Introduction
Microglia are the resident macrophages of the
central nervous system (CNS). Accumulating evidence has
demonstrated that the over-activation of microglia, for example, in
response to certain environmental toxins and endogenous proteins,
contributes to the progression of several neurodegenerative
diseases, including Alzheimer's disease, Parkinson's disease and
multiple sclerosis (MS) (1).
Previous studies have indicated that neurodegenerative diseases are
associated with the secretion of various proinflammatory and
cytotoxic factors by activated microglia in the brain (2–5).
Therefore, inhibiting the activation of microglia is an important
strategy for the prevention of neurodegeneration.
Microglia can be activated by lipopolysaccharide
(LPS), and serve a role in the innate and adaptive immune responses
through the production of proinflammatory mediators, including
myeloid differentiation primary response protein MyD88 (MyD88),
nuclear factor-κB, caspase-3 and heat shock protein 60 (HSP60)
(6,7). It has been demonstrated that HSP60 is
highly expressed by activated microglia, and that the extracellular
release of HSP60 increases the production of other proinflammatory
factors through binding to toll-like receptor 4 (TLR-4) and
stimulating neuronal cell death (8,9). Thus,
the regulation of HSP60 production is a potential therapeutic
option for the treatment of neurodegenerative disorders.
Matrine (MT), the major active component of members
of the Sophora genus, is used to treat inflammatory diseases
and cancer in traditional Chinese medicine (10,11). Kan
et al (12) demonstrated that
MT could attenuate the severity of experimental autoimmune
encephalomyelitis through reducing levels of chemokine ligand 2 and
C-X-C motif chemokine 10, and suggested that MT may be an effective
immunomodulatory therapeutic approach for MS through inhibiting
immune cell recruitment mechanisms. MT was also identified to
effectively protect neuronal axons from CNS inflammation-induced
damage by inhibiting risk factors, including β-secretase 1, and
upregulating neuroprotective factors, including brain-derived
neurotrophic factor (13). However,
the cellular and molecular mechanisms underlying the
anti-inflammatory activity of MT on microglia remain unclear
(14). The present study aimed to
investigate the neuroprotective effects of MT and determine whether
HSP60 was associated with these effects through inhibiting
microglial activation. The results demonstrated that MT could
inhibit the expression of HSP60, TLR-4, heat shock factor 1
(HSF-1), caspase-3 and MyD88 to prevent neuronal injury in
LPS-treated BV2 mouse microglial cells. These results suggest that
MT prevents microglial activation via inhibiting the
HSP60/TLR-4/MyD88 signaling pathway.
Materials and methods
Chemicals
BV2 mouse microglial cells were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). LPS
and MT were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). The anti-β-actin (cat. no. TA-09) antibody was purchased
from ZSGB-BIO Technology Co., Ltd. (Beijing, China). Antibodies
directed against HSP60 (cat. no. ADI-SPA-806-D) and HSF-1 (cat. no.
ADI-SPA-950-D) were purchased from Stressgen Biotechnologies (San
Diego, CA, USA). Antibodies directed against caspase-3 (cat. no.
9665), MyD88 (cat. no. 4283) and TLR-4 (cat. no. 2219) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Tumor necrosis factor TNF-α ELISA kit (mouse TNF-α ELISA
EMC102a.96) was acquired from Xinbosheng Biotechnology, Inc.
(Shenzhen, China), and HSP60 ELISA kit (mouse HSP-60 ELISA kit,
E-20344) was purchased from Beijing Chenglin Biotechnology, Inc.
(Beijing, China). Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum (FBS) were obtained from Gibco® (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The Cell Counting Kit-8
(CCK-8) was obtained from BestBio (Shanghai, China). The
bicinchoninic acid (BCA) and enhanced chemiluminescence (ECL) kits
were acquired from Pierce (Thermo Fisher Scientific, Inc.).
Cell culture
Mouse BV2 microglial cells were maintained in DMEM
supplemented with 10% FBS at 37°C with 5% CO2 in an
incubator. Cells were pretreated with LPS (1 µg/ml) for 30 min and
then incubated with different concentrations (5, 10, 20 or 50
µg/ml) of MT for 24 h prior to a variety of assays.
