperlipidemia. J
Med Food. 7:305–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esmaillzadeh A, Tahbaz F, Gaieni I,
Alavi-Majd H and Azadbakht L: Cholesterol-lowering effect of
concentrated pomegranate juice consumption in type II diabetic
patients with hyperlipidemia. Int J Vitam Nutr Res. 76:147–151.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sumner MD, Elliott-Eller M, Weidner G,
Daubenmier JJ, Chew MH, Marlin R, Raisin CJ and Ornish D: Effects
of pomegranate juice consumption on myocardial perfusion in
patients with coronary heart disease. Am J Cardiol. 96:810–814.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aviram M, Rosenblat M, Gaitini D, Nitecki
S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H
and Hayek T: Pomegranate juice consumption for 3 years by patients
with carotid artery stenosis reduces common carotid intima-media
thickness, blood pressure and LDL oxidation. Clin Nutr. 23:423–433.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davidson MH, Maki KC, Dicklin MR,
Feinstein SB, Witchger M, Bell M, McGuire DK, Provost JC, Liker H
and Aviram M: Effects of consumption of pomegranate juice on
carotid intima-media thickness in men and women at moderate risk
for coronary heart disease. Am J Cardiol. 104:936–942. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuhrman B, Volkova N and Aviram M:
Pomegranate juice oxidized LDL uptake and cholesterol biosynthesis
in macrophages. J Nutr Biochem. 16:570–576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das AK, Mandal SC, Banerjee SK, Sinha S,
Saha BP and Pal M: Studies on the hypoglycaemic activity of Punica
granatum seed in streptozotocin induced diabetic rats. Phytother
Res. 15:628–629. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scalbert A, Manach C, Morand C, Rémésy C
and Jiménez L: Dietary polyphenols and the prevention of diseases.
Crit Rev Food Sci Nutr. 45:287–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aviram M, Dornfeld L, Kaplan M, Coleman R,
Gaitini D, Nitecki S, Hofman A, Rosenblat M, Volkova N, Presser D,
et al: Pomegranate juice flavonoids inhibit low-density lipoprotein
oxidation and cardiovascular diseases: Studies in atherosclerotic
mice and in humans. Drugs Exp Clin Res. 28:49–62. 2002.PubMed/NCBI
|
|
24
|
Boussetta T, Raad H, Letteron P,
Gougerot-Pocidalo MA, Marie JC, Driss F and El-Benna J: Punicic
acid, a conjugated linolenic acid, inhibits TNFα-induced neutrophil
hyperactivation and protects from experimental colon inflammation
in rats. PLoS One. 4:64582009. View Article : Google Scholar
|
|
25
|
Lee CJ, Chen LG, Liang WL and Wanga CC:
Anti-inflammatory effects of Punica granatum Linne in vitro and in
vivo. Food Chemistry. 118:315–322. 2012. View Article : Google Scholar
|
|
26
|
Elfalleh W, Tlili N, Nasri N, Yahia Y,
Hannachi H, Chaira H, Ying M and Ferchichi A: Antioxidant
capacities of phenolic compounds and tocopherols from Tunisian
pomegranate (Punica granatum) fruits. J Food Sci. 76:C707–C713.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hmid I, Elothmani D, Hanine H, Oukablic A
and Mehinagic E: Comparative study of phenolic compounds and their
antioxidant attributes of eighteen pomegranate (Punica granatum L.)
cultivars grown in Morocco. Arab J Chem. http://dx.doi.org/10.1016/j.arabjc.2013.10.0112013.
|
|
28
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
29
|
Singer JM, Piotz CM, Parker E and Elster
SF: The latex fixation test. III. Agglutination test for C-reactive
proteins and comparison with the capillary precipitin method. Am J
Clin Path. 28:611–617. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Youdim KA, Shukitt-Hale B, MacKinnon S,
Kalt W and Joseph JA: Polyphenolics enhance red blood cell
resistance to oxidative stress: In vitro and in vivo. Biochim
Biophys Acta. 1523:117–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moskaug JØ, Carlsen H, Myhrstad MC and
Blomhoff R: Polyphenols and glutathione synthesis regulation. Am J
Clin Nutr. 81 1 Suppl:277S–283S. 2005.PubMed/NCBI
|
|
32
|
Gebicka L and Banasiak E: Hypochlorous
acid-induced heme damage of hemoglobin and its inhibition by
flavonoids. Toxicol In Vitro. 26:924–929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cohen ML and Rifkind D: The Pediatric
Abacus: Review of Clinical Formulas and How to Use Them. Parthenon
Pub. Group; New York, NY: 2002
|
|
34
|
Basu A and Penugonda K: Pomegranate juice:
A heart-healthy fruit juice. Nutr Rev. 67:49–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagababu E, Gulyani S, Earley CJ, Cutler
RG, Mattson MP and Rifkind JM: Iron-deficiency anemia enhances red
blood cell oxidative stress. Free Radic Res. 42:824–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|