Introduction
Diffuse large B-cell lymphoma (DLBCL) is a prevalent
type of non-Hodgkin lymphoma (NHL) that occurs in developed
countries (1) and the survival rate
of untreated patients is only 50% for <1 year (1). Remarkable progress has been made in
exploring the biological mechanism of DLBCL during the past decade,
and results have indicated that environmental factors (2,3), dietary
factors (4,5), genetic factors and clinical conditions
may influence the risk of DLBCL (6,7).
However, the pathology and mechanism of DLBCL still remains to be
elucidated.
Major studies have started to focus on microRNA
(miRNA), which are small non-coding RNAs composed of 20–22
nucleotides. miRNA have an important role in the lymphoid system,
which is critical to the differentiation and malignant
transformation of B-cells. A selection of miRNA function as
regulators in oncogenic or tumor-suppressive pathways in lymphoma
(8,9). Moreover, miR-21, miR-155 and miR-17-92
clusters have been acknowledged as oncogenic miRNA, which are
believed to target tumor-suppressive molecules in various types of
tumor, including glioblastomas, cholangiocarcinomas, lung cancer,
breast cancer and colon cancer (10). Furthermore, overexpression of miR-21,
miR-155 and miR-17-92 may be observed in lymphomas derived from B
cells, T cells or natural killer cells (8,11,12).
Notably, miR-21 has a vital role in regulating the chemosensitivity
of DLBCL cells (13) and Bcl-2, a
tumor-associated and anti-apoptotic molecule, has a key role in the
chemoresistance of NHL and has been considered as a prognostic
biomarker for DLBCL (14). However,
the role of miR-21 in regulating the expression of Bcl-2 in DLBCL
remains unclear, and there are no in-depth studies on the
relationship between miR-21 and Bcl-2 in DLBCL.
The aim of the present study was to analyze the
association between miR-21 and Bcl-2 expression levels in DLBCL
cells. Furthermore, cell transfection, MTT, and flow cytometry
analysis were used to investigate whether miR-21 has an important
role in modulating DLBCL cells.
Materials and methods
Patients and tissue samples
Specimens were obtained from 55 patients with DLBCL
(30 men and 25 women) diagnosed using hematology at the First
Affiliated Hospital, and College of Clinical Medicine of Henan
University of Science and Technology (Luoyang, Henan, China)
between November 2012 and December 2014. The age of included
patients ranged from 16 to 89 years, with a median age of 62.
Histologic diagnoses were established according to the
classification system outlined by the World Health Organization
(15). According to the immune
markers of cluster differentiation (CD)-10, Bcl-6, multiple myeloma
oncogene-1 and Hans type principles (16), 55 patients with DLBCL were divided
into germinal center B cell-like (GCB)-type (19 cases) and non-GCB
type (36 cases) groups, with adjacent healthy lymph node tissues
from the same patients as the control group. Tissue samples were
frozen in liquid nitrogen immediately following surgery and stored
at −80°C. A portion of the tumor tissues were fixed in 10% formalin
and embedded with paraffin. Sections of 4 µm thickness were
examined with immunohistochemistry. The present study was approved
by the Ethics Committee of the First Affiliated Hospital and
College of Clinical Medicine, Henan University of Science and
Technology, and all participants gave their written informed
consent.
Detection of miR-21 with reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissue samples was extracted using
TRIzol reagent and purified using a miRNeasy Mini Kit (Qiagen GmbH;
Hilden, Germany). Genomic DNA was removed with DNase treatment and
quantified using NanoDrop ND-2000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). A ratio of OD260/OD280
between 1.7 and 2.1 indicated a higher purity of RNA which was
considered to be satisfactory for follow-up experiments. The
Omniscript reverse transcription kit (Qiagen GmbH) was used to
reverse transcribe total RNA into cDNA according to the
manufacturer's protocol. Expression levels of miR-21 were detected
using the QuantiTect SYBR Green PCR Kit (Qiagen GmbH). The primer
sequences for miR-21 were: Sense, 5′-GCGCGTCGTGAAGCGTTC-3′;
antisense, 5′-GTGCAGGGTCCGAGGT-3′. The total reaction volume was 10
µl, containing the following: MirVana 56 RT buffer (2 µl), miR-21
RT primer (1 µl), ArrayScript Enzyme Mix (0.4 µl), total RNA (0.2
µl), and H2O (6.4 µl). The cycling conditions were as
follows: 95°C for 30 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 30 sec. All assays were repeated three times. The
relative expression quantity of miR-21 was calculated using the
2−ΔΔCq method (17) and
normalized to the expression of U6 snRNA.