Cell viability assay
Cell viability was assayed using the CCK-8 kit.
Cells were seeded into 96-well microtiter plates at a density of
5×104 cells/well and cultured for 24 h. Subsequently,
CCK-8 solution (10 µl) was added to each well according to the
manufacturer's instructions and the plates were incubated for a
further 2 h. The absorbance at 450 nm was measured using a
microplate reader in order to determine cell viability as a
percentage of the control (untreated with MT) value.
ELISA for TNF-α and HSP60
Levels of TNF-α and HSP60 released into the culture
media were determined using TNF-α and HSP60 ELISA kits according to
the manufacturer's protocol. The absorbance was detected at 450 nm
using a microplate reader in order to determine the amount of TNF-α
and HSP60.
Western blotting
Cells were washed three times with PBS (2 min for
each wash) and lysed (10 min, 4°C) with radioimmunoprecipitation
assay buffer. The lysate was centrifuged (1,200 × g, 15 min, 4°C)
and the supernatant was collected. Protein concentration was
measured using the Pierce BCA kit following the manufacturer's
protocol. Equal quantities of protein (10 µl) were resolved by
SDS-PAGE on a 12% gel for 90 min. The resolved protein was
transferred onto a polyvinylidene difluoride membrane, which were
blocked (60 min, room temperature) with 5% dried milk.
Subsequently, the membranes were incubated with primary antibodies
