Introduction
Micro-finite element (µFE) models, created from high
resolution micro-computed tomography (µ-CT) images, have become a
major computational tool for the assessment of the mechanical
properties of human trabecular bone. By simulating a loading
condition, this model then can be used to simulate the mechanical
behavior of trabecular bone (1,2), and has
shown excellent prediction power compared with experimental
measurements (3,4). µFE analysis, based on this model, is
potentially useful when evaluating the effects of bone diseases and
their subsequent treatment on the mechanical properties of
trabecular bone.
To date, µFE models of trabecular bone have
predominantly been based on cored samples extracted from sites with
high concentrations of the trabecular bone, for example, the
vertebral body or proximal part of femur (5,6). Among
the studies using µFE models, the shapes of µFE models are
typically cubical and cylinder. Gross et al (6) constructed cubic µFE models within the
vertebral body in their study to investigate morphology-elasticity
relationships. Cubic µFE models with a side length of 4 mm within
the vertebral body were set to obtain the biomechanical properties
of human trabecular bone (7).
Cylindrical trabecular µFE models were also constructed to
investigate the mechanical properties of vertebral trabecular bone
(3,8).
To the best of our knowledge, whether there are
differences between cubic and cylindrical µFE models has not yet
been studied. Therefore, the purpose of our study was to
investigate the influence of the shape of the µFE model on the
mechanical properties calculated from µFE analysis, and to
determine whether there were differences between cubic and
cylindrical µFE models.
Materials and methods
Specimens preparation
In total, 5 lumbar vertebral bodies (L1-5) were
collected form one embalmed human cadaver (61 years old; male)
provided by the Department of Human Anatomy at the Fourth Military
Medical University (Xi'an, China). Written informed consent from
the donor was obtained for the use of these specimens in research.
Collection and preparation procedures were approved by the Ethics
Committee of the Fourth Military Medical University. All specimens
were physically evaluated and radiographed to exclude bone
diseases, bone cancers, and previous fractures. Soft tissues were
carefully removed from the bone with a scalpel. Lateral and
posterior elements including the pedicle, transverse process, and
spinous process were excised from the vertebral body using a band
saw (Isomet 1000, Buehler, Plymouth, MN, USA).
µ-CT scanning. µ-CT scans of all samples (5
vertebral bodies) were performed with a high-resolution µ-CT system
(Siemens Inveon; Siemens AG, Munich, Germany) with an isotropic
resolution of 33.355 µm. Image processing included the application
of a modest Gauss global filter and segmentation according to the
method described by Otsu (9), which
is a popular and established method in the field of threshold
segmentation. Images were obtained using the following parameters:
i) X-ray tube voltage, 100 kV; ii) anode current, 100 µA; and iii)
shutter speed, 2,500 msec. High resolution images were obtained to
produce µFE models for subsequent studies.
