Introduction
Stress urinary incontinence (SUI) is a common and
embarrassing urologic problem (1,2). Among
the various treatment techniques available, the sling procedure has
become the mainstay of surgical treatment for the management of
SUI. However, these slings are associated with complications,
including infection and urethral erosion (3–6).
Following the advancements in tissue engineering research, a
biodegradable tissue engineered sling may offer a promising
alternative for the treatment of SUI (7,8).
Over the past 10 years, numerous studies have
reported injectable stem cell therapies that achieved a high
success rate in repairing SUI models. Muscle-derived stem cells
(9), adipose-derived stem cells
(ADSCs) (10,11), bone-marrow-derived mesenchymal stem
cells (BMSCs) (12), fibroblasts and
myoblasts (13) have been used for
the repair of damaged sphincter muscle, which reveals a promising
approach for sling engineering in vitro.
In the present study, a polyglycolic acid (PGA)
scaffold was selected as the suburethral sling material because of
its good biocompatibility and suitable degradation rate (14). Considering the fact that the majority
of sling engineering studies have reported the short-term results
of in vivo sling engineering, ADSCs and PGA were employed to
explore the possibility of generating a sling complex in
vitro for a relatively longer observation period. In future, it
would be optimal to provide off-the-shelf engineered sling products
so that patients can benefit from sling grafts that are immediately
available.
Materials and methods
Isolation and culture of ADSCs
The Sprague-Dawley rats were obtained from the
Animal Research Center of Fudan University (Shanghai, China). A
total of 20 4-month-old female Sprague-Dawley rats (body weight,
240±20 g) were housed in pairs at 23°C and 50–70% humidity, with a
12 h light/dark cycle and free access to water and food pellets.
Adipose tissues were obtained from the inguinal regions of
Sprague-Dawley rats. The experimental protocol was approved by the
Research Ethics Committee of the Shanghai Jiao Tong University
Affiliated Sixth People's Hospital (Shanghai). Isolation and
culture of ADSCs was performed as described previously (15,16).
Briefly, the samples were digested with 0.10% collagenase I
(Sigma-Aldrich, Inc.; Merck KGaA, Darmstadt, Germany) through
shaking at 37°C for 1 h. Following digestion, collagenase I was
neutralized with an equal volume of basic growth medium containing
Low Glucose Dulbecco's Modified Eagle's Medium (LG-DMEM; HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin (both
Gibco; Thermo Fisher Scientific, Inc.). After centrifugation at
37°C for 5 min at 1,500 × g, cells were resuspended in the basic
growth medium and cultured at 37°C with 5% CO2. The
culture medium was changed every 3 days. When the culture dishes
reached 80–85% confluence after ~4 days, the cells were passaged
with trypsin-EDTA. ADSCs at passage 2 were used for the following
experiments.
Characterization of ADSCs in
vitro
The specific cell surface antigens, cluster of
differentiation (CD) 90, 44 and 34, of ADSCs were characterized by
flow cytometry analysis. Briefly, cells were incubated with
fluorescein isothiocyanate-tagged antibodies, including anti-rat
CD90 (1:100; cat. no. 11-0900; eBioscience; Thermo Fisher
Scientific, Inc.), anti-rat CD44 (1:100; cat. no. MCA643FA; Bio-Rad
Laboratories, Inc., Hercules, CA, ISA) and anti-rat CD34 (1:100;
cat. no. 11-0341, eBioscience; Thermo Fisher Scientific, Inc.) for
30 min at 4°C, then washed three times using PBS containing 4% FBS.
Flow cytometry was performed using fluorescence-activated cell
sorting (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's protocol. Data were analyzed by
FlowJo software version 7.6 (Tree Star, Inc., Ashland, OR, USA).
The ADSCs were cultured and induced using an appropriate medium.
For osteogenic differentiation, ADSCs were induced in DMEM
supplemented with 10% FBS, β-glycerol phosphate, dexamethasone and
ascorbic acid for 3 weeks, and analyzed with Alizarin Red S
staining as previously described (17,18). For
adipogenic differentiation, ADSCs were incubated in DMEM
supplemented with 10% FBS, 1-methyl-3-isobutylxanthine,
dexamethasone, insulin and indomethacin for 2 weeks, and analyzed
by Oil Red O staining as previously described (19).