in Tris-buffered saline-Tween-20 (TBST) directed against HSP60
(1:1,000 dilution), MYD88 (1:1,000 dilution), HSF-1 (1:1,000
dilution), TLR-4 (1:1,000 dilution), caspase-3 (1:2,000 dilution)
or β-actin (1:1,000 dilution) at 4°C overnight. After washing with
PBS, the membranes were incubated with anti-mouse (cat. no.
ZB-2305; 1:5,000 dilution) or anti-rabbit (cat. no. ZB-2301;
1:5,000 dilution) secondary antibodies diluted in TBST for 1 h at
room temperature. Proteins bands were then visualized using the ECL
kit and X-ray films. The results were quantified using Quantity One
software, version 4.6.9 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Results are presented as the mean ± standard error
of the mean of three independent experiments. One-way analysis of
the variance followed by a post hoc Student-Newman-Keuls test was
used statistically analyze the significance of differences between
groups. P<0.05 was considered to indicate a statistically
significant difference. SPSS 19.0 software (IBM Corp., Armonk, NY,
USA) was used for statistical analysis.
Results
MT increases LPS-treated BV2 cell
viability
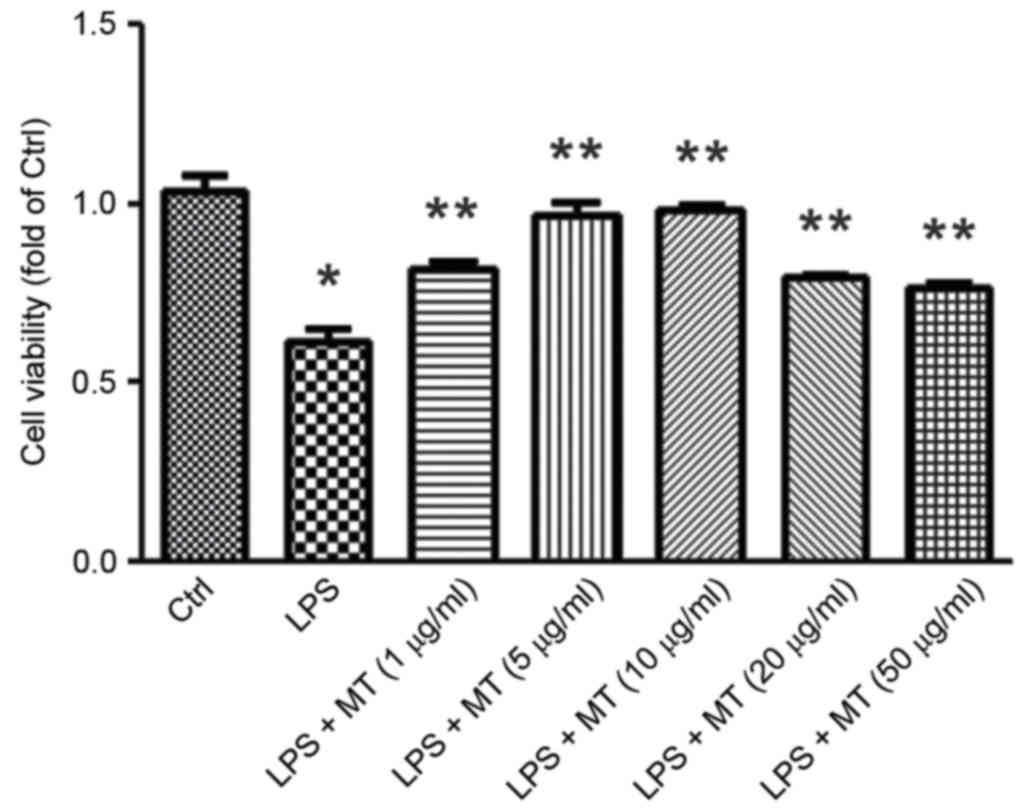
The CCK-8 assay was performed to detect the effect
of MT on the viability of LPS-stimulated BV2 cells. The results
indicated that after treatment with 1 µg/ml LPS for 30 min the
viability of BV2 cells significantly decreased compared with the
control group (P<0.05; Fig. 1).