Detection of Bcl-2 with
immunohistochemistry
Bcl-2 expressed in DLBCL tissues was analyzed using
immunohistochemical streptoavidin-biotin peroxidase. Tissues were
fixed with 4% formalin at 4°C for 12 h. Formalin-fixed
paraffin-embedded tissues obtained from 55 DLBCL tissues and 12
normal lymphoma tissues were cut into 4-µm slices. Subsequently,
the following procedures were applied: Conventional dewaxing,
graded ethanol dehydration, antigen retrieval were performed, and
membranes were incubated with 3% hydrogen peroxide to block
endogenous peroxidase (4°C for 10 min), and normal sheep serum
(Cappel Laboratories, Cochranville, PA, USA) to block the tissue
sample (27°C for 20 min). Primary mouse anti-human Bcl-2 monoclonal
antibody (1:500; cat. no. IS61430, Dako, Glostrup, Denmark) was
applied, incubated at 4°C overnight and stored at room temperature
for 20 min. Membranes were washed three times with Tris-buffered
saline with Tween 20 (TBST) for 10 min and subsequently incubated
with the secondary antibody (anti-IgG; 1:2,000 dilution; cat. no.
P0448, Dako) that was labeled with biotin (Dako), and the
streptomycin-avidin that was labeled with horseradish peroxidase
(Dako) for 30 min at room temperature. Membranes were washed again
with TBST three times for 10 min, staining was performed using
diaminobenzidine and slices were counterstained with hemalum.
Phosphate-buffered saline instead of a primary antibody was
considered as a negative control (NC) and a known positive antibody
anti-CD38 (1:100; cat. no. TA353695, Origene Technologies, Inc.,
Rockville, MD, USA) was set as a positive control. Moreover, the
product of staining intensity (3, brown; 2, yellow; 1, light
yellow; and 0, colorless) and the percentage of positive cells (4,
>75%; 3, 51–75%; 2, 26–50%; 1, 6–25%; and 0, <5%) were
complied with the integral calculation method for Bcl-2. Cells were
randomly selected from five high power fields under a light
microscope (magnification, ×400) in each slice and 100 cells were
counted in each field. Based on the two types of scores, the
integral levels were evaluated as: Negative (−), 0 points; weak
positive (+), 1–2 points; positive (++), 3–5 points; strongly
positive (+++), >5 points. The slides were independently
evaluated by two blinded pathologists.
miRNA target prediction and 3
untranslated region (UTR) luciferase-reporter assay
MiRNA targets were predicted using the TargetScan
database version 7.1 (http://www.targetscan.org/vert_71/). Wild-type and
mutant-type Bcl-2 3′UTR luciferase reporter vectors were
constructed. miR-21 mimics or control were co-transfected with
constructed wild-type or mutant-type luciferase reporter vector
into DLBCL OCI-LY3 cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific Inc.). The pRL-TK control vector (Promega
Corporation; Madison, WI, USA) was transfected and served as a
control. Subsequently, luciferase activity was analyzed using the
Dual-Luciferase Reporter Assay System (E1910; Promega Corporation)
following cell transfection for 48 h.
Cell culture and cell
transfection
OCI-LY3 cells were cultured in RPMI 1640 medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin). Cells were cultured in an incubator containing
5% CO2 at 37°C and the OCI-LY3 cell line was provided by
the Chinese Academy of Sciences (Guangzhou, China). Cells were
divided into five different groups: Control, NC, miR-21 mimics,
miR-21 inhibitor, and Bcl-2 siRNA groups. Cells without any
treatment were in the control group, while the other four groups
were transfected with negative control (empty vector), miR-21
mimics, miR-21 inhibitor and Bcl-2 siRNA, respectively. The
corresponding vectors were purchased from Shanghai GenePharma Co.,
Ltd (Shanghai, China). Cells were transfected using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific Inc.) and cultured in an
incubator containing 5% CO2 at 37°C. Complete medium was
replaced every 6 to 8 h until the culture process was
completed.
MTT assay
MTT [3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyl-tetrazolium bromide] assays were used to evaluate cell
viability. Transfected cells, which were washed twice with PBS,
were cultured to a confluence of 80%, digested with trypsin and
constructed into cell suspensions and the number of cells were
counted manually. OCI-LY3 cells were inoculated into 96-well plates
at a cell density of 3–6×103 cells/well. Six wells were
replicated. Cells were detected following transfection for 24, 48,
72, and 96 h, respectively. MTT (20 µl; 5 mg/ml; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) was added and the cell culture
was sustained for 4 h at 37°C in an incubator with 5%
CO2. Subsequently, DMSO (150 µl) was added into each
well and the cells were lightly shaken for 10 min to dissolve the
crystals. Samples were detected using a microplate reader
(SpectraMAX Plus; Molecular Devices, Sunnyvale, CA, USA) at a
wavelength of 490 nm. An MTT curve was drawn with the absorbance
value as the vertical axis and the time interval as abscissa.