µFE model building
µFE models were generated directly from the
segmented images using a voxel conversion process (10). Firstly, all the DICOM image files
were imported into the ScanIP software package (version 3.2; Build
1, Simpleware Ltd., Exeter, UK) to crop the different volumes of
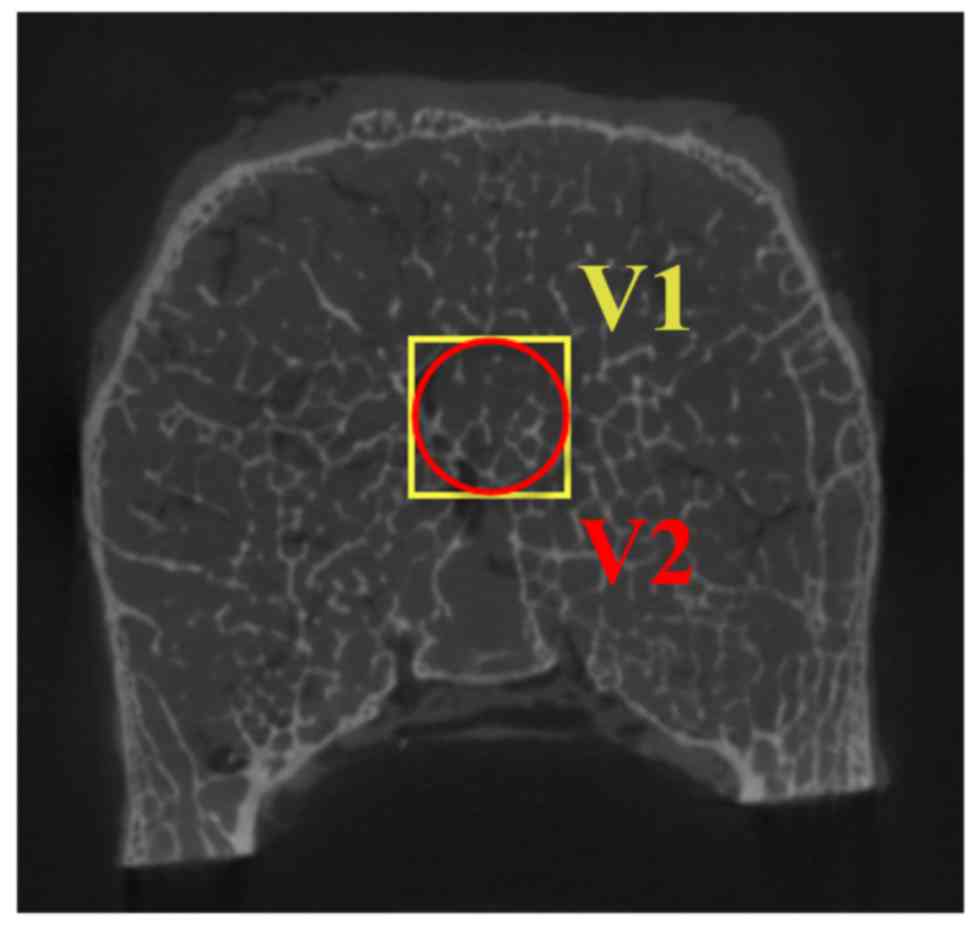
interest (VOI). As shown in Fig. 1,
cubic cores (V1 in Fig. 1) of 8×8×8
mm3 were cropped to build cubic µFE models. Inscribed
cylindrical cores (V2 in Fig. 1) of
8-mm diameter and 8-mm height were cropped to build cylindrical µFE
models. Images were then segmented with the optimal threshold to
match the bone volume fraction measured from µ-CT analysis. The
Floodfill function in the ScanIP software was used to remove all
the floating or disconnected structures. Two layers of voxel
elements were added at the superior and inferior surface to mimic
stainless steel layers in axial compression tests.
Subsequently, the files were imported into ScanFE
(version 3.1.2, Build 2, Simpleware Ltd., Exeter, UK) to construct
the µFE model. With this approach, voxels representing bone tissue
were converted to equally sized eight-node hexahedral voxel
elements, whereas voxels representing the bone marrow were ignored.
All the models were imported to ANSYS (release 14.0; ANSYS, Inc.,
Township, PA, USA) to perform further calculations. For all models,
the element material properties of bone were considered to be
isotropic, linear elastic, and uniform with a tissue Young's
modulus of 10 GPa and a tissue Poisson's ratio of 0.3 (11). An isotropic homogenous tissue modulus
of 200 GPa and a tissue Poisson's ratio of 0.3 was assigned to the
element of stainless steel layers (12).
Computational process of µFE
analysis
A 1% axial strain was prescribed to the top surface
of the model, and axial displacements at the bottom surface were
constrained, simulating an axial compression test along the
superior-inferior direction. Contact between the upper and lower
surfaces of the specimen and the steel plates were modeled using
contact elements with a zero friction value to ensure that only
compressive forces were transmitted (13). All analyses were performed on a
workstation computer (ThinkStation; Intel Xeon CPU E3-122, 3.10
GHz, Lenovo Group Ltd., Beijing, China). The apparent stresses were
calculated as the total reaction force per apparent area. Based on
that, the E values were calculated by dividing the apparent strain
by 0.01 (14).