However, when LPS treatment was followed by MT treatment (5, 10, 20
or 50 µg/ml) for 24 h BV2 cell viability was significantly
increased compared with the group treated with LPS alone (all
P<0.05; Fig. 1). BV2 cells
treated with 5–10 µg/ml MT exhibited the optimal increase in
viability; thus, 7.5 µg/ml MT was used in subsequent
experiments.
HSP60 expression is inhibited by MT in
LPS-stimulated BV2 cells
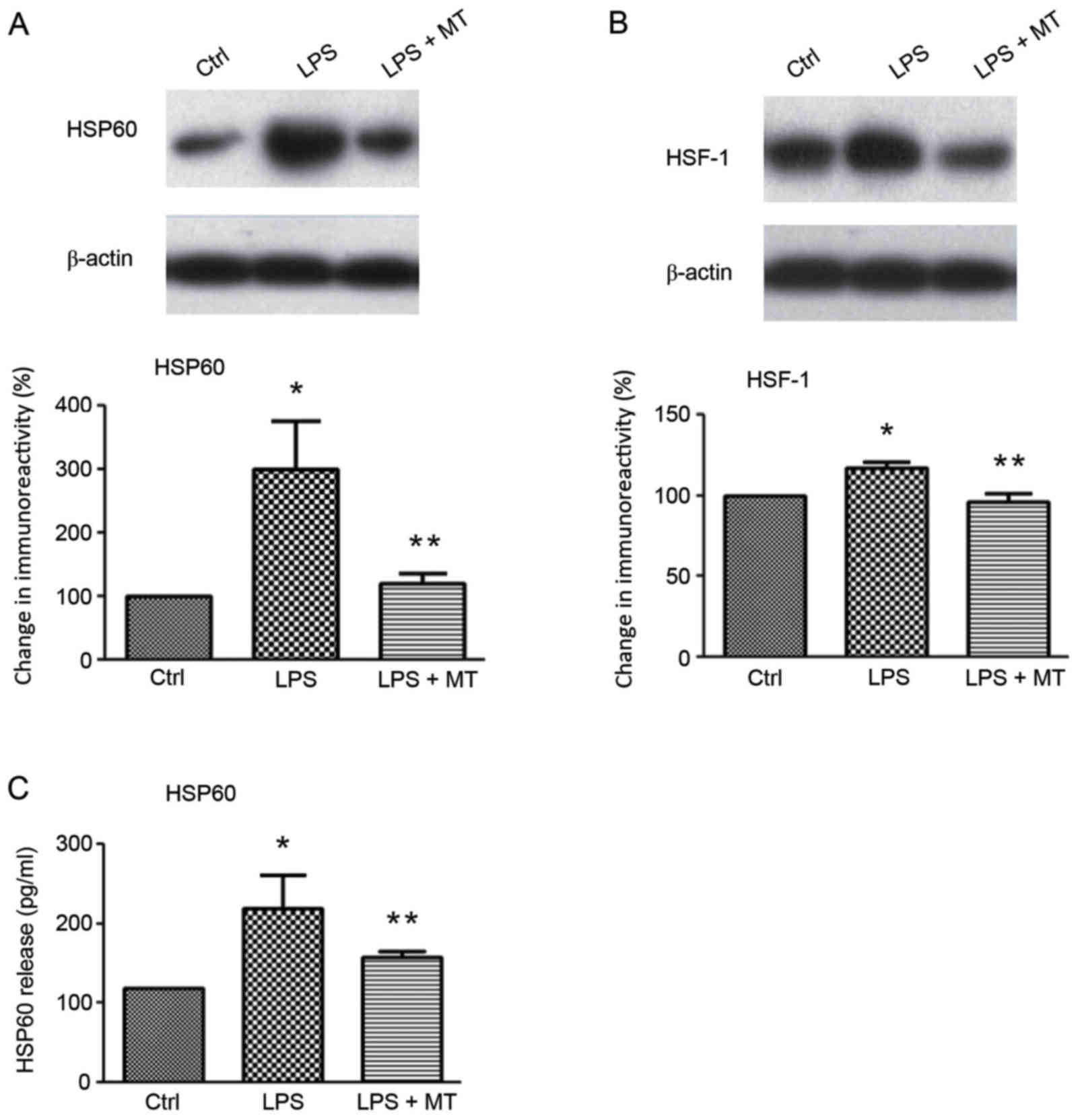
Western blotting and ELISA were used to investigate
the effects of MT on HSP60, and HSF-1 expression (Fig. 2). The results indicated that LPS
stimulation significantly increased the level of HSP60 compared
with the control group; however, MT treatment following LPS
stimulation significantly reduced this increase (both P<0.05;
Fig. 2A). Similarly, the ELISA
results demonstrated that the release of extracellular HSP60 in
LPS-stimulated BV2 cells was significantly inhibited by MT compared
with cells treated with LPS alone (P<0.05; Fig. 2C). HSF-1 is a transcription factor
that regulates HSP60 expression and release (9). Western blot analysis revealed that the
expression of HSF-1 was significantly upregulated by LPS compared
with the control group; however, subsequent MT treatment
significantly reduced this increase (both P<0.05; Fig. 2B). A previous study reported that
HSP60 may translocate extracellularly during cellular stress in
order to induce apoptosis (15).
