Introduction
In the last 5 years, gastric cancer has become the
most frequent cause of cancer-related fatality, making it the fifth
most common cancer in the world, with approximately 952,000 new
cases diagnosed in 2012 (1,2). In China, gastric cancer is one of the
most common cancers and the incidence ranks third among all
malignant tumors, after lung and liver cancer in men and after
breast and lung cancer in women (3).
Despite the fact that diagnostic and therapeutic approaches have
advanced in the past decade, the prognosis is still unclear due to
the high recurrence (4).
5-fluorouracil (5-FU) is a chemotherapeutic agent
that is currently the most widely used drug choice for the
treatment of solid tumors, such as metastatic colorectal cancer and
gastric cancer (5–8). Alternatively, Traditional Chinese
Medicine (TCM) is a multi-channel, multi-layer and multi-target
method for treating cancer as an alternative to surgery,
radiotherapy, chemotherapy and biological therapy (9). TCM involves the use of complicated
ingredients that may promote the inhibition and apoptosis of cancer
cells (9). Compound cantharides
capsules (CCC) are one of the most common TCMs that contain
cantharidin, bear gallbladder powder, Panax ginseng,
Astragalus mongholicus root, Eleutherococcus
senticosus, as well as other ingredients (10). Clinically, CCC have been used to
treat primary liver cancer, lung cancer, rectal cancer and
malignant lymphoma (11). Some of
the ingredients have been demonstrated to have direct effects on
tumor cells. For example, ginsenoside and ginseng polysacchride
have been demonstrated to directly inhibit tumor cells (12). Research has indicated that
Eleutherococcus senticosus is able to induce apoptosis in
human gastric cells (13).
Clinically, various TCMs, including CCC, are used in combined
therapy to strengthen chemotherapy effects (14).
Multiple factors are involved in gastric cancer
pathogenesis, including tumor suppressor genes, oncogenes and
growth factors (15–17). Thus, advanced understanding of the
molecular mechanisms involved in gastric cancer therapy is of great
clinical significance.
Apoptosis is a genetically controlled process.
Cantharidin has been indicated to induce apoptosis via cytochrome c
release in a pancreatic β cell line (18) and has been demonstrated to have a
role in the cell cycle and growth (19,20).
Cytochrome c release and caspase activation are mediated by the
translocation of cytosolic B-cell lymphoma-2 associated X protein
to the mitochondria in response to various apoptotic stimuli, which
is regulated by a tumor suppressor protein, p53 (21–23). The
p53 protein has been well-acknowledged to regulate cellular
response to various cellular stresses during cancer progression
(24). p53 phosphorylation is
mediated by protein kinases, such as extracellular signal-regulated
kinase (ERK)-1/2, p38 kinase and c-Jun N-terminal kinase (JNK) by
mitogen-activated protein kinases (MAPKs) (25). Dual phosphorylation of threonine and
tyrosine within the motif Thr-Glu-Tyr in ERK, Thr-Gly-Tyr in p38 or
Thr-Pro-Tyr in JNK induces MAPK activation of the protein kinases
(26). Genotoxic agents and
apoptosis regulate the p38 and JNK pathways (27,28).
Controversial evidence has indicated that the complex roles of
different pathways exist and interact to perform distinct cellular
effects in various cell lineages (29,30).
In the present study, the aim was to explore whether
CCC was able to facilitate 5-FU chemotherapy in human gastric
cancer cells by altering cell viability and apoptosis, and to
evaluate the mRNA expression levels of proliferation-related genes
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis to determine the differential mRNA expression
levels of specific markers. Furthermore, the present study explored
the potential effects of combined therapy treatment on cancer cells
and findings suggested that combined therapy promoted the
inhibition of gastric cancer cell viability through inhibiting JNK
and p38 phosphorylation.
Materials and methods
Serum preparation
In the present study, 20 male Sprague Dawley (SD)
rats at 6 weeks and weighing 180–220 g were randomized into a CCC
group and a control group for serum collection. Animals were
maintained on in a 12-h light/dark cycle at 22°C with 55% humidity
and had ad libitum access to food and water. The present
study was approved by the Ethics Committee of Zibo City Hospital of
Traditional Chinese Medicine (Zibo, China). CCC was purchased from
Huaxi Pharmaceutical Co., Ltd. (Baoji, China). In the CCC group,
CCC was administered intragastrically to SD rats. In human
patients, 4.5 g medication is given to adults with an average
weight of 65 kg (31). As the
average weight of rats was 200 g, medication conversion rate was
6.25. Therefore, 10 rats in the CCC group received a total CCC
dosage of 5.4 g over the course of 5 days. In the first 3 days of
the experiment, 2 mg/ml was administered twice a day to the rats.
On days 4 and 5, rats received 4 ml (2 mg/ml) CCC, followed by
blood sampling from the femoral artery. The same dosage and same
frequency of saline was administered to rats in the control group
(n=10). A total of 2 ml of CCC serum or control serum was taken
from each SD male rat for the following experiments.
Cell culture
Human gastric cancer cell lines, BGC-823 and
SGC-7901, which are low- and mid-level differentiated,
respectively, were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China) and cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10 % fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.) and 50 U/ml penicillin and 50 µg/ml
streptomycin. Cell culture was maintained at 37°C in a humidified
atmosphere containing 5% CO2 prior to switching to a
different concentration of CCC serum or control serum for the cell
treatment. Each cell line was divided into five groups for
treatment: The CCC serum group; 10 µl/ml 5-FU group (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany); CCC serum + 10 µl/ml 5-FU; control
serum; and control cells without treatment. Each group included the
following subgroups: 5% CCC serum, 10% CCC serum and 20% CCC serum
subgroup in the CCC serum group; 5% CCC serum with 10 µl/ml 5-FU,
10% CCC serum with 10 µl/ml 5-FU and 20% CCC serum with 10 µl/ml
5-FU subgroup in the CCC serum + 10 µl/ml 5-FU group; and 5%
control serum, 10% control serum and 20% control serum subgroup in
the control serum group. In each cell line, 24 h after treatment,
cells from each group were transferred to normal RPMI-1640
supplemented with 10% FBS, 50 U/ml penicillin and 50 µg/ml
streptomycin.
CCC treatment, cell viability assay
and concentration gradient infection rate
The effect of CCC serum and/or combined therapy on
the cell viability of gastric cancer cells was evaluated by MTT
assays. BGC-823 and SGC-7901 cells were seeded into 96-well culture
plates at a density of 105 cells/well in quintuplicate.
