Introduction
Rheumatoid arthritis (RA) is one of the most
prevalent chronic autoimmune diseases, with an prevalence of 0.5 to
1% (1). Although the causes are not
completely known, the imbalance of CD4+ helper T cells
plays a crucial role in the initiation and perpetuation of the
immune response in RA patients. Activated Th0 cells differentiate
into distinct CD4+ T cell lineages which drive and
constrain immune-mediated pathology. Pathogenic T cells, such as
Th1 and Th17, are thought to be necessary for the initiation and
maintenance of RA (2,3). In addition to a breakdown of immune
tolerance, bone erosion, as a consequence of osteoclastogenesis,
occurs during the progression of RA. Although therapeutic advances
in the past decades have transformed articular and systemic
outcomes of RA (4,5), there are still considerable unmet
needs. Efforts to develop novel treatments are ongoing.
MicroRNAs (miRNAs or miRs) are endogenous noncoding
RNAs, 18–22 nt long, that bind one or more mRNAs, thereby
modulating protein expression by either repression of translation
or the increase of mRNA turnover and degradation. The molecular
mechanisms of miRNA synthesis and function have been extensively
reviewed (6). Many miRNAs have been
reported to participate in the pathogenesis of RA (7,8) and
emerge as key regulators of the immune system, with some even
posessioning significant therapeutic potential.
miR-34a has been widely studied as a tumor
suppressor. Recent studies have demonstrated its functions in
immune system, including the expression in immune cells and the
modulation of development, function, and survival of dendrite
cells, T lymphocytes, B lymphocytes, macrophages and mast cells.
Except for activating T lymphocytes and macrophages (9,10),
miR-34a was found to be elevated in the lesions of patients with
multiple sclerosis (11) and newly
diagnosed type II diabetes (12).
Krzeszinski et al have reported that miR-34a inhibited bone
loss by blocking osteoclastogenesis (13). miR-34a has been linked to inhibition
of chondrogenesis (14) and
angiogenesis by blocking vascular endothelial growth factor
production (15). miR-34a is thus
essential to immune responses, bone metabolism, chondrogenesis, all
of which are involved in the pathogenesis of RA.
Intrigued by these findings, we were prompted to
explore the important roles of miR-34a in the murine arthritis
in vivo and its implications in the autoimmunity and bone
metabolism, then further to get insight into the underlying
mechanisms of miR-34a in the experimental arthritis, which may
provide a novel strategy for preventing arthritis.
Materials and methods
Induction of collagen-induced
arthritis (CIA)
This study was approved by the Ethics Committee of
Harbin Medical University, Harbin, China. Male DBA/1j mice (6–8
weeks of age) were purchased from Shanghai Laboratory Animal Center
(Shanghai, China). Bovine type II collagen (CII; Chondrex, Inc.,
Washington, DC, USA), 2 mg/ml in 0.05 M acetic acid, was emulsified
with an equal volume of complete Freund's adjuvant (Sigma-Aldrich,
St. Louis, MO, USA). On day 0, 0.1 ml emulsion was injected
subcutaneously into mice at the base of the tails. On day 21, a
booster injection, 0.1 ml CII emulsified with incomplete Freund's
adjuvant, was administered near the primary injection site. The CIA
model mice were then given an injection of 10 nmol miR-34a agomir
or 50 nmol miR-34a antagomir (Guangzhou RioBio Co., Ltd.,
Guangzhou, China), which were a modified miR-34a mimic or inhibitor
respectively on the day of 28, 31, 35, 38 and 41 post the 1st
immunization. The mice were sacrificed on the day 44 after the 1st
immunization, and spleens, lymph nodes, synovium, serum were
obtained for further detection.
Clinical assessment of arthritis
Mice were monitored every other day for signs of
arthritis beginning when they were given the booster injection.