MT decreases the expression of TLR-4,
MyD88 and caspase-3 proteins in LPS-stimulated BV2 cells
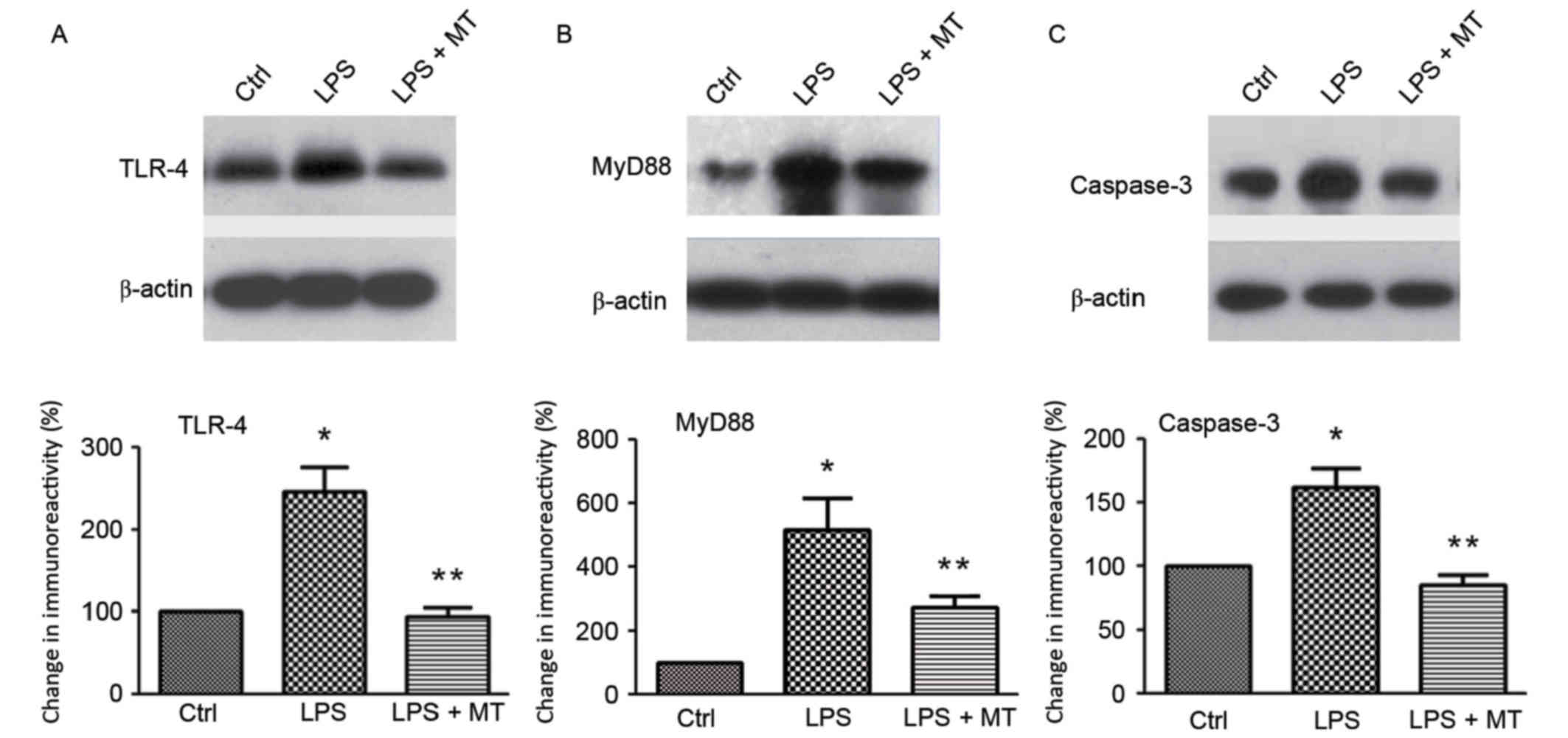
The expression of proteins in the TLR-4/MyD88
signaling pathway was measured by western blotting after treatment
with LPS and MT. The results demonstrated that the expression of
TLR-4 was significantly inhibited by MT in LPS-stimulated BV2 cells
compared with cells treated with LPS alone (P<0.05; Fig. 3A). MyD88 and caspase-3 are downstream
signaling molecules of TLR-4. Western blot analysis demonstrated
that MT significantly suppressed the expression of MyD88 (Fig. 3B) and caspase-3 (Fig. 3C) compared with cells treated with
LPS alone (both P<0.05). These results indicate that MT exerts
its neuroprotective effects through inhibiting the TLR-4/MyD88
signaling pathway, which subsequently inhibits microglial
activation.