Preparation of cell-PGA
constructs
A custom-made spring formed with a stainless steel
frame was used to provide constant strain as described previously
(20). Briefly, 50 mg PGA fibers
(~20–30 µm in diameter) were arranged into a cord shape with a
length of 4.5 cm and a diameter of 0.4 cm, and then secured onto a
custom-made spring. The scaffolds were sterilized with 75% ethanol,
washed with PBS and then pre-incubated at 37°C in DMEM supplemented
with 10% FBS to enhance cell attachment. Subsequently, the ADSCs
were collected and resuspended in culture medium at a density of
4×107 cells/ml followed by seeding onto the PGA fibers.
After 7 days of culture, the cell-scaffold construct was examined
using a scanning electron microscope (SEM) as reported previously
(21). After the SEM examination,
the subsequent culture period included a 4-week-long myoblast
differentiation phase and an 8-week-long proliferation phase.
Induction of myoblast differentiation was performed by adding 10
µmol/l 5-azacytidine (5-Aza, Sigma-Aldrich, Inc.; Merck KGaA), 5%
FBS and 5% horse serum (Gibco; Thermo Fisher Scientific, Inc.) to
LG-DMEM. After the 4-week-long differentiation phase, the medium
was replaced with basal culture medium containing LG-DMEM
supplemented with 10% FBS.
Analysis of the cell scaffolds
At 4 weeks (following the 4-week-long myoblast
differentiation phase), 8 weeks (4-weeks into the proliferation
phase) and 12 weeks (following the 8-week-long proliferation
phase), specimens of in vitro engineered slings were
collected, and their length and diameter was measured. Randomly
selected samples were taken and fixed with 4% paraformaldehyde at
4°C for 24 h followed by three washes in PBS, prior to being
paraffin embedded and sectioned to a 5–8 mm thickness for
hematoxylin and eosin (HE) staining as reported previously
(21) in order to examine the tissue
structure, particularly the cellular density and rate of PGA
degradation. In addition, the sections were subjected to Masson's
trichrome staining as described previously (21) in order to examine collagen
production. Myoblast formation was evaluated by immunohistochemical
staining. The tissue sections were stained with primary antibodies
directed against desmin (1:100; Abcam, Cambridge, UK) and α-smooth