Statistical analysis
Statistical analyses were performed using the
SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA). Paired-t
tests were performed to determine whether there were differences
between the E values obtained from cubic and cylindrical models.
P<0.05 was considered to indicate a statistically significant
difference.
Results
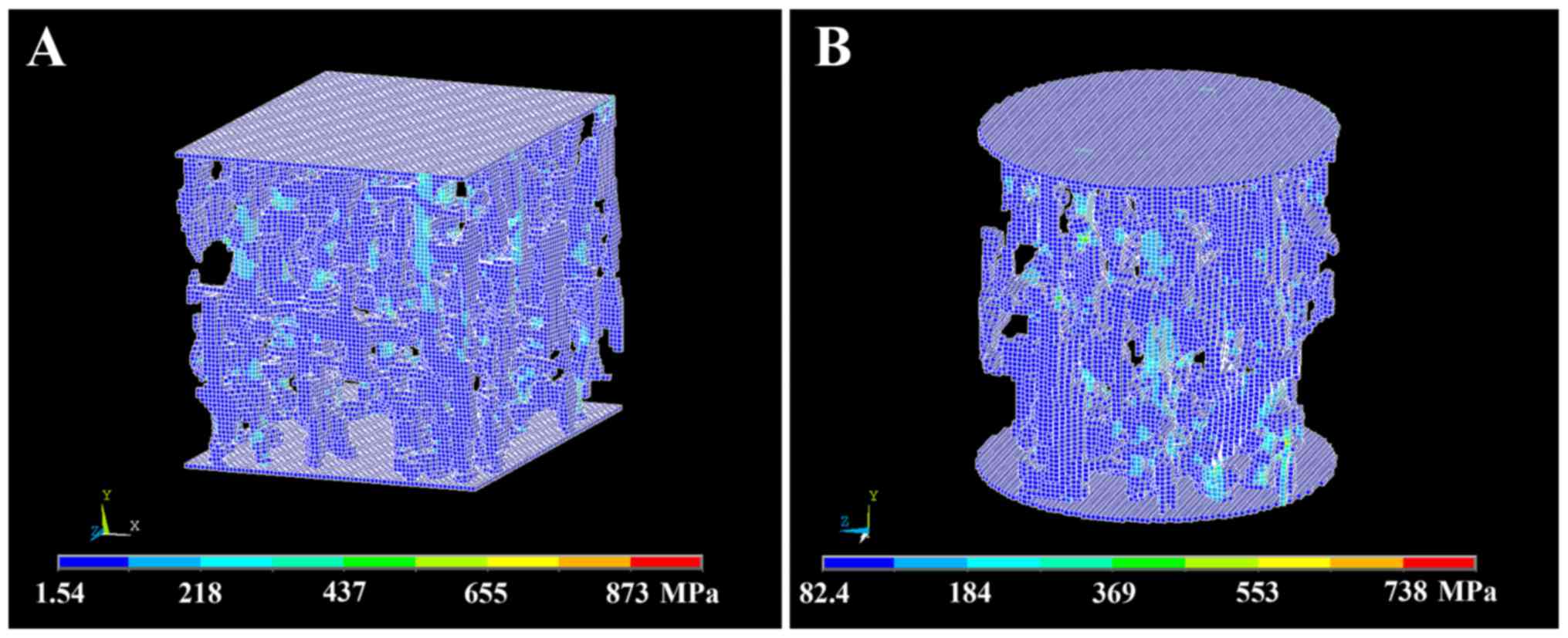
Von Mises stress distributions
Von Mises stress distributions in the trabecular
bone structure of cubic and cylindrical µFE models are shown in
Fig. 2. There were no significant
differences of Von Mises stress distributions between cubic and
cylindrical µFE models.