Serum treatment subgroups from all groups were added to the plates
to a concentration of 0.01% and allowed to adhere for 24 h before
being transferred to normal cell culture (RPMI 1640 supplemented
with 10% FBS, 50 U/ml penicillin and 50 µg/ml streptomycin).
Following incubation at 25°C for 30 min, 10 µl MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well for 24, 48 or 72 h, after switching back to cell culture, and
cells were incubated at 25°C for a further 4 h. Media was then
removed and 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA)
was then added. After incubation at 37°C for 30 min, absorbance (A)
was measured at 570 and 630 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) for obtaining optical density
(OD) values. Relative cell viability (%) was calculated using the
following equation: Relative viability rate (%) =
(A570nm-A630nm) of study
group/(A570nm-A630nm) of control group ×
100%. The optimal incubation time was determined and used for
subsequent analysis.
Time gradient infection rate
Each cell line was divided into the control, CCC
serum (concentration selected from previous experiment), 5-FU,
combined treatment (concentration selected from previous
experiment) and serum control groups. Cells (2×105
cells/ml) were cultured in RPMI-1640 supplemented with 10% FBS, 50
U/ml of penicillin and 50 µg/ml of streptomycin at 37°C and the
appropriate treatment sera were added to the cultures of the
various group. A total of 24, 48 and 72 h after treatment, cells
were transferred to normal cell culture for 4 h prior to MTT assay
for calculating the inhibition rate. The percentage of inhibition
was calculated as follows: Inhibition ratio = (1-OD of study
group/OD of control group) × 100%. The optimal CCC concentration
was determined and used for subsequent analysis.
Cell growth curve
Each cell line was divided into the control, CCC
serum, 5-FU, combined treatment and serum control groups. In each
group, cells were cultured into a 96-well plate at a density of
105 cells/well. A total of 10 µl of MTT (5 mg/ml) was
added to each well, after serum incubation, the cells were
incubated for 24, 48 or 72 h at 37°C, respectively. Cells were
incubated at 25°C for a further 4 h, cell number was subsequently
calculated using a hemocytometer and a microscope (Olympus, Tokyo,
Japan) and cell growth curves were analyzed.
Apoptosis assays by flow
cytometry
Each cell line was divided into control, CCC serum,
5-FU, combined treatment and serum control groups for different
treatments. In each group, cells were plated in 6-well culture
plates at density of 3×106 cells/well. After treatment
with different conditions, the cell culture medium was removed and
the cells were fixed using 0.5 ml stationary liquid (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) for 10
min. Following the removal of stationary liquid, the cells were
pre-incubated with the different serum treatments for 24 h before
they were harvested by centrifugation at 6,000 × g for 20 min at
4°C and washed twice with PBS (pH=7.4). Cells were re-suspended in
a 2×106 cells/ml concentration. A total of 1 ml of the
cells were transferred into a 15-ml polypropylene tube on ice and 3
ml cold absolute ethanol was added. The cells were incubated for at
least 1 h at 4°C for fixation. Subsequently cells were washed twice
with PBS and 1 ml propidium iodide (PI; 40 µg/ml) staining solution
was added to the cell pellets and mixed well. Apoptosis of cells
was analyzed using FACScalbur flow cytometry (BD Biosciences, San
Jose, CA, USA) according to manufacturer's protocol.
Cell cycle detection by flow
cytometry
Each cell line was divided into control, CCC serum,
5-FU, combined treatment and serum control groups for different
treatments. In each group, cells were plated in 6-well culture
plates at a density of 3×106 cells/well. Cells were
pre-incubated with the different serum treatment for 24 h before
they were harvested by centrifugation at 6,000 × g for 20 min at
4°C and washed twice with PBS (pH=7.4). The cell solution was then
stained using a cell cycle kit (cat. no. YN-28; Forevergen
Biosciences Co., Ltd., Guangzhou, China), according to the protocol
of the manufacturer. Flow cytometry was performed using a BD
FASAria Cell Sorter (BD Biosciences).
Western blot analysis to identify
expression levels of key proteins from different signaling
pathways
BGC-823 and SGC-7901 cell lines were grown on 6-well
culture plates at a density of 3×106 cells/well. After
24 h of treatment, cells from the control, CCC serum, 5-FU,
combined treatment and serum control group, were obtained for
protein extraction with cold radioimmunoprecipitation assay buffer
(50 mM Tris pH 7.4, 150 mM NaCl, 1% triton X-100, 0.1% SDS, 1%
sodium deoxycholate, 5 mM EDTA, 30 mM Na2HPO4
and 50 mM NaF). Cells were immediately scraped off followed by
centrifugation at 12,000 × g for 15 min at 4°C. The supernatants
were collected and protein concentrations were determined using
Bradford reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) by
measuring absorbance at 595 nm. A total of 2 µg of protein was
loaded and separated using 12.5% SDS-PAGE with a total volume of 20
µl/well. The protein from the acrylamide gel was transferred to a
nitrocellulose membrane. The membrane was blocked with skimmed milk
in Tris-buffered saline-Tween 20 at 4°C overnight and subsequently
incubated with primary antibodies, including anti-proliferating
cell nuclear antigen (PCNA; 1:500; sc25280; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), phosphorylated (p)-P38
(1:500; sc7973; Santa Cruz Biotechnology, Inc.), P38 (1:1,000;
cst74451), p-ERK1/2 (1:2,000; cst9106), ERK1/2 (1:1,000; cst4695),
p-JNK (1:750; cst4668), JNK (1:1,000; cst9258; all CST Biological
Reagents Co., Ltd., Shanghai, China), p-IκBα (1:500; sc8404; Santa
Cruz Biotechnology, Inc.) and IκBα (1:1,000; cst4814; CST
Biological Reagents Co., Ltd.) overnight at room temperature.
Subsequently, blots were incubated with secondary antibody
horseradish peroxidase (1:4,000; SRE0082; Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature and washed again as described
above. Band intensity was determined using chemiluminescent
reagents (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and analyzed using ImageJ software v.14.8 (National Institutes of
Health, Bethesda, MD, USA).
RT-qPCR analysis
Total RNA was extracted from each of the control,
CCC serum, 5-FU, combined treatment and serum control groups in the
two cell lines at 24 h post treatment for the detection of the
c-Myc and p53 genes using TRIzol (Takara Bio, Inc., Otsu, Japan).