muscle actin (α-SMA; 1:100), followed by the addition of a goat
anti-rabbit secondary IgG conjugated to horseradish peroxidase
(EnVision+ system; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA). The antibody staining was visualized with the Liquid
DAB+ Substrate Chromogen system (cat. no. K3467; Dako;
Agilent Technologies, Inc.) prior to counterstaining with
hematoxylin at 37°C for 15 min.
Biomechanical analysis
The in vitro engineered slings were collected
at 4, 8 and 12 weeks as described above. The constructs were
subjected to mechanical tests using a biomechanical analyzing
instrument (Instron Model 4411; Instron, Norwood, MA, USA). The
length of the tested slings was set to 2 cm between two grippers.
The grippers were then gradually moved apart at a speed of 25
mm/min until complete rupture of the tissue in order to calculate
the maximal (max) load. Additionally, the Young's modulus (MPa) was
calculated from the linear slope of a stress-strain curve as
described previously (22).
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. A one-way analysis of variance was performed to
analyze the differences in mechanical properties between different
time points. P<0.05 was considered to indicate a statistically
significant difference. SPSS software (version 11.0; SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis.
Results
Morphological and differentiation
characteristics of ADSCs
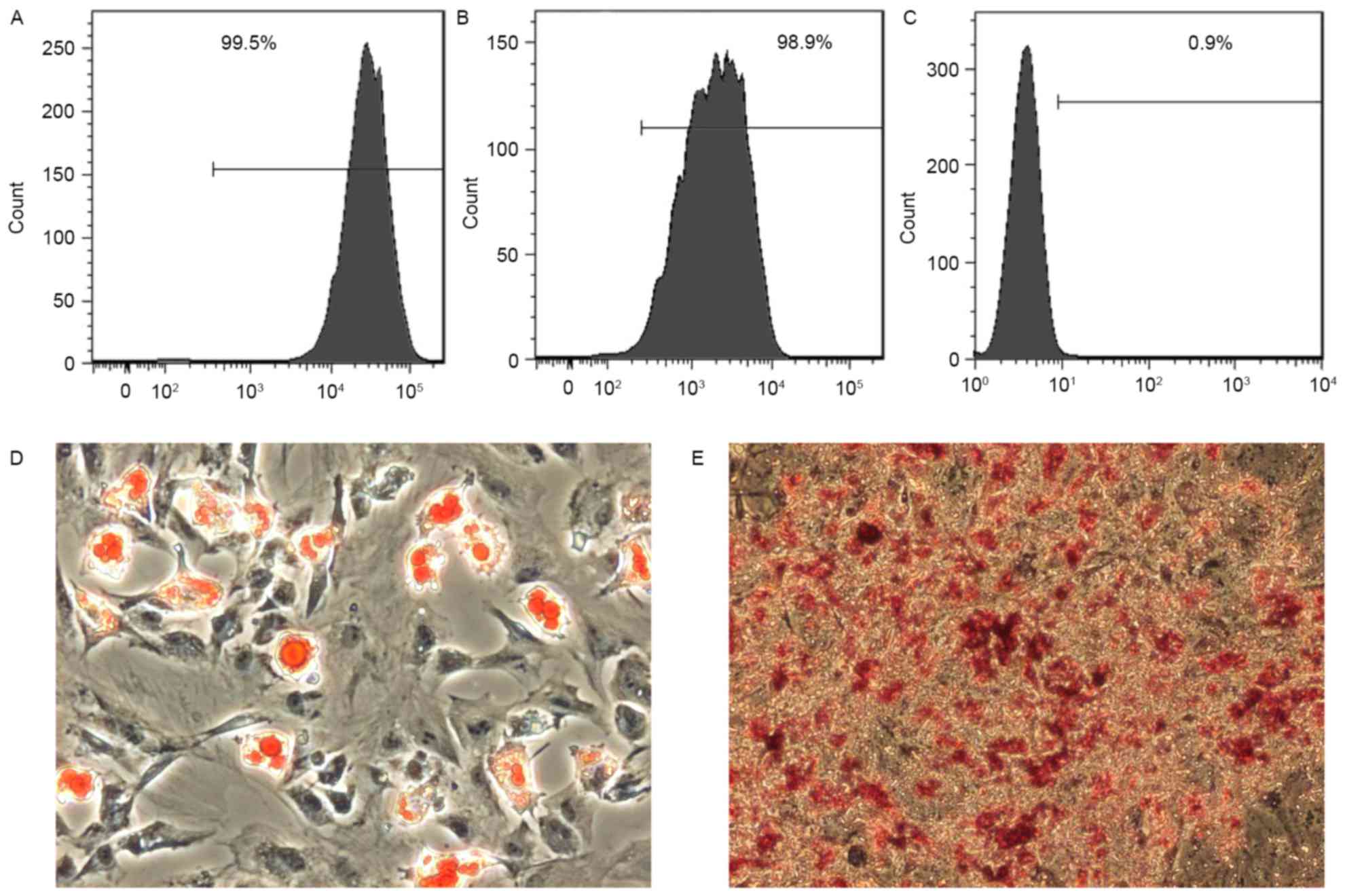
Primary cultured ADSCs generated from fresh rat
adipose tissue proliferated rapidly, reaching 80% confluence within
5 days. These cells were assessed for the expression of cell
surface markers that are considered to define adult stem cells, and
identified to express CD90 (Fig. 1A)
and CD44 (Fig. 1B). However, the
cells were negative for the hematopoietic stem cell marker CD34
(Fig. 1C). Additionally, ADSCs were
tested for their ability to differentiate into other cell types.
ADSCs cultured in adipogenic medium accumulated lipid vacuoles,
which were confirmed by Oil Red O staining (Fig. 1D). Furthermore, ADSCs cultured in
osteogenic medium deposited calcium as detected by Alizarin Red S
staining (Fig. 1E). These data
indicate that ADSCs exhibits multipotentiality.