MT reduces proinflammatory cytokine
TNF-α production in LPS-stimulated BV2 cells
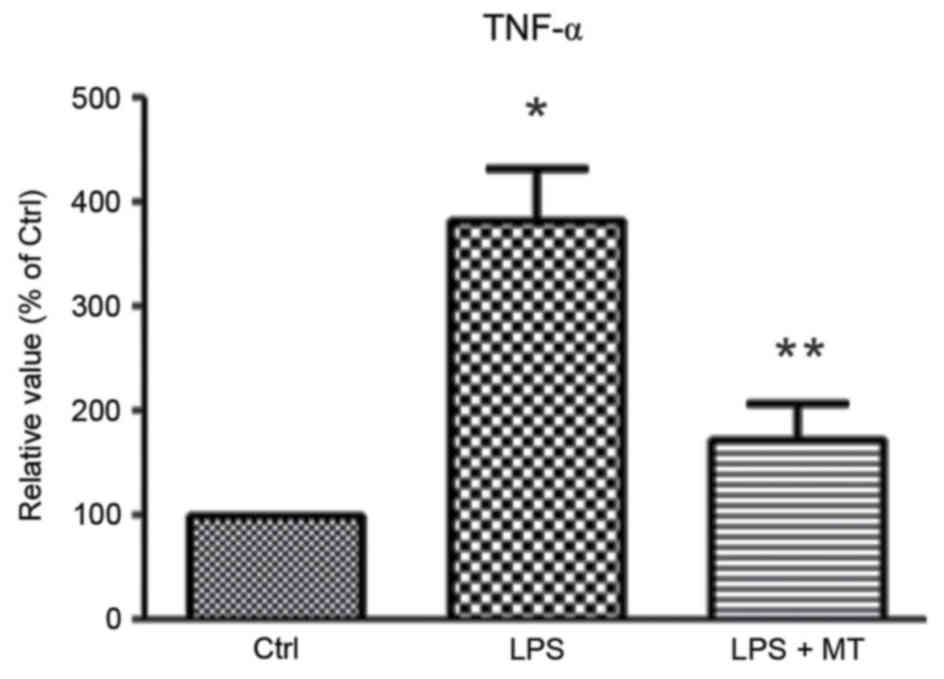
To evaluate the effect of MT on the production of
inflammatory factors, the level of TNF-α in the culture media was
measured using an ELISA. This revealed that MT significantly
inhibited the release of TNF-α in LPS-induced BV2 cells compared
with cells treated with LPS alone (P<0.05; Fig. 4). This indicates that MT may prevent
neuronal cell death by suppressing the production of inflammatory
factors.
Discussion
Microglia serve a primary role in the immune
response in the CNS, and activated microglia secrete numerous
proinflammatory and neurotoxic factors, which are responsible for
inflammation-associated and neurodegenerative diseases (16). Inhibiting microglial activation, in
order to reduce the production of proinflammatory and neurotoxic
factors, may be an effective method for the prevention and
treatment of neurodegenerative diseases.
LPS has been demonstrated to induce the activation
of microglia (17). Here, 1 µg/ml
LPS was used to stimulate microglia BV2 cells for 30 min in order
to establish a microglial activation model. The effect of MT on
activated microglia was then investigated via cell viability
assays, western blotting and ELISAs. Western blotting results
revealed that the expression of HSF-1, MyD88, HSP60 and TLR-4
increased significantly after LPS stimulation, and that MT
treatment could significantly reduce this increase. ELISA results
demonstrated that MT could significantly reduce the expression of
HSP60 and TNF-α. These results indicate that MT inhibits microglial
activation via inhibiting the HSP60/TLR-4/MyD88 signaling
pathway.
MT, an alkaloid with low toxicity, is widely used in
clinical treatment, for example, to treat silicosis and prevent
liver function damage of anti-tumor drugs (18,19). MT
has been demonstrated to exert anti-inflammatory, antitumor,
antiarrhythmia, antipyretic, analgesic and anticonvulsant effects
in the CNS (12,13). However, it remains unclear whether MT
has neuroprotective effects on microglia.
HSP60 is a mitochondrial protein with dual roles.
Under normal conditions, HSP60 acts as a molecular chaperone,
assisting in polypeptide transposition, folding and assembly. When
HSP60 is overexpressed or ectopically expressed under conditions of
stress, HSP60 acts as a self-antigen, which is recognized by the
immune system and causes an immune response (20). HSP60 also acts as a signal molecule,
serving a role in signal transduction (21–23).
Activated HSP60 is primarily localized in the plasma membrane or
extracellular space, where it exerts a proapoptotic effect by
enhancing caspase activation (24).