E values
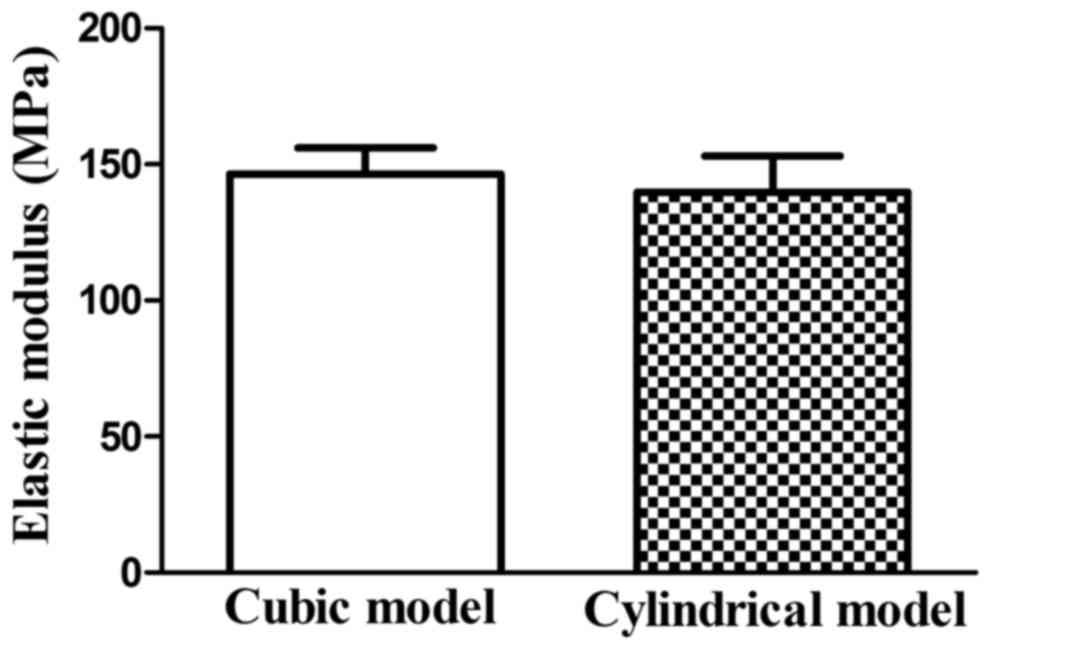
E values obtained from µFE analyses are shown in
Fig. 3. E of the cubic models was
146.34±9.76 MPa, and of cylindrical models was 139.35±13.21 MPa.
Paired t-tests showed that there were no statistically significant
differences in E values between the cubic and cylindrical
models.
Discussion
In the present study, cubic and cylindrical µFE
models were built to investigate if there were significant
differences between these two models. No significant differences in
the E values were detected between the cubic and cylindrical µFE
models.
µFE analysis is now widely used to investigate bone
mechanical properties; these mechanical properties have been
demonstrated to relate to bone microarchitecture (15). Based on high resolution 3D images
obtained from µCT, µFE models were constructed for further
simulation calculations. By simulating a loading condition, this
model can be used to derive the elastic modulus of the bone, as
well as the distribution of stresses and strains in the bone tissue
(1,16).
Obtained from µFE models, trabecular bone modulus
has a good correlation with experimental modulus and strength
(15,17). Elastic modulus obtained in our
studies were similar to former experimental compression studies on
trabecular bone samples (18,19).
Compression tests on vertebral trabecular bone cores have shown
that the apparent elastic modulus on axial direction was 189.7 MPa
(18), which is simular to the value
of 139.96–146.34 MPa obtained in the present study. In another
study based on vertebral trabecular bone, apparent elastic modulus
calculated from µFE models was 146–154 MPa (19). These findings indicated that the µFE
models constructed in our study are reliable.