The quantity and purity of RNA were determined by OD measurements
at OD A260/A280 ratio with 1.8 or above using Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific Inc.). The cDNA was
synthesized from 1 µg RNA using a 5 Prime Masterscript RT-PCR
System (5 Prime, Inc., Gaithersburg, MD, USA), according to the
manufacturer's instructions, and stored at −20°C until assay. The
KAPA SYBR FAST qPCR kit (Kapa Biosystems, Woburn, MA, USA) was used
for qPCR. The 20 µl total reaction mixture for qPCR contained 20 ng
cDNA template, 10 µl of 2X KAPA SYBR FAST qPCR master mix, 200 nM
of forward and reverse primers and PCR-grade water. β-actin was
used as a reference gene. The sequences of the primers for the
reaction were as follows: H-p53-F, forward
5′-GTTGGTCGGTGGGTTGGTAGTTT-3′ and reverse
5′-GGTGTGGGATGGGGTGAGATTT-3′; H-c-Myc-F, forward
5′-CTTCTCTCCGTCCTCGGATTCT-3′ and reverse
5′-GAAGGTGATCCAGACTCTGACCTT-3′; and β-actin, forward
5′-TGCAGAGGATGATTGCTGAC-3′ and reverse 5′-GAGGACTCCAGCCACAAAGA-3′.
The reaction was performed in an Applied Biosystems 7500 Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with the following PCR cycling conditions: Enzyme activation for 3
min at 95°C, followed by 40 cycles of initial denaturation at 95°C
for 30 sec and annealing/extension at 58°C for 32 sec. Melting
curve analysis was performed for verifying specificity of each
primer after PCR to ensure amplification specificity. The threshold
cycle number was determined and used in the comparative Cq method.
The relative quantity of the target gene was estimated using the
2−ΔΔCq method (27). All
data were analyzed using ABI 7500 v. 2.0 software (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Statistical analysis
Experiments on cell viability, western blotting and
RT-qPCR were repeated three times in triplicate measurement.
Statistical analyses were performed with one-way analysis of
variance followed by a post hoc analysis (Tukey's multiple
comparison test) using GraphPad Prism v.5.0 software for Windows
(GraphPad Software, Inc., La Jolla, CA, USA). The results were
expressed as the mean + standard deviation for each group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
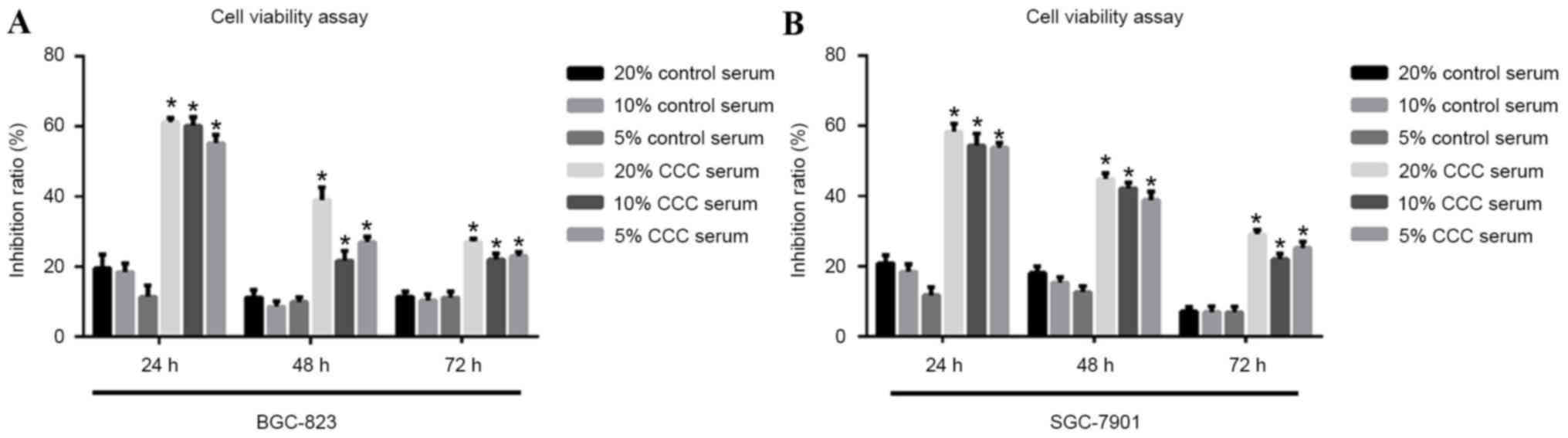
CCC serum concentration selection by
cell viability assay
To select the optimal CCC serum concentration for
the treatment, MTT assay was performed to measure cell viability of
low-level differentiated BGC-823 cells and mid-level differentiated
SGC-7901 cells. The results demonstrated an increase in inhibition
rate as the CCC serum concentration increased from 5 to 20% in both
the low-level differentiated BGC-823 cells and mid-level
differentiated SGC-7901 cells (Fig.
1). Thus, 20% CCC serum was selected for usage in the following
experiments.
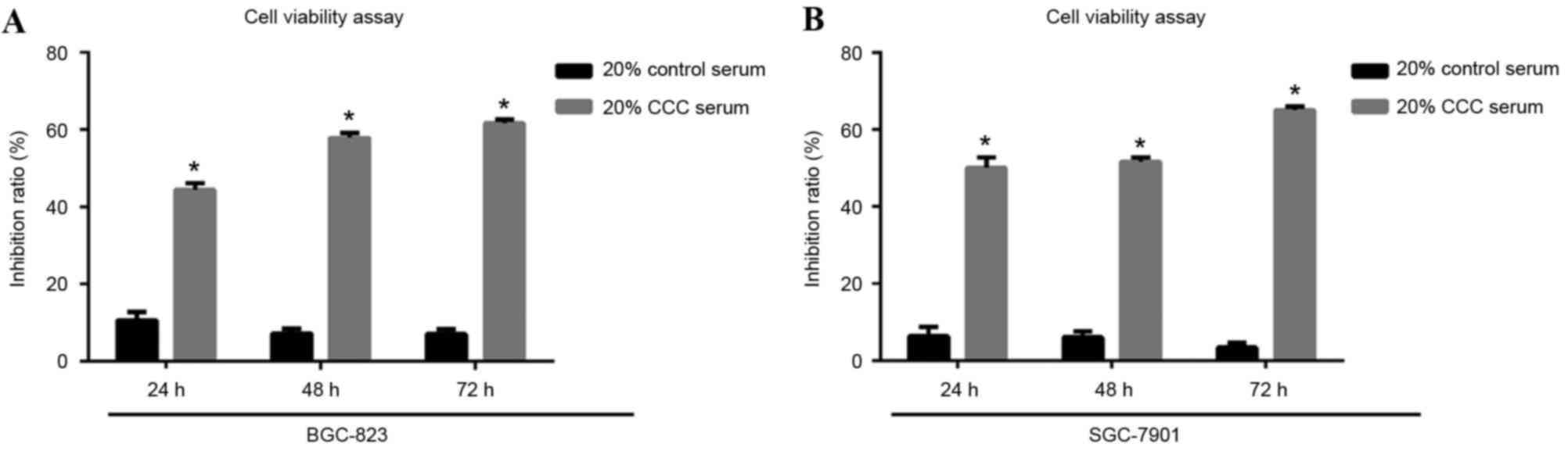
CCC serum incubation treatment time
selection by cell viability assay
To select the optimal CCC serum incubation time for
the treatment, cell viability percentage of low-level
differentiated BGC-823 cells and mid-level differentiated SGC-7901
cells were estimated by MTT following 24, 48 or 72 h exposure to
20% CCC serum. The results demonstrated a similar inhibition level
at 48 and 72 h, which was higher than that at 24 h, in BGC-832
cells. A similar inhibition level at 24 and 48 h was observed in
the SGC-7901 cells, which was lower than that at 72 h. All
inhibition ratios were significantly increased in the CCC
serum-treated groups compared with the control groups (P<0.05;
Fig. 2).