LPS-induced microglia cells are toxic when caspase-3 is active,
however activated microglia have been demonstrated not to be toxic
to neighboring neurons when caspase-3 is inhibited (25,26). The
transcription factor HSF-1 regulates the expression of HSP60 by
binding to its promoter (27). The
results of the present study demonstrated that MT significantly
decreased the expression and release of HSP60, caspase-3 and HSF-1
in LPS-stimulated BV2 microglia cells.
HSP60 is a ligand of TLR-4, which is a component of
two signaling pathways, one that is MyD88-dependent and another
that is independent of MyD88. MyD88 is a common receptor for all of
the known TLRs, excluding TLR-3 (28). The results of the present study
identified that the protein expression of TLR-4 and MyD88
significantly increased in LPS-stimulated BV2 cells, and that MT
significantly inhibited this increase. Activated microglia cells
release a large amount of proinflammatory cytokines, including
TNF-α and interleukin 1β. TNF-α serves important roles in the
immune response and apoptosis, and this increased expression of
TNF-α is associated with the destruction of dopaminergic neurons
(29). ELISA results from the
present study demonstrated that TNF-α was released by BV2 cells
upon LPS activation, and that this significant increase in
extracellular TNF-α could be inhibited by MT.
In conclusion, the results of the present study
indicate that MT inhibits the activation of microglia by
suppressing the HSP60/TLR-4/MyD88 signaling pathway, and that this
inhibited has a neuroprotective and anti-inflammatory effect. Thus,
MT is a potential neuroprotective agent. These findings may provide
a novel direction for the treatment of neurodegenerative
diseases.
Acknowledgements
The present study was supported by Ningixa 13th Plan
of Five-Year Major Scientific Program (grant no. 2016BZ07) and the
National Natural Science Foundation of China (grant nos. 31460257,
81460182, 81571098, 31560273 and 31260243).
References
|
1
|
Perry VH, Nicoll JA and Holmes C:
Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nature Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
3
|
Liu B and Hong JS: Role of microglia in
inflammation-mediated neurodegenerative disease: Mechanisms and
strategies for therapeutic intervention. J Pharmacol Exp Ther.
304:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanisch Uk and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gehrmann J, Matsumoto Y and Kreutzberg GW:
Microglia: Intrinsic immuneffector cell of the brain. Brain Res
Rev. 20:269–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch MA: The multifaceted profile of
activated microglia. Mol Neurobiol. 40:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YH, Teng P, Wang Y, Zhang YM, Ma CJ and
Pu J: Expression and regulation of HSP60 in activated microglia
cells. J Ningxia Med Univ. 8:712–714. 2011.
|
|
8
|
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J
and Gu J: Microglial LOX-1 reacts with extracellular HSP60 to
bridge neuroinflammation and neurotoxicity. Neurochem Int.
61:1021–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng W, Li Y, Hou X, Zhang N, Ma J, Ding
F, Li F, Miao Z, Zhang Y, Qi Q, et al: HSP60 is involved in the
neuroprotective effects of naloxone. Mol Med Rep. 10:2172–2176.