Arthritis severity was scored on a scale from 0 to 4 on each paw
(16), as normal (0); erythema and
swelling of one digit (1); erythema
and swelling of a pair of digits or erythema and swelling of the
ankle joint (2); erythema and
swelling of three digits or swelling of two digits and the ankle
joint (3); or erythema and swelling
of the ankle, foot, and digits with deformity (4). The maximum severity score was 16;
scores were recorded as means ± SEM.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of synovium, lymph nodes and spleen was
extracted with TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's protocol and
converted to cDNA along with random or specific primers. Then,
quantitative PCR (qPCR) was carried out by an ABI 7500 thermocycler
(Applied Biosystems Life Technologies, Foster City, CA, USA)
following the instructions. The relative mRNA expression of
cytokines and transcription factors was detected by RT-qPCR using
AccuPower® PreMix (Bioneer, Inc., Alameda, CA, USA) in accordance
with instruction of the manufacturer. β-actin was used as an
internal inference for mRNA and U6 was used as an internal
inference for miR-34a. For both miRNA and mRNA detection, the
reaction conditions were 95°C for 5 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 30 sec. The specific primers for
miR-34a and U6 were purchased from Guangzhou RioBio Co., Ltd. The
specific primers for interleukin (IL)-17A were as follows: forward,
5′-ATCCACCTCACACGAGGCACAA-3′ and reverse,
5′-AGATGAAGCTCTCCCTGGACTCAT-3′, for IL-6: forward,
5′-TTCCATCCAGTTGCCTTCTT-3′, reverse: 5′-ATTTCCACGATTTCCCAGAG-3′,
for interferon (IFN)-γ: forward, 5′-TGAAAGACAATCAGGCCATC-3′,
reverse, 5′-TTGCTGTTGCTGAAGAAGGT-3′, for IL-1β: forward,
5′-TTCAGGCAGGCAGTATCACTC-3′, reverse, 5′-GAAGGTCCACGGGAAAGACAC-3′,
for IL-21: forward, 5′-GGACCCTTGTCTGTCTGGTAG-3′, reverse,
5′-TGTGGAGCTGATAGAAGTTCAGG-3′, for IL-10: forward,
5′-CCAGGGAGATCCTTTGATGA-3′, reverse, 5′-CATTCCCAGAGGAATTGCAT-3′,
for GAT A3: forward, 5′-TGGATGGCGGCAAAGC-3′, reverse,
5′-CGGAGGGTAAACGGACAGAG-3′, for ROR-γt: forward,
5′-CGCCTCACCTGACCTACCC-3′, reverse, 5′-TGGCTGTCTGGACCCTGTTCT-3′,
for T-bet: forward, 5′-CCTGGACCCAACTGTCAACT-3′, reverse,
5′-AACTGTGTTCCCGAGGTGTC-3′, for Foxp3: forward,
5′-AAGTGCTTTGTGCGAGTGG-3′, reverse, 5′-TCAAGGGCAGGGATTGG-3′, for
β-actin: forward, 5′-GGCTGTATTCCCCTCCATCG-3′ and reverse,
5′-CCAGTTGGTAACAATGCCATGT-3′. qPCR primers of mRNA were synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China). The relative
expression level was quantified using the 2−ΔΔCq
method.
Detection of miR-34a expression
Expression of miR-34a in the spleen, lymph nodes,
synovium from CIA mice was detected by RT-qPCR on the 35th day
after the 1st immunization. On the same day, normal DBA/1j mice
were sacrificed for the analysis of miR-34a level as control.
For the efficiency of miR-34a agomir and antagomir,
the chemically modified miR-34a mimic and inhibitor, CIA mice were
treated with 10 nmol miR-34a agomir or 50 nmol antagomir or
nagetive control on the 28, 31 day after the 1st immunization. On
the 35th day, mice from each group were sacrificed and spleens,
lymph nodes, synovium were obtained for analysis of miR-34a
level.
Enzyme-linked immunosorbent assay
(ELISA)
The blood was obtained when the mice were sacrificed
on the 44th day after the 1st immunization, and clotted in the room
temperature for 2 h to get the serum. Tumor necrosis factor
(TNF)-α, IL-1β, IL-6, IFN-γ and IL-10 in the serum were measured
using the milliplex kit (Merck KGaA, Darmstadt, Germany).
Carboxy-terminal telopeptides of type II collagen (CTX1) and
amino-terminal propeptides of type I procollagen (P1NP) in the
serum were detected using ELISA according to the instruction of the
manufacturer (Shanghai BlueGene Biotech Co., Ltd., Shanghai,
China).
Flow cytometric analysis
The spleens were obtained when the mice were
sacrificed and single cell suspension was prepared. Cell-surface
markers were stained for 30 min with fluorescein isothiocyanate
(FITC)-labeled anti-mouse CD4 antibody or FITC-labeled anti-mouse
CD3 antibody or allophycocyanin (APC)-conjugated anti-mouse B220
antibody. For cytokines and transcript factors staining, cells were
fixed and permeated using a Foxp3 staining kit, and intracellular
staining was performed with phycoerythrin (PE)-conjuctated
anti-mouse IFN-γ antibody, PE-conjuctated anti-mouse IL-4 antibody,
PE-conjuctated anti-mouse IL-17A antibody or PE-conjuctated
anti-mouse Foxp3 monoclonal antibody at room temperature for 30
min. For cytokines staining, cells were stimulated with phorbol
myristate acetate (50 ng/ml) and ionomycin (1 µg/ml) in the
presence of bref A (3 µg/ml) and monomycin (1.4 µg/ml) for 4 h.
Isotype controls were used to confirm antibody specificity. All
cells were resuspended in washing buffer and analyzed by flow
cytometry. All reagents were purchased from eBioscience, Inc. (San
Diego, CA, USA). A total of 1×104 viable cells were
analyzed in a FACSCanto II flow cytometer (BD Biosciences, San
Diego, CA, USA) utilizing FACSDiva software.