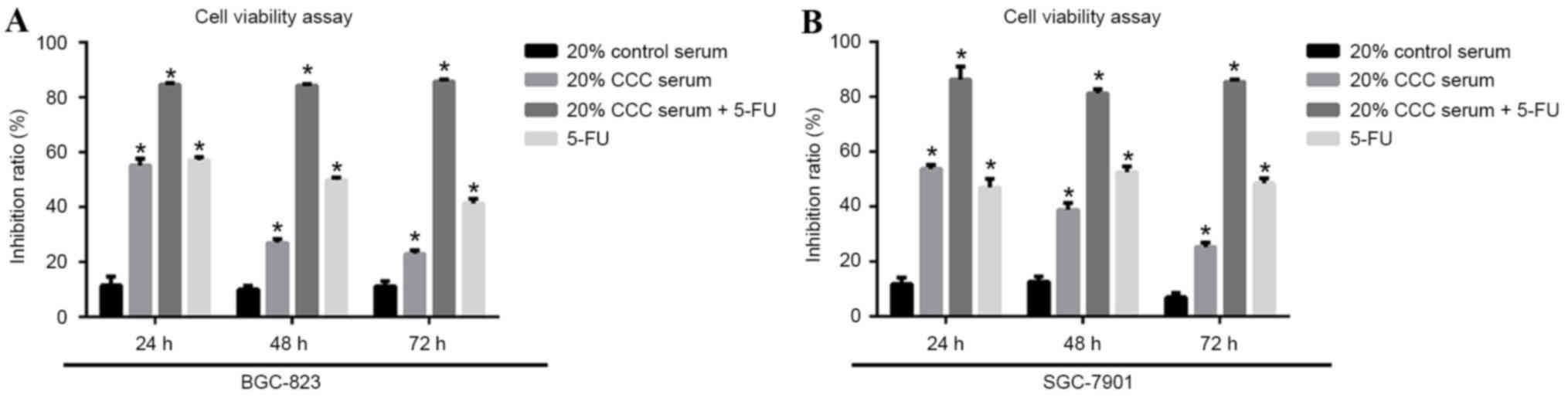
Both 20% CCC serum and combined
therapy inhibit tumorigenicity
In the BGC-823 and SGC-7901 cell lines, CCC serum
therapy, 5-FU and combined therapy, which consisted of 20% CCC
serum + 5-FU, demonstrated significant inhibition to cell viability
(P<0.05) compared with the control serum group (Fig. 3). Combined therapy provided a
significantly increased and stable inhibition rate of >80% when
compared with the control group over the 72-h observation time
(P<0.05). In both of the cell lines, combined therapy resulted
in a superior inhibition rate when compared with 20% CCC serum or
5-FU treatment alone (P<0.05; Fig.
3).
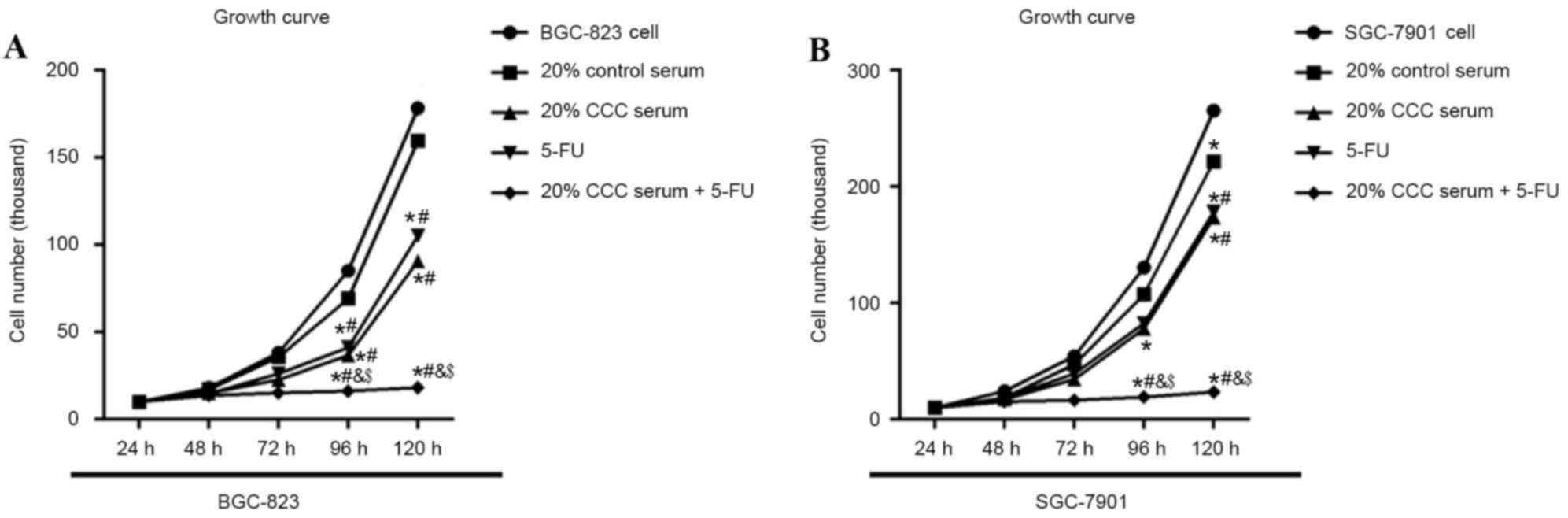
20% CCC serum synergistically enhances
the effect of 5-FU on cell viability in gastric cancer cell
lines
To examine the effect of 5-FU and 20% CCC on cell
viability and survival, low-level differentiated BGC-823 gastric
cancer cells and mid-level differentiated SGC-7901 gastric cancer
cells were each exposed to 20% CCC serum, 5-FU (10 µl/ml) or
combined therapy consisting of 20% CCC serum + 5-FU (10 µl/ml) for
24 h before cell numbers were counted for a continuous 5 days. On
day 2, cell numbers were similar between each treatment in both
BGC-823 and SGC-7901. On day 3, 4 and 5, cell numbers were
significantly decreased (P<0.05) in the 20% CCC serum + 5-FU
group when compared with control serum group in both cell lines
(Fig. 4). On day 3, 4 and 5, similar
inhibition effects were observed in the 20% CCC serum and 5-FU
groups in both cell lines. When both CCC serum and 5-FU were
incubated together with the cells, the combined treatment group
cells demonstrated a significant reduction in cell number compared
with separate treatment at 96 and 120 h (P<0.05).