2014.PubMed/NCBI
|
|
10
|
Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang
J, Niu Y, Sun T, Li YX and Yu JQ: Matrine attenuates focal cerebral
ischemic injury by improving antioxidant activity and inhibiting
apoptosis in mice. Int J Mol Med. 36:633–644. 2015.PubMed/NCBI
|
|
11
|
Rong B, Zhao C, Gao W and Yang S: Matrine
promotes the efficacy and safety of platinum-based doublet
chemotherapy for advanced non-small cell lung cancer. Int J Clin
Exp Med. 8:14701–14717. 2015.PubMed/NCBI
|
|
12
|
Kan QC, Pan QX, Zhang XJ, Chu YJ, Liu N,
Lv P, Zhang GX and Zhu L: Matrine ameliorates experimental
autoimmune encephalomyelitis by modulating chemokines and their
receptors. Exp Mol Pathol. 99:212–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kan QC, Lv P, Zhang XJ, Xu YM, Zhang GX
and Zhu L: Matrine protects neuro-axon from CNS
inflammation-induced injury. Exp Mol Pathol. 98:124–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ML, Zhang XJ, Kang J, Zhang HJ, Chen
XL, Liu N, Liu SQ, Ma WD, Zhang GX and Zhu L: Matrine promotes NT3
expression in CNS cells in experimental autoimmune
encephalomyelitis. Neurosci Lett. 649:100–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandra D, Choy G and Tang DG: Cytosolic
accumulation of HSP60 during apoptosis with or without apparent
mitochondrial release: evidence that its pro-apoptotic or
pro-survival functions involve differential interactions with
caspase-3. J Biol Chem. 282:31289–31301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kreutzberg GW: Microglia: A sensor for
pathological events in the CNS. Trends Neurosci. 19:312–328. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teng P, Li Y, Cheng W, Zhou L, Shen Y and
Wang Y: Neuroprotective effects Of Lycium barbarum polysaccharides
in lipopolysaccharide-induced BV2 microglia cells. Mol Med Rep.
7:1977–1981. 2013.PubMed/NCBI
|
|
18
|
Miao RM, Fang ZH and Yao Y: Therapeutic
efficacy of tetrandrine tablets combined with matrine injection in
treatment of silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za
Zhi. 30:778–780. 2012.(In Chinese). PubMed/NCBI
|
|
19
|
Lao Y: Clinical study of matrine injection
on preventing liver function damage of anti-tumor drugs during
chemotherapy of breast cancer. Zhong Yao Cai. 28:735–737. 2005.(In
Chinese). PubMed/NCBI
|
|
20
|
Quintana FJ and Cohen IR: HSP60 speaks to
the immune system in many voices. Novartis Found symp. 291:101–114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lehnardt S, Schott E, Trimbuch T, Laubisch
D, Krueger C, Wulczyn G, Nitsch R and Werber JR: A vicious cycle
involving release of heat shock protein 60 from injured cells and
activation of toll-like receptor 4 mediates neurodegeneration in
the CNS. J Neurosci. 28:2320–2331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen JJ, Bross P, Westergaard M, Nielsen
MN, Eiberg H, Børglum AD, Mogensen J, Kristiansen K, Bolund L and
Gregersen N: Genomic structure of the human mitochondrial
chaperonin genes: HSP60 and HSP10 are localised head to head on
chromosome 2 separated by a bidirectional promoter. Hum Genet.
112:71–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Z, Ma J and Yuan WJ: Heat shock
protein 60 in cell apoptosis. Sheng Li Ke Xue Jin Zhan. 39:267–270.
2008.(In Chinese). PubMed/NCBI
|
|
24
|
Gupta S and Knowlton AA: HSP60 trafficking
in adult cardiac myocytes: Role of the exosomal pathway. Am J
Physiol Heart Circ Physiol. 292:H3052–H3056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burguillos MA, Deierborg T, Kavanagh E,
Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P,
Englund E, Venero JL and Joseph B: Caspase signalling controls
microglia activation and neurotoxicity. Nature. 472:319–324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samali A, Cai J, Zhivotovsky B, Jones DP
and Orrenius S: Presence of a pre-apoptotic complex of
pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of
jurkat cells. EMBO J. 18:2040–2048. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Chen L, Hagiwara N and Knowlton
AA: Regulation of heat shock protein 60 and 72 expression in the
failing heart. J Mol Cell Cardiol. 48:360–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosenberger K, Dembny P, Derkow K, Engel
O, Krüger C, Wolf SA, Kettenmann H, Schott E, Meisel A and Lehnardt
S: Intrathecal heat shock protein 60 mediates neurodegeneration and
demyelination in the CNS through a TLR-4 and MyD88-dependent
pathway. Mol Neurodegener. 10:52015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montgomery SL and Bowers WJ: Tumor
necrosis factor-alpha and the roles it plays in homeostatic and
degenerative processes within the central nervous system. J
Neuroimmune Pharmacol. 7:42–59. 2012. View Article : Google Scholar : PubMed/NCBI
|