Voxels representing bone tissue were converted to
equally sized eight-node hexahedral voxel elements to construct µFE
models in our study. A number of studies have investigated the
effect of voxel size on the accuracy of biomechanical measurements
on human trabecular bone (20–22).
Voxel size predominantly refers to scanning and reconstruction
voxel size. Scanning voxel size is based on the µ-CT scanning
resolution. A previous study demonstrated that it is the resolution
of raw data that primarily determines the accuracy of models as the
bone volume fraction of bone volume/total volume was predominantly
affected by the scanning resolution (22). The scanning resolution of the µ-CT
system used in our study was 33.355 µm, which was enough to depict
the microarchitecture of human trabecular bone (23,24). In
another study, the recommended resolution in finite element models
of trabecular bone was one quarter of trabecular thickness
(25). The thickness of trabeculae
in our study was ~200 µm and the resolution of 33.355 µm,
fulfilling the conditions rule. Reconstruction voxel size is the
actual voxel size concerted to the 3D µFE model. The reconstruction
voxel size in our study was identical with scanning voxel size, and
this could avoid the inaccuracy of coarsening reconstruction voxel
size.
The size of cubic and cylindrical models in our
study has contained enough structural information for virtual
compressions. Pahr and Zysset (26)
suggested that the side length of the volume should be >5 mm as
it may provide the proposed boundary conditions. Another study
showed that the VOI of trabecular bone should be >6×6×6
mm3 to predict the microarchitecture of human trabecular
bone (27). The VOI of trabecular
bone in our study met these conditions. The µFE models constructed
in our study included a representation of steel plate located on
the upper and lower surfaces of the specimen. The lower steel plate
was constrained and compressive displacement was applied to the
upper steel plate. All these conditions stimulated the experimental
compression test better.
Notably, the cubic and cylindrical µFE models were
constructed from the same position of the vertebral body. It has
been confirmed that the architecture of the trabecular bone within
the vertebral body is inhomogeneous (17,28–31).
Within the vertebral body, the trabecular architecture in the
posterior region was superior to the anterior region (28,31).
Compared with the posterior region, the anterior region had lower
bone mass density, less trabecular bone volume fraction, less
trabecular number, and greater trabecular separation (28). Although there were no significant
differences between cubic and cylindrical µFE models, the positions
of the trabecular bone core should be identical.
In experimental compression tests, trabecular bone
cores extracted from vertebral bodies are often used (23,32). In
our study, cubic and cylindrical µFE models were constructed to
investigate if there were differences between the two models. This
indicated that the shape of trabecular bone core used in
experimental biomechanical tests may not affect the biomechanical
properties obtained from these real tests. These computational
models used in our study may enable us to make repeated virtual
compression tests on trabecular bone core extracted from the same
specimen. This may avoid the influence of variance among different
specimens and ensure the results are more reliable.
To construct µFE models of trabecular bone, choosing
the VOI was critical. Cubic and cylindrical VOI are the most
commonly used shapes of VOI (3,33). The
results demonstrated that there was no significant difference
between these two models. With regard to the Von Mises stress
distributions, there were also no significant differences between
cubic and cylindrical µFE models. Choosing between the cubic or
cylindrical models shall depend on the specific study design. A
limitation of the present study was that the trabecular bone
specimens used in our study were collected from vertebral bodies.
It has is well-documented that the morphologies of trabecular bone
harvested from different skeletal sites are quite different
(34,35). Further research is required to
confirm that the findings of the present study are applicable to
other skeletal sites.
In conclusion, to construct µFE models of vertebral
trabecular bone, cubic or cylindrical models were both feasible.
The present findings demonstrated that there were no significant
difference between these two shapes of models. To choose cubic or
cylindrical model, it depends on the specific study design.