20% CCC serum and 5-FU treatment
induce apoptosis in gastric cancer cells
In the present study, the apoptotic rate of gastric
cancer cells BGC-823 and SGC-7901 were quantified by flow cytometry
assay. As demonstrated in Fig. 5A,
both 20% CCC serum and 5-FU significantly induced apoptosis in the
gastric cancer cell lines compared with control serum and blank
control group (P<0.05), and combination treatment resulted in a
significantly greater increase in the apoptosis rate compared with
either treatment alone (P<0.05).
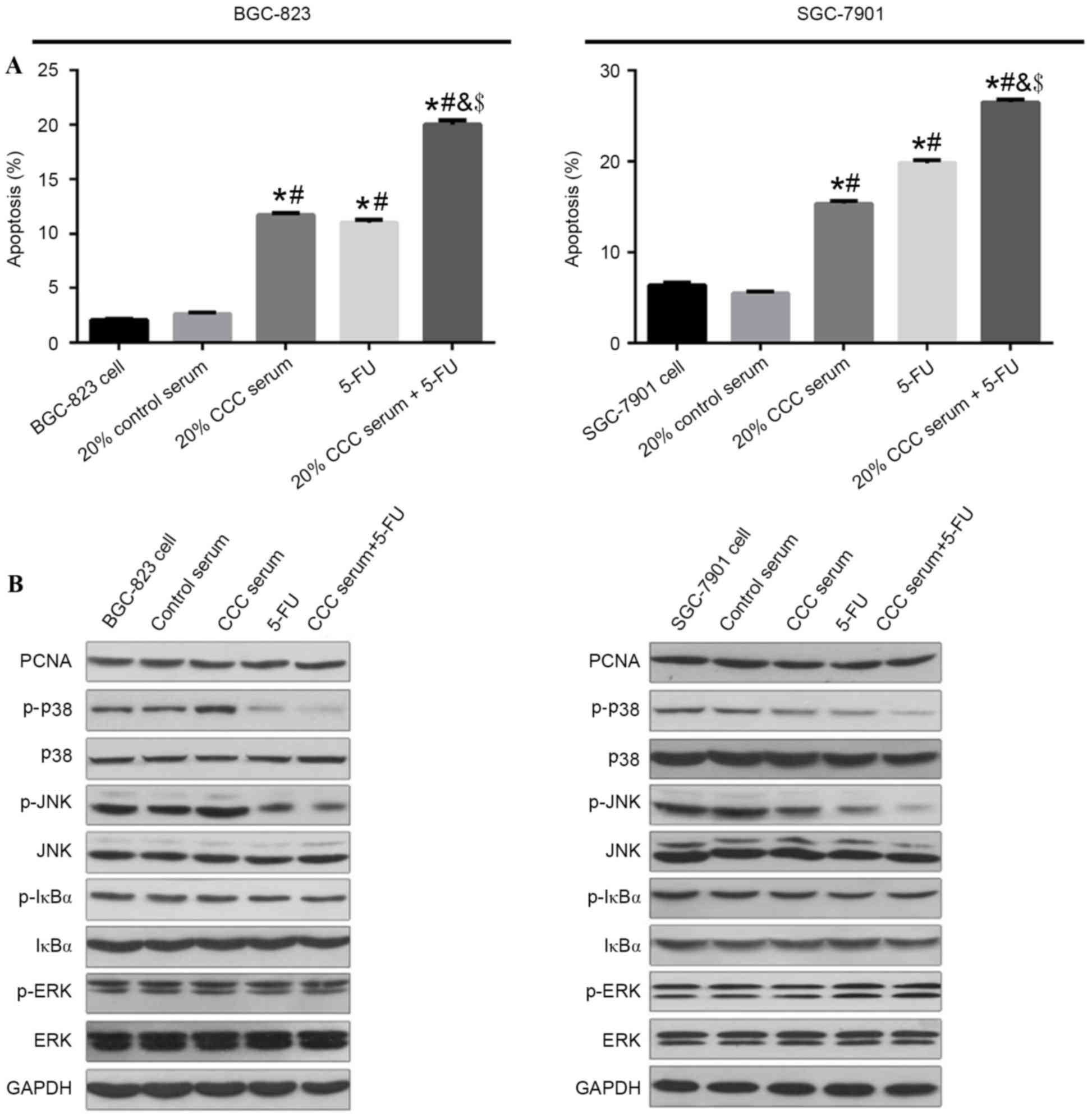 | Figure 5.(A) Cell apoptotic rate was
determined after combination treatment with 20% CCC serum and 5-FU
in low-level differentiated BGC-823 cells and mid-level
differentiated SGC-7901 cells by flow cytometric analysis of
apoptotic cells. (B) Protein expression levels of PCNA, p38, JNK,
IκBα, ERK1/2 and GAPDH in BGC-823 and SGC-7901 cells were detected
by western blot analysis. Data are presented as the mean + standard
deviation of three separate experiments. *P<0.05 vs. cells
without treatment in the same cell line; #P<0.05 vs.