Statistics
Data are expressed as the means ± standard deviation
(SD). Statistical analysis was performed with the SPSS 16.0 (SPSS,
Inc., Chicago, IL, USA). Statistical analysis of the difference
between three groups of mice was performed by ANOVA test, then the
difference between two groups were analyzed by SNK-q test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
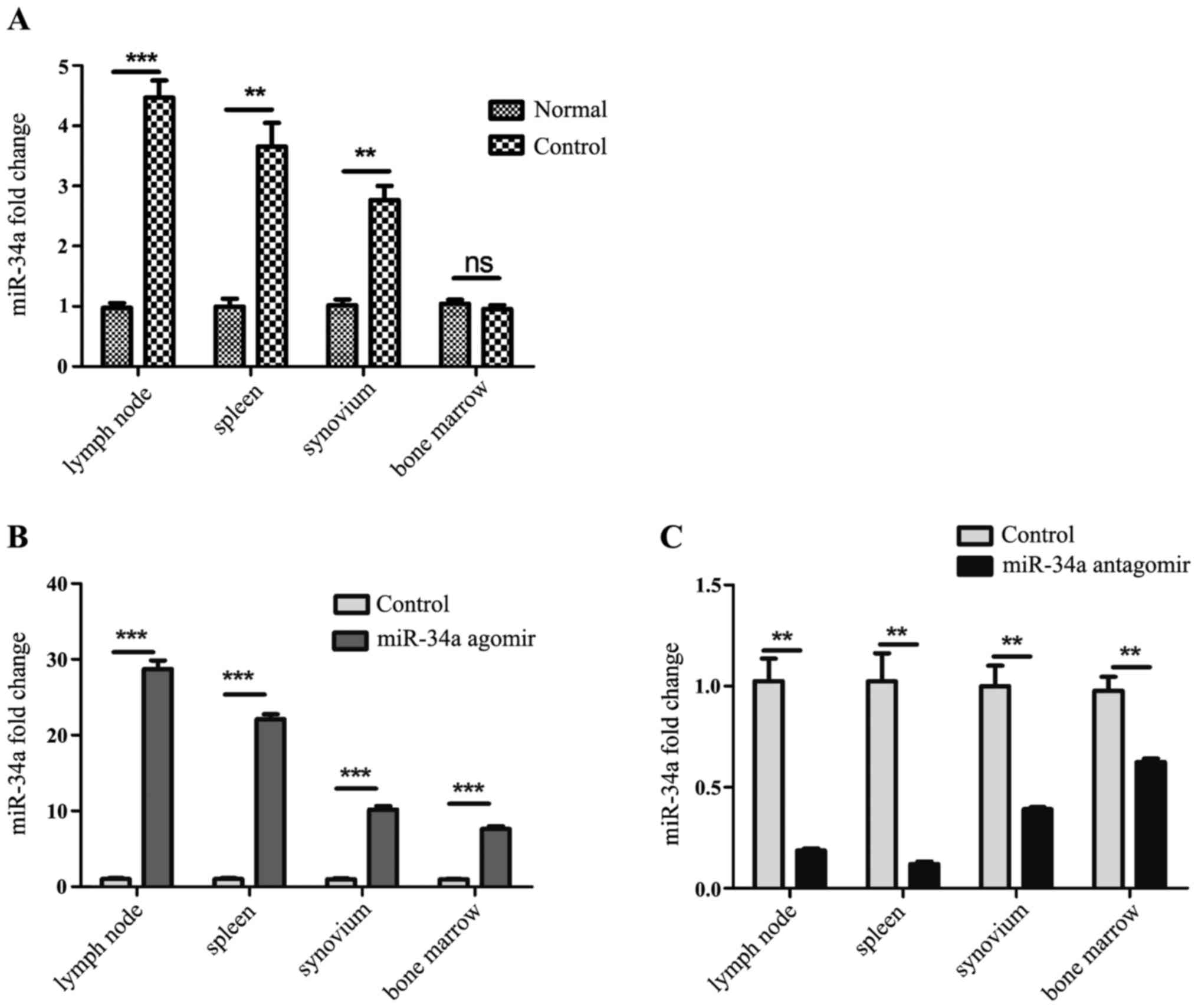
Expression of miR-34a was increased in
CIA mice and efficiently regulated by agomir and antagomir
To explore whether miR-34a participate in the
pathogenesis of experimental arthritis, established CIA model mice
were sacrificed on the 35th day after the 1st immunization, and the
expression of miR-34a in the lymph nodes, spleens, synovium from
CIA model mice and normal DBA/1j mice was analyzed by RT-qPCR. It
was shown that expression of miR-34a was increased in the CIA mice
(Fig. 1A).
Agomir and antagomir were chemically modified
microRNA mimic and inhibitor, to enhance spontaneous cellular
uptake and to increase resistance to various RNases. It exhibits
enhanced cellular uptake, stability and regulatory activity in
vivo. They can be given to the animals by either local or
systemic injection, inhaling or feeding and are good for long-term
upregulation or downregulation of the corresponding endogenous
miRNAs. In our work, miR-34a agomir and antagomir were injected
intravenously after the booster immunization twice a week, namely
the 28th and 31st day. On the 35th day after 1st immunization,
expression of miR-34a in each group was detected. It was shown that
miR-34a was increased significantly in spleens, lymph nodes and
synovium from agomir-treated CIA mice than CIA model mice (Fig. 1B), and decreased dramatically in
those from antagomir-treated CIA mice than CIA model mice (Fig. 1C).