Flow cytometric analysis
Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) apoptosis detection kits (BD Biosciences, San
Jose, CA, USA) were used to evaluate the apoptosis of OCI-LY3
cells. Following 48-h transfection, cells were washed twice with
cold PBS and re-suspended with binding buffer to a density of
0.5–1×106/ml. Subsequently, cell suspensions (100 µl)
were incubated with 5 µl of annexin V-FITC and PI in the dark for
15 min at room temperature. Binding buffer (400 µl) was added to
each tube and cells were analyzed using flow cytometry (Beckman FC
500 MCL/MPL; Beckman Coulter, Inc., Brea, CA, USA).
Detection of caspase-3 activity
Caspase-3 activity was detected using the caspase
colorimetric assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), following cell transfection for 48 h. Cells were lysed in
lysis buffer on ice for 20 min in order to detect the activity of
caspase-3. Following centrifugation at 28,341 × g for 5 min at 4°C,
supernatants were incubated with the caspase substrate in the
reaction buffer at 37°C for 4 h. Samples were detected with a
SpectraMAX Plus microplate reader at the wavelength of 405 nm.
Relative caspase-3 activity was calculated as the percentage of
A405 values in the experimental samples over those in the control
groups.
RT-qPCR for detecting the expression
levels of Bcl-2 mRNA
The extraction of cellular total RNA was conducted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
reverse transcribed from RNA using the Omniscript RT kit (Qiagen
GmbH). qPCR detection of Bcl-2 mRNA was performed using the
QuantiTect SYBR Green PCR Kit (Qiagen GmbH). Primers used for Bcl-2
(Invitrogen; Thermo Fisher Scientific Inc.) were as follows: Bcl-2
sense, 5′-CTGTGCTGCTATCCTGC-3′ and antisense,
5′-TGCAGCCACAATACTGT-3′. Relative expression levels of Bcl-2 were
calculated using the 2−ΔΔCq method (17) and β-actin was set as the
corresponding control.
Western blotting assay
Bcl-2 expression was detected by western blotting.
Cellular proteins were extracted after 48-h transfection and the
bicinchoninic acid method was used to evaluate the protein density.
Furthermore equal quantities of protein (50 µg) from each group
were loaded and separated by 10% SDS-PAGE, transferred onto
polyvinylidene fluoride membranes, and blocked with 5% non-fat milk
for 1 h at room temperature. Membranes were incubated with primary
mouse anti-human Bcl-2 monoclonal antibody (1:500) and GAPDH
antibody (1:1,000; cat. no. 5174, Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. Furthermore, membranes were
washed three times with TBST for 10 min each and incubated with
horseradish-peroxidase-linked anti-IgG (1:2,000; Origene
Technologies, Inc.) at room temperature for 1 h. Membranes were
washed again with TBST three times (10 min each) and signal
detection was performed using a Super Enhanced Chemiluminescence
Plus Detection Reagent (Applygen Technologies Inc., Beijing,
China). The samples were quantified using Lab Works software
version 4.5 (Mitov Software, Moorpark, CA, USA) with GAPDH as an
internal control.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM SPSS, Armonk, NY, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Significant differences in continuous data (mean ± standard
deviation) were analyzed using the analysis of variance with
Student Newman-Keuls post-hoc tests for comparisons between groups,
and differences in continuous data between two groups were analyzed
using unpaired Student's t-tests. Furthermore, results of
immunohistochemistry for Bcl-2 protein were analyzed by the rank
sum test. The association between miR-21 and Bcl-2 protein
expression was analyzed using the Spearman rank correlation.