20% control serum group; &P<0.05 vs. 20% CCC
serum group; $P<0.05 vs. 5-FU group. CCC, compound
cantharides capsule; 5-FU, 5-fluorouracil; PCNA, proliferating cell
nuclear antigen; JNK, c-Jun N-terminal kinase; ERK, extracellular
signal-related kinase; p, phosphorylated. |
Western blotting analysis was performed to analyze
the potential mechanism involved in 20% CCC serum and 5-FU-induced
apoptosis. Results demonstrated that the protein expression levels
of p-JNK and p-p38 kinase were markedly decreased after treatment
with CCC serum or combined therapy in the SGC-7901 cell line, and
only p-p38 kinase was notably decreased in the BGC-823 cell line.
Conversely, protein expression levels of PCNA, ERK1/2 and IκBα were
not affected (Fig. 5B). Together,
these results implied that combined therapy induced cell apoptosis
through deactivation of JNK and p38 kinase pathways but
demonstrated no effect on IκBα and ERK phosphorylation.
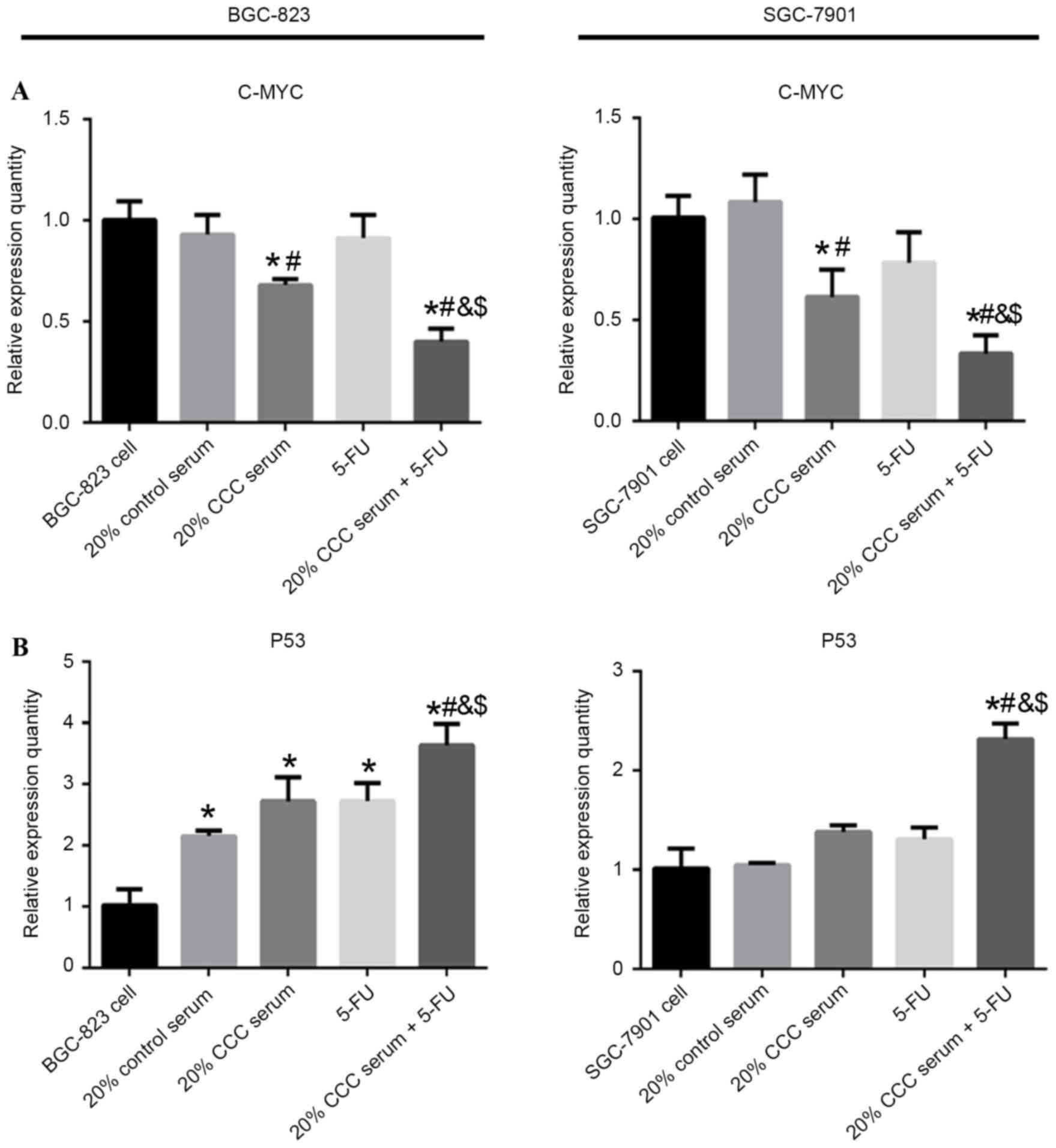
Expression of apoptosis-related genes
in gastric cancer cells
To observe the gene expression levels of
apoptosis-related genes in low-level differentiated BGC-823 cells
and mid-level differentiated SGC-7901 cells that were exposed to
combination treatment with 20% CCC serum and 5-FU or each treatment
alone, the gene expression levels of C-MYC and p53 were
investigated using RT-qPCR analyses. mRNA expression levels of
C-MYC were significantly decreased with 20% CCC serum (P<0.05)
and 20 % CCC serum + 5-FU treatment (P<0.05) in both cell lines
compared with 20% control serum treatment or cells without
treatment. P53 mRNA expression levels were significantly increased
in the 20% control serum group (P<0.05) compared with cells
without treatment in BGC-823 cell line. Furthermore, significantly
increased p53 mRNA expression levels were demonstrated in the 20%
CCC serum + 5-FU treatment group compared with 20% control serum,
20% CCC serum or 5-FU treatment in BGC-823 (P<0.05) and SGC-7901
(P<0.05) cells. The present results indicated that combined
therapy demonstrated the greatest significant change in C-MYC and
p53 mRNA expression levels in both of the cell lines compared with
cells without treatment (Fig.
6).
Discussion
The present study indicated that CCC serum treatment
reduced cell viability by inducing apoptosis in human gastric
cancer cells (SGC-7901 and BGC-823). Notably, to the best of our
knowledge, the present findings demonstrated, for the first time,
that CCC serum treatment was able to enhance the effects of 5-FU to
reduce cell viability and induce apoptosis in human gastric cancer
cells. These findings suggest that cantharide may be a potential
chemosensitizer for 5-FU-based chemotherapy regimens against
gastric cancer.
Gastric cancer is ranked as the fourth most common
type of cancer and the third leading cause of cancer mortality
among men and women worldwide (1).
The most common toxicities of chemotherapy or radiotherapy in the
treatment of gastric cancer were leukopenia, nausea/vomiting,
alopecia and proteinuria (32,33).