Culture and characterization of the
ADSC-PGA complex
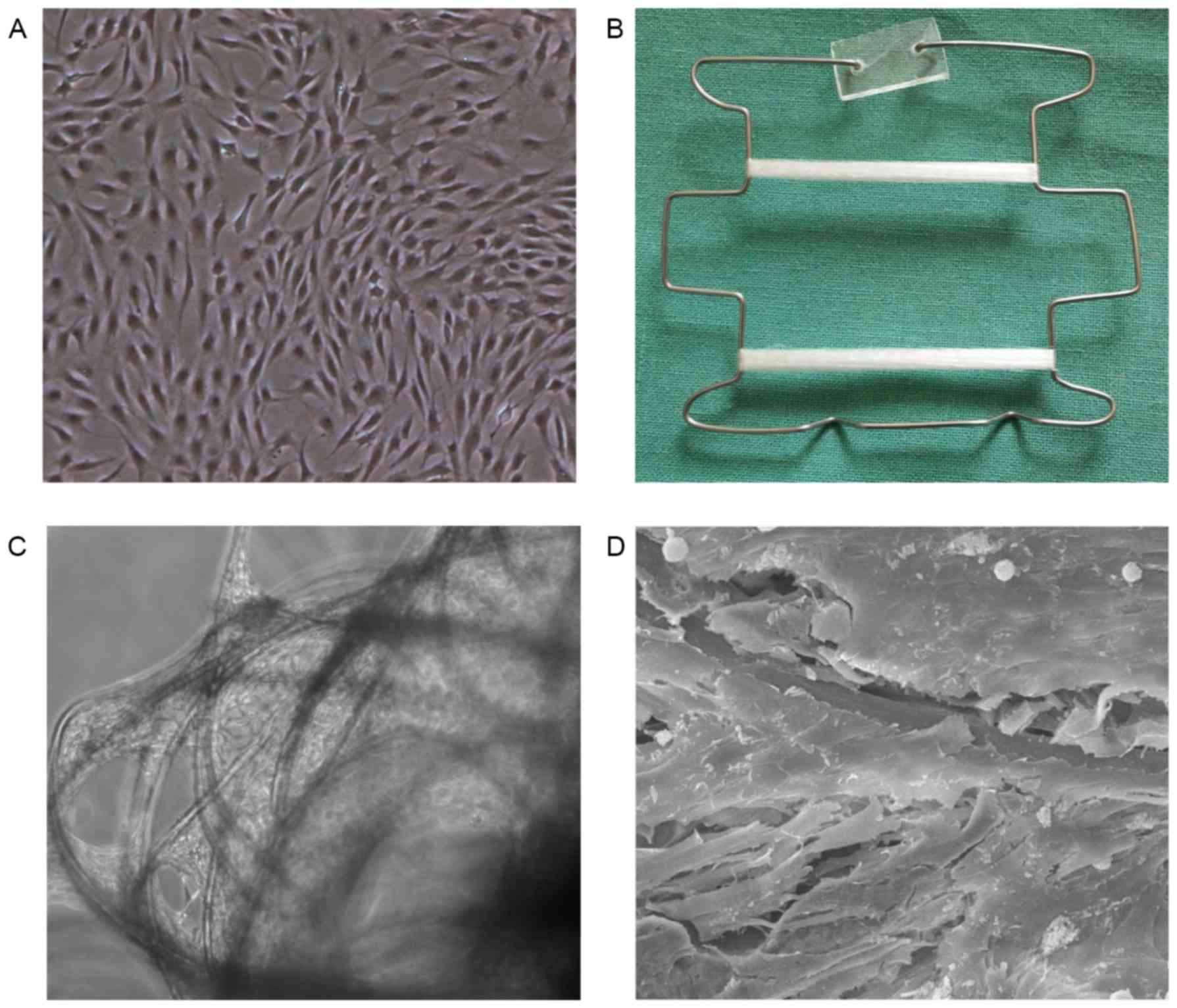
At passage 2, the ADSCs were observed to be able to
maintain good proliferation (Fig.
2A), and were thus used for sling engineering by seeding them
onto a PGA scaffold under a constant strain (Fig. 2B). A total of 7 days after seeding,
phase-contrast microscopy and SEM revealed good cell attachment on
the scaffold and secreted extracellular matrices filling the space
between the fibers (Fig. 2C and D),
indicating good biocompatibility between the ADSCs and PGA
fibers.
Gross observation and mechanical
properties of the engineered slings
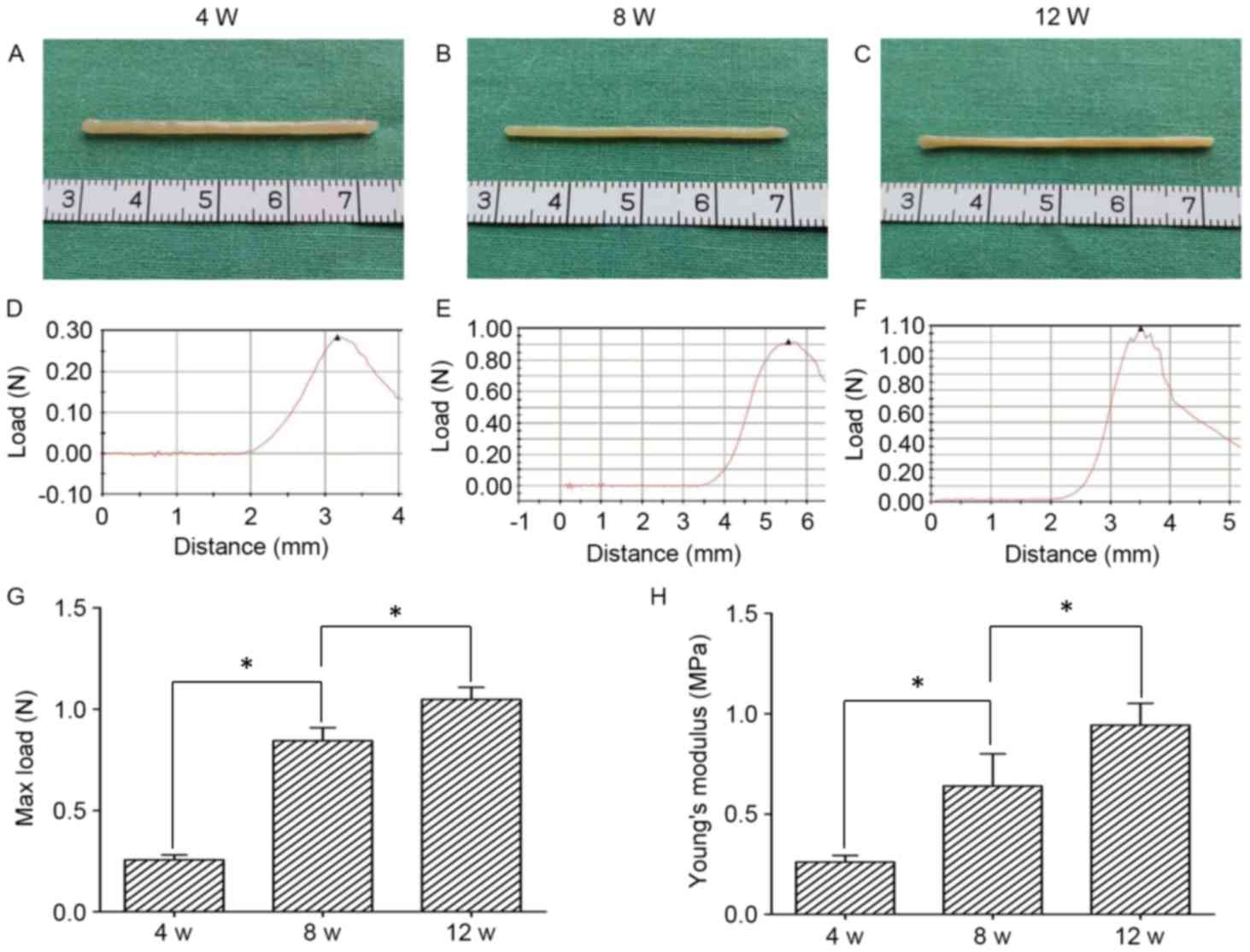
As illustrated in Fig.
3A, the ADSC-PGA construct appeared as a sling-like structure
with a cord-like shape during the first 4 weeks of in vitro
culture. The engineered slings exhibited a smoother surface at 8
(Fig. 3B) and 12 (Fig. 3C) weeks of culture. However, at 12
weeks the sling-like structure became much thinner with a more
mature tissue appearance when compared with the constructs at 4
weeks. Furthermore, a biomechanical analyzing instrument was used
to examine the mechanical properties of the in vitro
engineered slings. As presented in Fig.
3D-F, the engineered slings exhibited different patterns of
stress/strain curves when they were subjected to mechanical testing
at 4, 8 and 12 weeks, respectively. In order to further associate
tissue structure features and their mechanical properties in the
slings, the max load and Young's modulus were investigated. After
4, 8, and 12 weeks of culture, the max load reached 0.26±0.02 N,
0.84±0.06 N and 1.05±0.06 N, respectively (Fig. 3G). Furthermore, Young's modulus at 4
(0.26±0.03 MPa), 8 (0.64±0.16 MPa) and 12 weeks (0.94±0.11 MPa)
increased with time (Fig. 3H). These
results demonstrate that the engineered slings had gradually
increased stress/strain curves, max load and Young's modulus values
over time, with significant differences between slings cultured for
4, 8 and 12 weeks of culture (P<0.05).