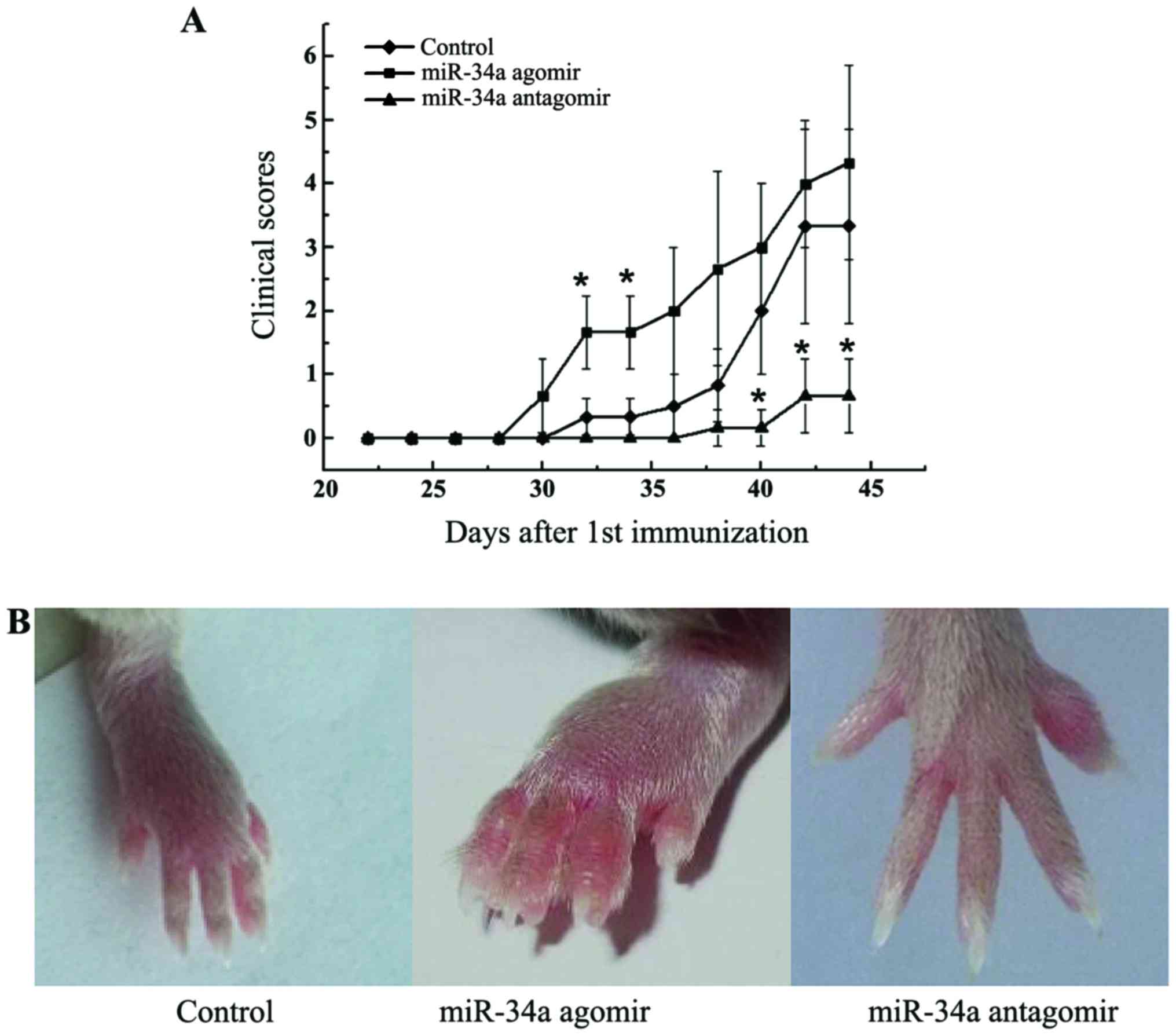
miR-34a antagomir delayed the onset
and suppressed the severity of arthritis
miR-34a has pleiotropic effects on immune
activation, angiogenesis, and bone metabolism, all of which are
critical processes in the pathogenesis of RA. To determine the
effect of miR-34a on arthritis, we investigated the influence of
miR-34a treatment on a CIA murine model. Modification of miR-34a
function by agomir and antagomir resulted in a corresponding
exacerbation and amelioration of arthritis. miR-34a antagomir
significantly alleviated the clinical manifestations of arthritis
(P<0.05, Fig. 2A and B). miR-34a
agomir treatment, on the other hand, dramatically accelerated the
development compared with CIA control mice with more swollen joints
(Fig. 2A and B).