Acknowledgements
The authors would like to thank Jia-Ji Yang at the
School of Stomatology of Fourth Military Medical University for
their technical assistance at µCT analysis. This study was
supported by the National Nature Science Foundation of China (grant
no. 81301292).
References
|
1
|
Niebur GL, Feldstein MJ, Yuen JC, Chen TJ
and Keaveny TM: High-resolution finite element models with tissue
strength asymmetry accurately predict failure of trabecular bone. J
Biomech. 33:1575–1583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verhulp E, Van Rietbergen B, Muller R and
Huiskes R: Micro-finite element simulation of trabecular-bone
post-yield behaviour-effects of material model, element size and
type. Comput Methods Biomech Biomed Engin. 11:389–395. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolfram U, Wilke HJ and Zysset PK: Valid
micro finite element models of vertebral trabecular bone can be
obtained using tissue properties measured with nanoindentation
under wet conditions. J Biomech. 43:1731–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chevalier Y, Pahr D, Allmer H, Charlebois
M and Zysset P: Validation of a voxel-based FE method for
prediction of the uniaxial apparent modulus of human trabecular
bone using macroscopic mechanical tests and nanoindentation. J
Biomech. 40:3333–3340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hambli R: Apparent damage accumulation in
cancellous bone using neural networks. J Mech Behav Biomed Mater.
4:868–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gross T, Pahr DH and Zysset PK:
Morphology-elasticity relationships using decreasing fabric
information of human trabecular bone from three major anatomical
locations. Biomech Model Mechanobiol. 12:793–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ulrich D, van Rietbergen B, Laib A and
Rüegsegger P: The ability of three-dimensional structural indices
to reflect mechanical aspects of trabecular bone. Bone. 25:55–60.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bevill G, Eswaran SK, Farahmand F and
Keaveny TM: The influence of boundary conditions and loading mode
on high-resolution finite element-computed trabecular tissue
properties. Bone. 44:573–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otsu N: A threshold selection method from
gray-level histograms. IEEE Trans Syst Man Cybern. 9:62–66. 1979.
View Article : Google Scholar
|
|
10
|
Ruffoni D, Wirth AJ, Steiner JA, Parkinson
IH, Müller R and van Lenthe GH: The different contributions of
cortical and trabecular bone to implant anchorage in a human
vertebra. Bone. 50:733–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ulrich D, van Rietbergen B, Weinans H and
Rüegsegger P: Finite element analysis of trabecular bone structure:
A comparison of image-based meshing techniques. J Biomech.
31:1187–1192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamaya M: Stress-strain curve estimation
procedures for stainless steels based on yield and ultimate
strengths. Eng Fract Mech. 127:194–210. 2014. View Article : Google Scholar
|
|
13
|
Hambli R: Micro-CT finite element model
and experimental validation of trabecular bone damage and fracture.
Bone. 56:363–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cowin SC: Bone mechanics handbook. 2nd.
Boca Raton: CRC Press; 2001
|
|
15
|
Hou FJ, Lang SM, Hoshaw SJ, Reimann DA and
Fyhrie DP: Human vertebral body apparent and hard tissue stiffness.
J Biomech. 31:1009–1015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Rietbergen B and Ito K: A survey of
micro-finite element analysis for clinical assessment of bone
strength: The first decade. J Biomech. 48:832–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DG, Hunt CA, Zauel R, Fyhrie DP and
Yeni YN: The effect of regional variations of the trabecular bone
properties on the compressive strength of human vertebral bodies.
Ann Biomed Eng. 35:1907–1913. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aiyangar AK, Vivanco J, Au AG, Anderson
PA, Smith EL and Ploeg HL: Dependence of anisotropy of human lumbar
vertebral trabecular bone on quantitative computed tomography-based
apparent density. J Biomech Eng. 136:0910032014.PubMed/NCBI
|
|
19
|
Depalle B, Chapurlat R, Walter-Le-Berre H,
Bou-Saïd B and Follet H: Finite element dependence of stress
evaluation for human trabecular bone. J Mech Behav Biomed Mater.