Histological assessment of the in
vitro engineered slings
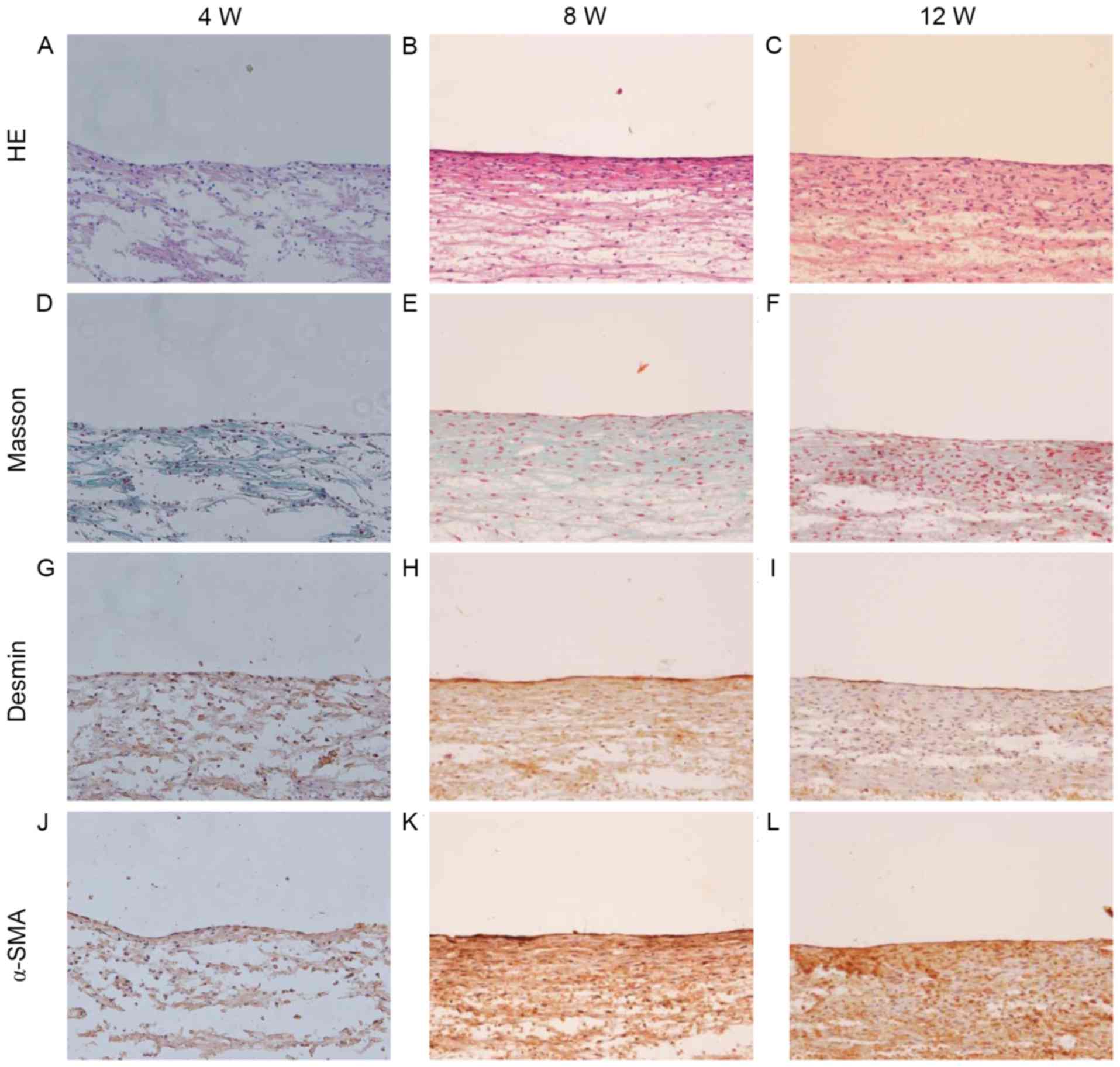
The in vitro engineered slings were assessed
by HE and Masson's trichrome staining (Fig. 4). This revealed that the ADSC-PGA
constructs were primarily composed of PGA fibers, with less matrix
deposition observed at 4 weeks of culture (Fig. 4A and D). When cultured for 8 weeks,
the slings exhibited a structure of longitudinally aligned collagen
fibers and cells with increased matrix deposition (Fig. 4B and E). At 12 weeks, the parallel
alignment of the cells and collagen fibers was enhanced further
(Fig. 4C and F), suggesting that a
longer cultivation enhances tissue remodeling and maturation. To
further investigate whether ADSCs could be induced to differentiate
into myoblasts and whether the differentiated ADSCs could maintain
their contractile phenotype when seeded onto the PGA scaffold,
immunohistochemical staining for desmin and α-SMA was performed. As
illustrated in Fig. 4G-L, the tissue
of the engineered slings was identified to contain cells expressing
desmin and α-SMA, which increased over time. Complete degradation
of the scaffold material was identified after 12 weeks of culture
(Fig. 4C and F).