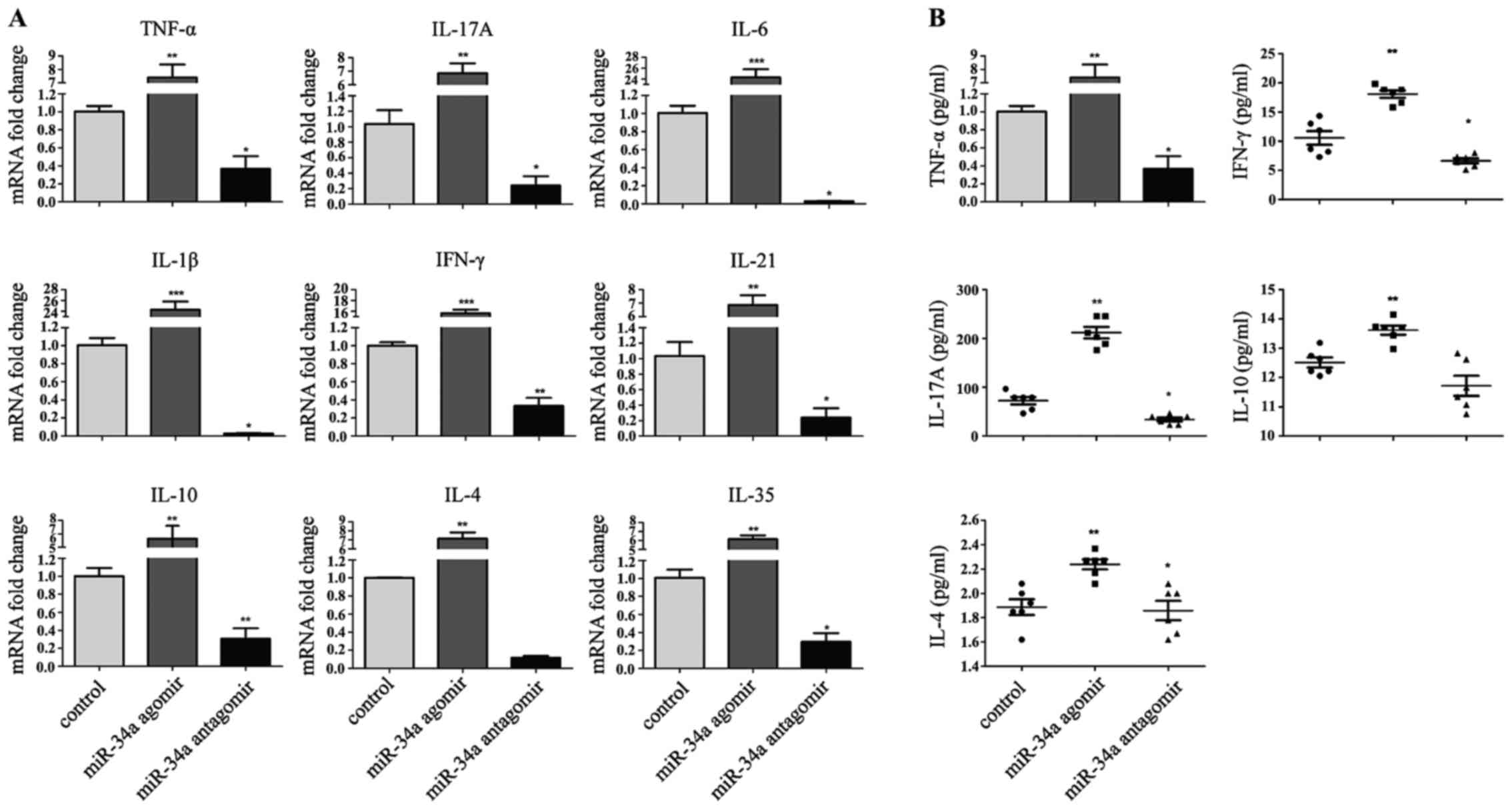
Decreased expression of proinflammtory
cytokines in miR-34a antagomir-treated CIA mice
The amelioration of arthritis in response to
injection of miR-34a antagomir prompted us to investigate the
pattern of cytokines expression in the joints of CIA mice. As
expected, RT-qPCR confirmed a significant decrease of pathogenic
TNF-α, IL-1β, IL-6, IFN-γ, IL-17A, IL-21 transcripts in the
synovium (Fig. 3A).
Anti-inflammatory cytokines decreased simultaneously. A similar
decrease in serum cytokines was also observed, with the largest
change in IL-17A (Fig. 3B).
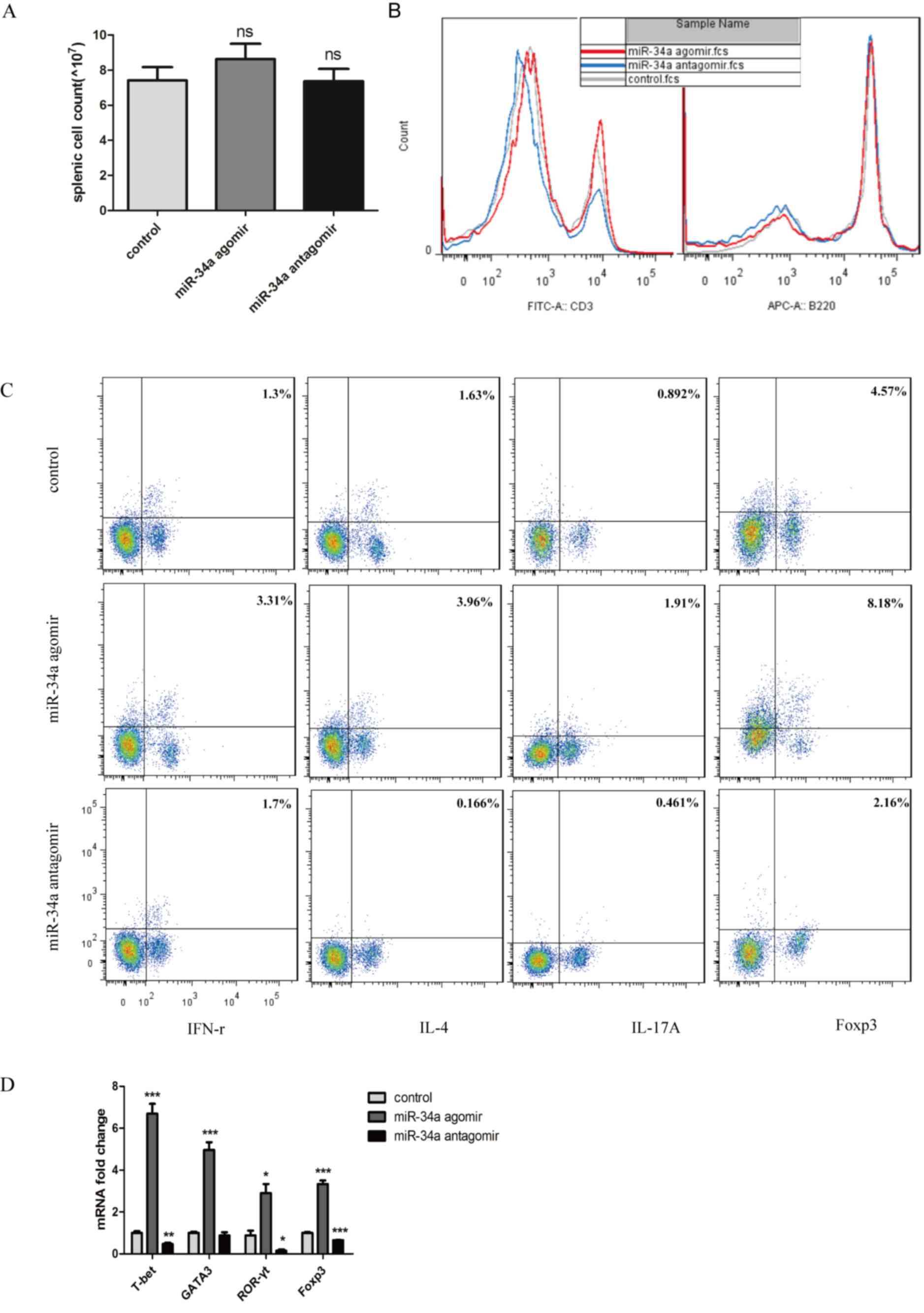
Percentage of proinflammatory Th1 and
Th17 cells decreased with miR-34a inhibition
Because of the pattern of cytokines expression in
the joints and to further investigate the mechanism of remission in
CIA mice caused by miR-34a antagomir, we assayed the splenic
lymphocyte population. Before flowcytometry, splenocytes were
counted, and no significant changes were seen in the spenic cell
count which indicated that no toxic effects were observed following
each treatment (Fig. 4A). The
percentage of CD3+ cells decreased significantly from
24.4 to 14.4%, but no significant difference of B220+
cells between the antagomir-treated mice and CIA control mice was
observed (Fig. 4B). To evaluate cell
subsets associated with aggravation of arthritis, we assayed
functional CD4+ T-cell subpopulations. Both
IFN-γ-producing Th1 and IL-17A-produing Th17 cell populations
decreased significantly in response to miR-34 antagomir (Fig. 4C).
To confirm the differences in peripheral Th cells in
the mice from different experimental groups, lymph nodes were
obtained and transcription factors specifically from Th1, Th2,
Th17, Treg cells were analyzed. All the transcriptional factors
were decreased. In response to antagomir, those from the
anti-inflammatory Th2 and Treg cells were decreased to 0.87-fold
and 0.65-fold respectively compared with CIA control mice, whereas