18:200–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim DG, Christopherson GT, Dong XN, Fyhrie
DP and Yeni YN: The effect of microcomputed tomography scanning and
reconstruction voxel size on the accuracy of stereological
measurements in human cancellous bone. Bone. 35:1375–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maloul A, Fialkov J and Whyne C: The
impact of voxel size-based inaccuracies on the mechanical behavior
of thin bone structures. Ann Biomed Eng. 39:1092–1100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeni YN, Christopherson GT, Dong XN, Kim
DG and Fyhrie DP: Effect of microcomputed tomography voxel size on
the finite element model accuracy for human cancellous bone. J
Biomech Eng. 127:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bauer JS, Sidorenko I, Mueller D, Baum T,
Issever AS, Eckstein F, Rummeny EJ, Link TM and Raeth CW:
Prediction of bone strength by µCT and MDCT-based
finite-element-models: How much spatial resolution is needed? Eur J
Radiol. 83:e36–e42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Isaksson H, Töyräs J, Hakulinen M, Aula
AS, Tamminen I, Julkunen P, Kröger H and Jurvelin JS: Structural
parameters of normal and osteoporotic human trabecular bone are
affected differently by microCT image resolution. Osteoporos Int.
22:167–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niebur GL, Yuen JC, Hsia AC and Keaveny
TM: Convergence behavior of high-resolution finite element models
of trabecular bone. J Biomech Eng. 121:629–635. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pahr DH and Zysset PK: Influence of
boundary conditions on computed apparent elastic properties of
cancellous bone. Biomech Model Mechanobiol. 7:463–476. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan YB, Qi W, Wang J, Liu LF, Teo EC,
Tianxia Q, Ba JJ and Lei W: Relationship between architectural
parameters and sample volume of human cancellous bone in micro-CT
scanning. Med Eng Phys. 33:764–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Owoc JS, Boyd SK, Videman T and
Battié MC: Regional variations in trabecular architecture of the
lumbar vertebra: Associations with age, disc degeneration and disc
space narrowing. Bone. 56:249–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wegrzyn J, Roux JP, Arlot ME, Boutroy S,
Vilayphiou N, Guyen O, Delmas PD, Chapurlat R and Bouxsein ML: Role
of trabecular microarchitecture and its heterogeneity parameters in
the mechanical behavior of ex vivo human L3 vertebrae. J Bone Miner
Res. 25:2324–2331. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Shoumura S, Emura S and Bunai Y:
Regional variations of vertebral trabecular bone microstructure
with age and gender. Osteoporos Int. 19:1473–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hulme PA, Boyd SK and Ferguson SJ:
Regional variation in vertebral bone morphology and its
contribution to vertebral fracture strength. Bone. 41:946–957.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parkinson IH, Badiei A, Stauber M,
Codrington J, Müller R and Fazzalari NL: Vertebral body bone
strength: The contribution of individual trabecular element
morphology. Osteoporos Int. 23:1957–1965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lü L, Meng G, Gong H, Zhu D, Gao J and Fan
Y: Tissue level microstructure and mechanical properties of the
femoral head in the proximal femur of fracture patients. Acta Mech
Sinica. 31:259–267. 2015. View Article : Google Scholar
|
|
34
|
Hildebrand T, Laib A, Müller R, Dequeker J
and Rüegsegger P: Direct three-dimensional morphometric analysis of
human cancellous bone: Microstructural data from spine, femur,
iliac crest, and calcaneus. J Bone Miner Res. 14:1167–1174. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eckstein F, Matsuura M, Kuhn V, Priemel M,
Müller R, Link TM and Lochmüller EM: Sex differences of human
trabecular bone microstructure in aging are site-dependent. J Bone
Miner Res. 22:817–824. 2007. View Article : Google Scholar : PubMed/NCBI
|