Discussion
Previous studies have reported the success of sling
engineering for treating stress urinary incontinence (7,8). An
important issue in sling engineering is finding an appropriate cell
source for engineering a transplantable sling graft. ADSCs have
proven to be an excellent cell source, which have the advantage of
being able to be harvested in abundance and causing fewer traumas
to the donor site. Furthermore, ADSCs possess a similar
pluripotency and self-renewal potential to BMSCs (23). Previously, the periurethral injection
of ADSCs has been demonstrated to allow the active functional
recovery of deficient sphincters (24,25).
Therefore, ADSCs are likely to become the primary cell source for
sling engineering. PGA has been widely used in tissue engineering
due to its biocompatibility and degradability. Previous studies
have revealed that by forming a preliminary tissue structure PGA
could substantially degrade in vitro, avoiding the
accumulation of degradation products at the implantation site,
which may adversely affect tissue regeneration by causing fibrosis
(26,27).
The present study investigated the feasibility of
engineering a relatively thick sling by promoting the myoblast
differentiation of ADSCs seeded onto PGA scaffolds under mechanical
loading stress. This revealed that a relatively thick sling could
be engineered after 12 weeks of in vitro culture.
Furthermore, the engineering of a sling requires a relatively long
time to allow for tissue maturation, which leads to improved
mechanical properties. In the present study, the engineered sling
could reach a gross diameter of >2 mm and exhibited enhanced
mechanical properties in a time-dependent manner. Furthermore,
histological examination revealed that the collagen fibers and
myoblasts were highly compacted, which increased with increased
culture times, and complete degradation of the scaffold material
was identified after 12 weeks of culture.
The present preliminary study demonstrated the
feasibility of engineering a sling in vitro using a tissue
engineering approach. The method used in the present study may aid
in the future clinical treatment of SUI. In addition, following
in vitro mechanical loading, the engineered sling tissue
matured and demonstrated enhanced mechanical properties, which
demonstrated that mechanical loading serves an important role in
the maturation of tissue-engineered slings. However, there were
several limitations in the present study. The tension-loaded slings
were observed to be thinner with increased engineering times.
Therefore, a constant strain without relief, which is
non-physiological, may not be the best way to exert mechanical
loading stress. Previous studies have demonstrated that tissue
quality and mechanical strength could be markedly improved when
periodic mechanical loading was applied in a bioreactor system
(28,29). Thus, a dynamic strain may be a more
appropriate approach to pursue in future.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that a sling-like
tissue structure could be generated in vitro by culturing
the cell scaffold for a relatively long time. The results of the
present study identified that differentiated ADSCs could maintain
their myoblast phenotype when cultured on the PGA scaffold under a
static strain. In addition, as engineering times increased, the
engineered sling tissues exhibited a notable improvement in
histological structure and mechanical properties. However, the
slings under constant strain were observed to be thinner over time,
and so the application of a bioreactor system to exert dynamic
strain in future is warranted.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81270780), the Youth Project
of the Shanghai Municipal Commission of Health and Family Planning
(grant no. 20164Y0059) and the Natural Science Foundation of
Minhang (grant no. 2016MHZ37).
References
|
1
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, van Kerrebroeck P, Victor A and Wein A: The
standardisation of terminology of lower urinary tract function:
Report from the Standardisation Sub-committee of the International
Continence Society. Am J Obstet Gynecol. 187:116–126. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klausner AP and Vapnek JM: Urinary
incontinence in the geriatric population. Mt Sinai J Med. 70:54–61.
2003.PubMed/NCBI
|
|
3
|
Rutner AB, Levine SR and Schmaelzle JF:
Processed porcine small intestine submucosa as a graft material for
pubovaginal slings: Durability and results. Urology. 62:805–809.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hung MJ, Liu FS, Shen PS, Chen GD, Lin LY
and Ho ES: Analysis of two sling procedures using polypropylene
mesh for treatment of stress urinary incontinence. Int J Gynaecol
Obstet. 84:133–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stav K, Dwyer PL, Rosamilia A, Schierlitz
L, Lim YN, Chao F, De Souza A, Thomas E, Murray C, Conway C and Lee
J: Repeat synthetic mid urethral sling procedure for women with
recurrent stress urinary incontinence. J Urol. 183:241–246. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walter AJ, Morse AN, Leslie KO, Zobitz ME,
Hentz JG and Cornella JL: Changes in tensile strength of cadaveric
human fascia lata after implantation in a rabbit vagina model. J
Urol. 169:1907–1910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim IG, Piao S, Hong SH, Kim SW, Hwang TK,
Oh SH, Lee JH and Lee JY: The effect of a bioactive
tissue-engineered sling in a rat of stress incontinence model. J
Biomed Mater Res A. 100:286–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou XH, Zhi YL, Chen X, Jin HM, Wang LL,
Jiang YZ, Yin Z and Ouyang HW: Mesenchymal stem cell seeded knitted
silk sling for the treatment of stress urinary incontinence.