those from proinflammatory Th1 and Th17 cells decreased to
0.46-fold and 0.15-fold respectively (Fig. 4D).
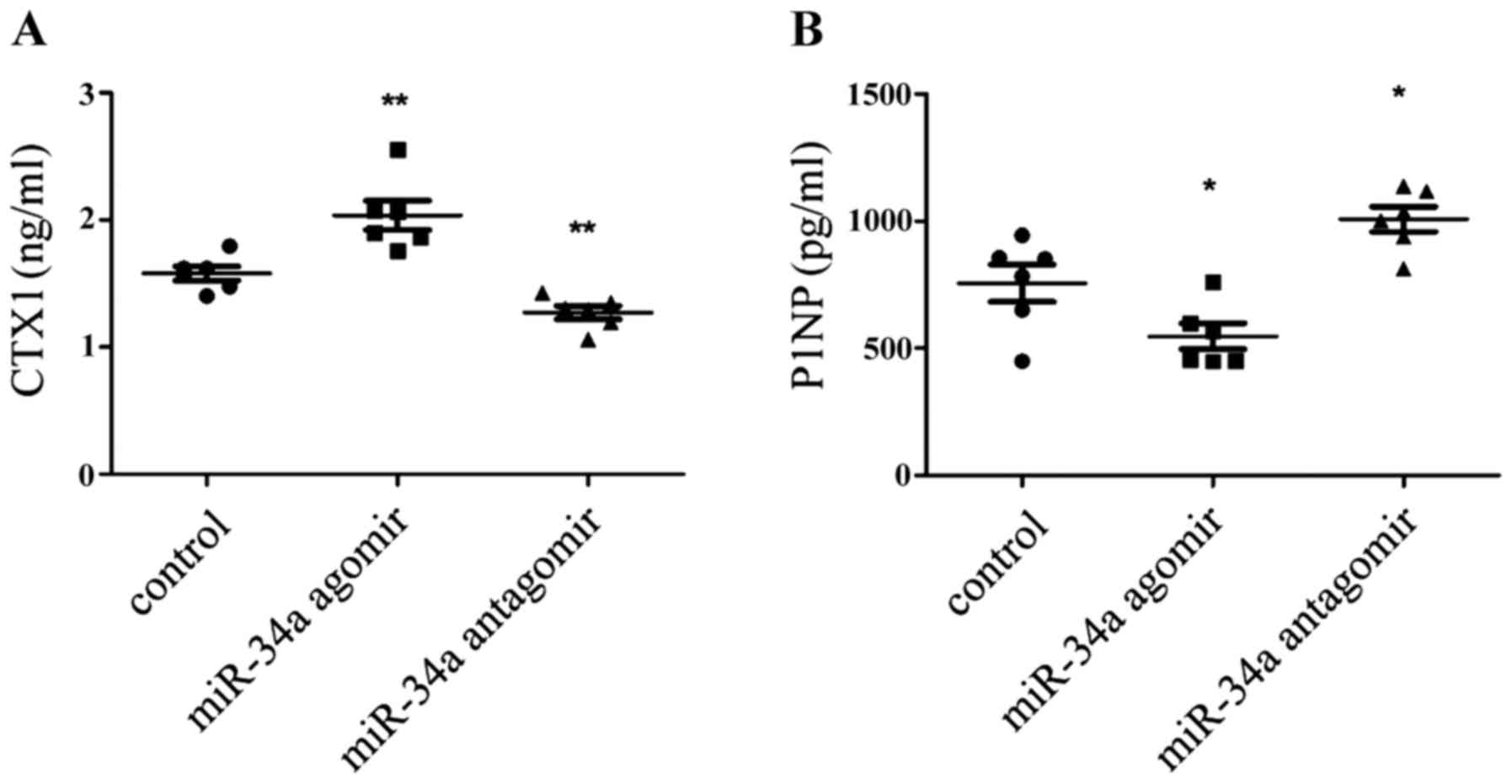
miR-34a antagomir inhibited bone loss
during inflammatory arthritis
Recently miR-34a was shown to suppress
osteoclastogenesis in an ovariectomized murine model of
postmenopausal osteoporosis and bone metastases (13). But inflammation itself also had
effects on the osteoclast differentiation (17). So would miR-34a alone affect
inflammatory bone loss? To explore the pattern of bone turn over in
each group, the serum markers of CTX-1 and P1NP were mearsured to
indicate bone resorption and formation. It was shown that CTX-1 was
decrease in the antagomir-treatment CIA mice (Fig. 5A), meanwhile, P1NP was augmented
(Fig. 5B). So it can be inferred
that bone loss is supressed in miR-34a antagomir-treated CIA
mice.
Discussion
This study was designed to determine the role of
miR-34a in experimental arthritis. CIA mice were used as an
autoimmune arthritis model. Changes in miR-34a function in response
to transfer of modified miR-34a mimics and inhibitors were
reflected by corresponding exacerbation or amelioration of
arthritis. Inhibition of miR-34a improved autoimmune arthritis
progression accompanied with downregulated percentage of peripheral
T lymphocyte and decreased bone loss. As far as we know, the
results suggest, for the first time, that miR-34a inhibition could
ameliorate experimental arthritis in vivo.
miR-34a is a well-known tumor suppressor that has
already been evaluated in clinical studies of liver tumor and blood
carcinoma. Previous studies have shown that miR-34a is widely
involved in immune responses (9,10) in
vitro and also upregulated in autoimmune disease lesions
(11). Many molecular targets of
miR-34a, such as Sirtuin 1 and Foxp1 are associated with generation
of Th17 cells (18) or follicular
helper-T cells (19) that regulate
B-cell immunity. These findings are of major interest in the field
of T cell-dependent B cell-mediated autoimmune diseases, such as