Therefore, novel therapeutic agents with reduced toxicity for
patients are in high demand. In the past decades, various natural
products have demonstrated to be less toxic than anticancer agents,
clinically (34). CCCs consist of a
compound based on cantharides, which includes bear gallbladder
powder, Panax ginseng, Astragalus mongholicus root
and Eleutherococcus senticosus (35). CCCs are among numerous TCMs used for
cancer treatment and have been used in clinical settings in China
for various types of diseases, such as bronchial asthma, breast,
liver, lung and digestive tract tumors (9). It has been demonstrated in leukemia
cells that cantharides are inhibitors of protein phosphatase1 and
2A (36). Cantharidin has been
demonstrated to induce apoptosis by a p53-dependent mechanism
(29). Such effects may be mediated
by any of the various mechanisms in which cantharides are involved
(36–41). However, studies regarding the
molecular basis of the effects of cantharides in gastric cancer
cell lines have been limited. Also, previous studies investigating
cantharide were using cantharidin, a chemical compound, which was
added into the cell culture (42).
However, when orally ingesting CCCs, cantharide in the blood serum
is the most effective medicine (31). To the best of our knowledge, the
present study indicates for the first time that, by mimic the
pharmacological change in human being, and directly adopted the
blood serum after SD rats intake the capsules, which is a more
direct model to study the effects of cantharide.
In the present study, the effect of cantharide in
gastric cancer cells and its potential modulation were explored. A
CCC serum model was established to mimic cantharide serum after
human oral intake. Previous studies have demonstrated that
cantharide induces apoptosis in colon cancer, human hepatoma, oral
buccal carcinoma and liver carcinoma cells in vitro
(37,43,44).
Therefore, we speculated that cantharide, a potent inhibitor of
cancer cell proliferation, also has antitumor effects on human
gastric cancer cells. It was demonstrated that cantharide had
inhibitory effects on gastric cancer cell viability and induced
apoptosis in gastric cancer cells. The inhibition level of cells
from the 20% CCC serum group was not as high as the cells that
underwent 5-FU treatment. Combined therapy indicated the strongest
inhibition effect in human gastric cancer cells, demonstrating a
strong clinical potential for adopting CCC in chemotherapy to
enhance 5-FU effects. The cell viability interruption of gastric
cancer cells in the five groups revealed that 20% CCC exhibited a
similar effect to 5-FU. However, cell growth was significantly
reduced following combined therapy treatment compared with 5-FU or
CCC serum alone, which indicated a mutual promotion between CCC and
5-FU.
In addition, previous reports have suggested that
cantharide induced apoptosis via p38, MAPK and JNK activation in
pancreatic cancer and leukemia cells (29,45).
Consistent with the previous studies, the present results indicated
that the protein expression levels of p-p38 and p-JNK were also
increased through CCC treatment in gastric cancer cells; however,
the MAPK-associated factors, IkBα and ERK, were not activated.
These findings differ from a previous report in U937 human
myelocytic leukemic cells (29),
which indicated that cantharidin may not activate all MAPK pathways
during human gastric cancer cell apoptosis and viability
inhibition. The phosphorylation of p53 at serine 15 was reported to
be a key phosphorylation target during the p53 activation process
in apoptosis (25,46). There has not yet been evidence to
correlate phosphorylation and p53 by ERK1/2, and in the present
study, the relationship between ERK-1/2 and p53 was not fully
clarified. Overall, the present findings suggested that cantharidin
is able to induce apoptosis by increased the expression of the
p-p38 and p-JNK associated with C-MYC and p53.
In conclusion, to the best of our knowledge, the
present study reported, for the first time, that the administration
of CCCs, an effective serum containing cantharide, induces
apoptosis to varying degrees in differentiated gastric cells.
Furthermore, CCCs were able to promote 5-FU chemotherapy effects as
a combined therapy and inhibit the cell viability of gastric cancer
cells. Additionally, CCC administration promoted downregulation of
c-Myc and upregulation of p53 gene expression levels. These
findings suggested that downregulation of JNK and p38 kinase
signaling pathways have an important role in cantharide-induced
apoptosis in human gastric cancer cells. Therefore, CCCs may be
promising candidates for effective therapy to treat gastric
cancer.
Glossary
Abbreviations
Abbreviations:
|
CCC
|
compound cantharides capsules
|
|
5-FU
|
5-fluorouracil
|
|
TCM
|
Traditional Chinese Medicine
|
|
MAPK
|
mitogen-activated protein kinases
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics, 2012. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. Ca Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ychou M, Boige V, Pignon JP, Conroy T,
Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM,
Saint-Aubert B, et al: Perioperative chemotherapy compared with
surgery alone for resectable gastroesophageal adenocarcinoma: An
FNCLCC and FFCD multicenter phase III trial. J Clin Oncol.
29:1715–1721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Qi Y, Liu H, Wang X, Zhu H and Wang
Z: AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric
cancer cells. Mol Cell Biochem. 411:299–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camacho LH, Garcia S, Panchal AM, Lim J,
Hong DS, Ng C, Madoff DC, Fu S, Gayed I and Kurzrock R: Exploratory
study of hepatic arterial infusion oxaliplatin with systemic
5-fluorouracil/bevacizumab in patients with refractory solid tumor
and extensive liver metastases. Clin Colorectal Cancer. 9:311–314.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu MN, Liu AY, Pei FH, Ma X, Fan YJ, Du
YJ and Liu BR: Functional mechanism of the enhancement of
5-fluorouracil sensitivity by TUSC4 in colon cancer cells. Oncol
Lett. 10:3682–3688. 2015.PubMed/NCBI
|
|
8
|
He Q, Ma L, Li Y and Li G: A pilot study
of an individualized comprehensive treatment for advanced gastric
cancer with para-aortic lymph node metastasis. BMC Gastroenterol.