Biomaterials. 31:4872–4879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JY, Cannon TW, Pruchnic R, Fraser MO,
Huard J and Chancellor MB: The effects of periurethral
muscle-derived stem cell injection on leak point pressure in a rat
model of stress urinary incontinence. Int Urogynecol J Pelvic Floor
Dysfunct. 14:31–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao W, Zhang C, Jin C, Zhang Z, Kong D,
Xu W and Xiu Y: Periurethral injection of autologous
adipose-derived stem cells with controlled-release nerve growth
factor for the treatment of stress urinary incontinence in a rat
model. Eur Urol. 59:155–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li GY, Zhou F, Gong YQ, Cui WS, Yuan YM,
Song WD, Xin H, Liu T, Li WR, Gao ZZ, et al: Activation of VEGF and
ERK1/2 and improvement of urethral function by adipose-derived stem
cells in a rat stress urinary incontinence model. Urology.
80:953.e1–e8. 2012. View Article : Google Scholar
|
|
12
|
Kim SO, Na HS, Kwon D, Joo SY, Kim HS and
Ahn Y: Bone-marrow-derived mesenchymal stem cell transplantation
enhances closing pressure and leak point pressure in a female
urinary incontinence rat model. Urol Int. 86:110–116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitterberger M, Marksteiner R, Margreiter
E, Pinggera GM, Frauscher F, Ulmer H, Fussenegger M, Bartsch G and
Strasser H: Myoblast and fibroblast therapy for post-prostatectomy
urinary incontinence: 1-year followup of 63 patients. J Urol.
179:226–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niklason LE, Abbott W, Gao J, Klagges B,
Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri
S and Langer R: Morphologic and mechanical characteristics of
engineered bovine arteries. J Vasc Surg. 33:628–638. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arrigoni E, Lopa S, de Girolamo L, Stanco
D and Brini AT: Isolation, characterization and osteogenic
differentiation of adipose-derived stem cells: From small to large
animal models. Cell Tissue Res. 338:401–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhao L and Hantash BM: Support of
human adipose-derived mesenchymal stem cell multipotency by a
poloxamer-octapeptide hybrid hydrogel. Biomaterials. 31:5122–5130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YH, Ho ML, Chang JK, Chu HC, Lai SC
and Wang GJ: Microporation is a valuable transfection method for
gene expression in human adipose tissue-derived stem cells. Mol
Ther. 17:302–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen B, Wang B, Zhang WJ, Zhou G, Cao Y
and Liu W: In vivo tendon engineering with skeletal muscle derived
cells in a mouse model. Biomaterials. 33:6086–6097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Fu Q, Zhao RY and Deng CL:
Muscular tubes of urethra engineered from adipose-derived stem
cells and polyglycolic acid mesh in a bioreactor. Biotechnol Lett.
36:1909–1916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen W, Chen X, Chen J, Yin Z, Heng BC,
Chen W and Ouyang HW: The effect of incorporation of exogenous
stromal cell-derived factor-1 alpha within a knitted silk-collagen
sponge scaffold on tendon regeneration. Biomaterials. 31:7239–7249.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin G, Wang G, Banie L, Ning H, Shindel
AW, Fandel TM, Lue TF and Lin CS: Treatment of stress urinary
incontinence with adipose tissue-derived stem cells. Cytotherapy.
12:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu G, Song Y, Zheng X and Jiang Z:
Adipose-derived stromal cell transplantation for treatment of
stress urinary incontinence. Tissue Cell. 43:246–253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao D, Liu W, Wei X, Xu F, Cui L and Cao
Y: In vitro tendon engineering with avian tenocytes and
polyglycolic acids: A preliminary report. Tissue Eng. 12:1369–1377.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Y, Rodriguez A, Vacanti M, Ibarra C,
Arevalo C and Vacanti CA: Comparative study of the use of poly
(glycolic acid), calcium alginate and pluronics in the engineering
of autologous porcine cartilage. J Biomater Sci Polym Ed.
9:475–487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou
GD, Liu W and Cao Y: Engineering of an elastic large muscular
vessel wall with pulsatile stimulation in bioreactor. Biomaterials.
29:1464–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y
and Cui L: A small diameter elastic blood vessel wall prepared
under pulsatile conditions from polyglycolic acid mesh and smooth
muscle cells differentiated from adipose-derived stem cells.
Biomaterials. 31:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|