RA. Furthermore, in our work, level of miR-34a increased
dramatically in CIA mice. However, the effect of miR-34a in the
pathogenesis of autoimmune diseases in vivo and lymphocyte
distributions remains unknown.
The results of the experiments in CIA mice suggest
that downregulation of miR-34a could ameliorate CIA, with lower
clinical severity scores. Inflammatory cells invade the synovial
cavity and produce inflammatory cytokines such as TNF-α, IL-1β and
IL-6. These cytokines accelerate pannus formation and eventually
cause cartilage damage and bone destruction (20). Consistent with alleviation of
clinical symptoms, the expression of cytokines in the joints and in
serum from miR-34a antagomir-treated mice was significantly
decreased. As for the reason for the change of cytokines and
transcription factors, we searched target mRNAs of miR-34a in the
Targetscan and miRBase, and found that all the mRNAs of cytokines
and transcription factors involved in our work were not included in
the category. So cytokines and transcription factors production are
not induced by miR-34a directly. The change of cytokines production
maybe is just a result of disease remission.
The inflammatory status of local lesions prompted us
to investigate peripheral lymphocyte distribution. We found a
significant decrease of CD3+ T cells, but not of
B220+ cells. Because of the prominent roles and
significant changes of T cells associated with RA, Th subsets were
evaluated, it became clear that miR-34a antagomir led to lower
level of Th cell percentage, including Th1, Th2, Th17, Treg cells,
which was confirmed by the expression of transcription factors in
the lymph nodes. It is not amazed that both Th17 and Treg decreased
in the same time, which is probably bacause of the multiple target
moleculars of microRNAs or because that one of the miR-34a target
genes is diacylglycerol kinase ζ (DGKζ), a pivotal negative
regulator of T cell activation signal which is downstream of CD3
(10). The phenomenon can also be
caused by miR-155 (8).