16:82016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xun L, Yang G, Li X, Zhang Y, Yang J,
Chang J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese
medicine in cancer care: A review of controlled clinical studies
published in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Éwe GE: Chinese cantharides (mylabris
Cichorii). A worthy candidate for admission to the u. s. p. J Pharm
Sci. 9:257–263. 1920.
|
|
11
|
Wang GS: Medical uses of mylabris in
ancient China and recent studies. J Ethnopharmacol. 26:147–162.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia JM, Wang ZQ, Wu LJ and Wu YL: Advance
of pharmacological study on ginsenoside Rb_1. Zhongguo Zhong Yao Za
Zhi. 33:1371–1377. 2008.(In Chinese). PubMed/NCBI
|
|
13
|
Li W, Zhao H, Qian W, Li H, Zhang L, Ye Z,
Zhang G, Xia M, Li J, Gao J, et al: Chemotherapy for gastric cancer
by finely tailoring anti-Her2 anchored dual targeting
immunomicelles. Biomaterials. 33:5349–5462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han JJ, Yu JM, Wu HY, Liu JB, Song B and
Xue DW: Inhibitory effect of compound cantharides capsule on the
proliferation of xenografts of human hepatocellular carcinoma HepG
(2)215 in mice. Zhonghua Zhong Liu Za Zhi. 34:821–825. 2012.(In
Chinese). PubMed/NCBI
|
|
15
|
Tamura G: Alterations of tumor suppressor
and tumor-related genes in the development and progression of
gastric cancer. World J Gastroenterol. 12:192–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Otani K, Li X, Arakawa T, Chan FK and Yu
J: Epigenetic-mediated tumor suppressor genes as diagnostic or
prognostic biomarkers in gastric cancer. Expert Rev Mol Diagn.
13:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee EY and Muller WJ: Oncogenes and tumor
suppressor genes. Cold Spring Harb Perspect Biol.
10:a0032361997.
|
|
18
|
Krautheim A, Brechlin P, Becker K, Winkler
M and Steinfelder HJ: Hamster pancreatic beta cell lines with
altered sensitivity towards apoptotic signalling by phosphatase
inhibitors. Br J Pharmacol. 129:687–694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke PR, Hoffmann I, Draetta G and
Karsenti E: Dephosphorylation of cdc25-C by a type-2A protein
phosphatase: Specific regulation during the cell cycle in Xenopus
egg extracts. Mol Biol Cell. 4:397–411. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor BK, Stoops TD and Everett AD:
Protein phosphatase inhibitors arrest cell cycle and reduce
branching morphogenesis in fetal rat lung cultures. Am J Physiol
Lung Cell Mol Physiol. 278:L1062–L1070. 2000.PubMed/NCBI
|
|
21
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schuler M, Bossy-Wetzel E, Goldstein JC,
Fitzgerald P and Green DR: p53 induces apoptosis by caspase
activation through mitochondrial cytochrome c release. J Biol Chem.
275:7337–7342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marchenko ND, Zaika A and Moll UM: Death
Signal-induced localization of p53 protein to mitochondria a
potential role in apoptotic signaling. J Biol Chem.
275:16202–16212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pflaum J, Schlosser S and Müller M: p53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taylor CA, Zheng Q, Liu Z and Thompson JE:
Role of p38 and JNK MAPK signaling pathways and tumor suppressor
p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung
cancer cells. Mol Cancer. 12:352013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weilbacher A, Gutekunst M, Oren M,
Aulitzky WE and van der kuip H: RITA can induce cell death in
p53-defective cells independently of p53 function via activation of
JNK/SAPK and p38. Cell Death Dis. 5:e13182014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huh JE, Kang KS, Chae C, Kim HM, Ahn KS
and Kim SH: Roles of p38 and JNK mitogen-activated protein kinase
pathways during cantharidin-induced apoptosis in U937 cells.
Biochem Pharmacol. 67:1811–1818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shou LM, Zhang QY, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX and Tao M: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013.PubMed/NCBI
|
|
31
|
Huang K and Ma W: Clinical observation on
the patients with advanced esophageal cancer treated by
chemotherapy combined with compound cantharides capsule. Zhongliu
Jichu Yu Linchuang. 2014.(In Chinese).
|
|
32
|
Poon M, Hwang J, Dennis K, DeAngelis C,
Zhang L, Chung H, Stinson J, Wong S, Pulenzas N and Chow E: A novel
prospective descriptive analysis of nausea and vomiting among
patients receiving gastrointestinal radiation therapy. Supportive
Care Cancer. 24:1545–1561. 2016. View Article : Google Scholar
|
|
33
|
Huang J, Zhao Y, Xu Y, Zhu Y, Huang J, Liu
Y, Zhao L, Li Z, Liu H, Wang QL and Qi X: Comparative effectiveness
and safety between oxaliplatin-based and cisplatin-based therapy in
advanced gastric cancer: A meta-analysis of randomized controlled
trials. Oncotarget. 7:34824–34831. 2016.PubMed/NCBI
|
|
34
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ledermann DW: Simon Bolivar and the
cantharides. Rev Chilena Infectol. 24:409–412. 2007.(In Spanish).
PubMed/NCBI
|
|
36
|
Efferth T, Rauh R, Kahl S, Tomicic M,
Böchzelt H, Tome ME, Briehl MM, Bauer R and Kaina B: Molecular
modes of action of cantharidin in tumor cells. Biochem Pharmacol.
69:811–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu D and Chen Z: The effects of
cantharidin and cantharidin derivates on tumour cells. Anticancer
Agents Med Chem. 9:392–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kadioglu O, Kermani NS, Kelter G,
Schumacher U, Fiebig HH, Greten HJ and Efferth T: Pharmacogenomics
of cantharidin in tumor cells. Biochem Pharmacol. 87:399–409. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H and Yan X: Cantharidin modulates
the E2F1/MCM7-miR-106b-93/p21-PTEN signaling axis in MCF-7 breast
cancer cells. Oncol Lett. 10:2849–2855. 2015.PubMed/NCBI
|
|
41
|
Sagawa M, Nakazato T, Uchida H, Ikeda Y
and Kizaki M: Cantharidin induces apoptosis of human multiple
myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci.
99:1820–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kok SH, Chui CH, Lam WS, Chen J, Lau FY,
Cheng GY, Wong RS, Lai PP, Leung TW, Tang JC and Chan AS: Apoptotic
activity of a novel synthetic cantharidin analogue on hepatoma cell
lines. Int J Mol Med. 17:945–949. 2006.PubMed/NCBI
|
|
43
|
Chen YN, Chen JC, Yin SC, Wang GS, Tsauer
W, Hsu SF and Hsu SL: Effector mechanisms of norcantharidin-induced
mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer.
100:158–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK,
Kuo YS, Chiang CP and Kuo MY: Norcantharidin-induced apoptosis in
oral cancer cells is associated with an increase of proapoptotic to
antiapoptotic protein ratio. Cancer Lett. 217:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest and
apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Amaral JD, Xavier JM, Steer CJ and
Rodrigues CM: The role of p53 in apoptosis. Discov Med. 9:145–152.
2010.PubMed/NCBI
|