The ability of miR-34a to suppress
osteoclastogenesis was demonstrated by its effect on osteoclast
differentiation assays (13).
Whereas inflammation itself also influence bone metabolism
(17). Even though miR-34a could
block the osteoclastgenesis, miR-34a antagomir led to decreased
level of CTX-1 in the serum, which means the miR-34a antagomir did
indeed inhibit bone resorption. The protective effect on bone loss
of miR-34a antagomir is consistent with its suppression on the
autoimmunity.
In summary, our findings in the experimental
arthritic model suggest that inhibition of miR-34a could ameliorate
autoimmune arthritis and decrease T lymphocytes percentage and
inhibit bone loss. The critical roles of miR-34a in arthritis
pathogenesis suggest that inhibition of miR-34a is a potential
novel target for RA treatment.
Acknowledgements
This present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81373202
and 30972739) and the National High Technology Research and
Development Program of China (grant no. 2015AA042401).
References
|
1
|
Alamanos Y, Voulgari PV and Drosos AA:
Incidence and prevalence of rheumatoid arthritis, based on the 1987
American College of Rheumatology criteria: A systematic review.
Semin Arthritis Rheum. 36:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goronzy JJ and Weyand CM: T-cell
regulation in rheumatoid arthritis. Curr Opin Rheumatol.
16:212–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leipe J, Grunke M, Dechant C, Reindl C,
Kerzendorf U, Schulze-Koops H and Skapenko A: Role of Th17 cells in
human autoimmune arthritis. Arthritis Rheum. 62:2876–2885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smolen JS and Aletaha D: Rheumatoid
arthritis therapy reappraisal: Strategies, opportunities and
challenges. Nat Rev Rheumatol. 11:276–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoffer MA, Schoels MM, Smolen JS, Aletaha
D, Breedveld FC, Burmester G, Bykerk V, Dougados M, Emery P,
Haraoui B, et al: Evidence for treating rheumatoid arthritis to
target: Results of a systematic literature search update. Ann Rheum
Dis. 75:16–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao C and Rajewsky K: MicroRNA control in
the immune system: Basic principles. Cell. 136:26–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez-Martin A, Adams BD, Lai M,
Shepherd J, Salvador-Bernaldez M, Salvador JM, Lu J, Nemazee D and
Xiao C: The microRNA miR-148a functions as a critical regulator of
B cell tolerance and autoimmunity. Nat Immunol. 17:433–440. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Connell RM, Kahn D, Gibson WS, Round JL,
Scholz RL, Chaudhuri AA, Kahn ME, Rao DS and Baltimore D:
MicroRNA-155 promotes autoimmune inflammation by enhancing
inflammatory T cell development. Immunity. 33:607–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang P, Liu R, Zheng Y, Liu X, Chang L,
Xiong S and Chu Y: MiR-34a inhibits lipopolysaccharide-induced
inflammatory response through targeting Notch1 in murine
macrophages. Exp Cell Res. 318:1175–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin J, Xie D and Zhong XP: MicroRNA-34a
enhances T cell activation by targeting diacylglycerol kinase ζ.
PLoS One. 8:e779832013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Junker A, Krumbholz M, Eisele S, Mohan H,
Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R and Meinl
E: MicroRNA profiling of multiple sclerosis lesions identifies
modulators of the regulatory protein CD47. Brain. 132:3342–3352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao
Y, Dong Q, Pang Z, Guan Q, Gao L, et al: Significance of serum
microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A
clinical study. Acta Diabetol. 48:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krzeszinski JY, Wei W, Huynh H, Jin Z,
Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, et
al: miR-34a blocks osteoporosis and bone metastasis by inhibiting
osteoclastogenesis and Tgif2. Nature. 512:431–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim D, Song J, Kim S, Park HM, Chun CH,
Sonn J and Jin EJ: MicroRNA-34a modulates cytoskeletal dynamics
through regulating RhoA/Rac1 cross-talk in chondroblasts. J Biol
Chem. 287:12501–12509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar B, Yadav A, Lang J, Teknos TN and
Kumar P: Dysregulation of microRNA-34a expression in head and neck
squamous cell carcinoma promotes tumor growth and tumor
angiogenesis. PLoS One. 7:e376012012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida Y, Ogata A, Kang S, Ebina K, Shi
K, Nojima S, Kimura T, Ito D, Morimoto K, Nishide M, et al:
Semaphorin 4D contributes to rheumatoid arthritis by inducing
inflammatory cytokine production: Pathogenic and therapeutic
implications. Arthritis Rheumatol. 67:1481–1490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schett G and Teitelbaum SL: Osteoclasts
and arthritis. J Bone Miner Res. 24:1142–1146. 2009. View Article : Google Scholar
|
|
18
|
Lim HW, Kang SG, Ryu JK, Schilling B, Fei
M, Lee IS, Kehasse A, Shirakawa K, Yokoyama M, Schnölzer M, et al:
SIRT1 deacetylates RORγt and enhances Th17 cell generation. J Exp
Med. 212:607–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Geng J, Wen X, Bi E, Kossenkov AV,
Wolf AI, Tas J, Choi YS, Takata H, Day TJ, et al: The transcription
factor Foxp1 is a critical negative regulator of the
differentiation of follicular helper T cells. Nat Immunol.
15:667–675. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|