Results
miR-21 and Bcl-2 protein expression
levels in DLBCL clinical specimens
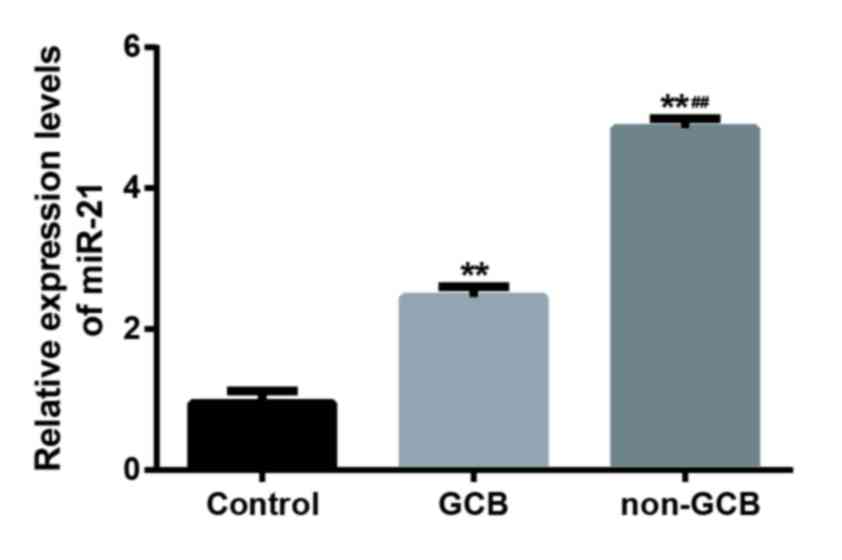
RT-qPCR was used to evaluate the expression level of
miR-21 in 19 cases with GCB-DLBCL, 26 cases without GCB and 20
normal lymph node tissues (Fig. 1).
The expression of miR-21 in DLBCL tissues was significantly
increased when compared to that in normal tissues (P<0.01) and
the expression level of miR-21 in non-GCB DLBCL tissues was
significantly higher than GCB-DLBCL tissues (P<0.01; Fig. 1). In addition, positive Bcl-2
expression was predominantly detected in the cell cytoplasm or cell
membrane with yellow granules. Bcl-2-positive cells appeared to be
focally gathered or unevenly distributed (Fig. 2). Bcl-2 expression levels were
significantly increased in non-GCB DLBCL tissues when compared with
normal tissues (P<0.01) and GCB-DLBCL tissues (P<0.05); the
positive expression of Bcl-2 in GCB-DLBCL tissues was significantly
increased when compared with normal tissues (P<0.05; Table I).
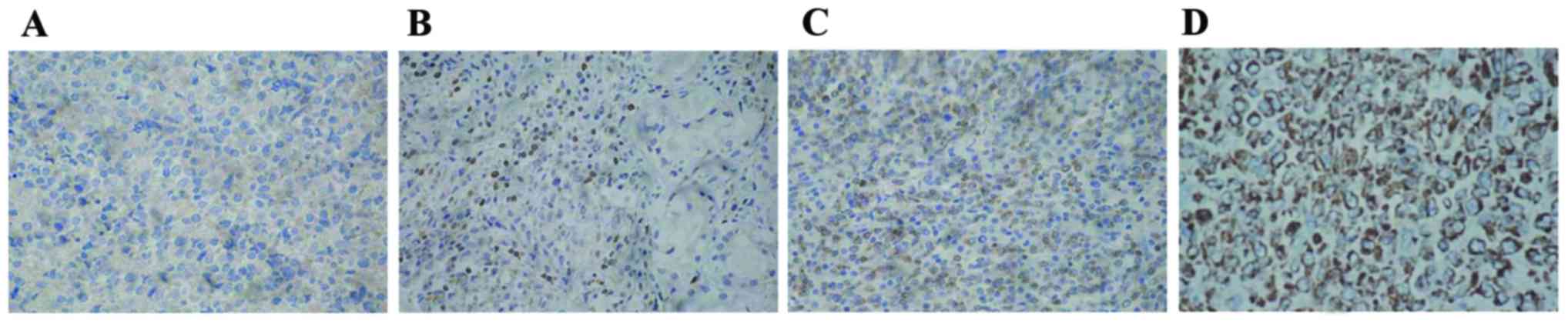 | Figure 2.Relative expression levels of Bcl-2 in
clinical specimens were detected via the immunohistochemical
streptoavidin-biotin peroxidase method (magnification, ×400). (A)
Bcl-2-cells; (B) Bcl-2+ cells; (C) Bcl-2++ cells; and (D) Bcl-2+++
cells. Bcl-2, B-cell lymphoma-2. Staining intensity was indicated
as follows: 3, brown; 2, yellow; 1, light yellow; and 0, colorless.
The percentage of positive cells (4, >75%; 3, 51–75%; 2, 26–50%;
1, 6–25%; and 0, <5%) were complied with the integral
calculation method for Bcl-2. OCI-LY3 cells were randomly selected
from five high power fields (magnification, ×400) in each slice and
100 cells were counted in each field. Based on the two types of
scores, the integral levels were evaluated as: Negative (−), 0
points; weak positive (+), 1–2 points; positive (++), 3–5 points;
or strongly positive (+++), >5 points. |
 | Table I.Expression of Bcl-2 in diffuse large B
cell lymphoma and control groups. |
Table I.
Expression of Bcl-2 in diffuse large B
cell lymphoma and control groups.
|
|
| Bcl-2 |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | N | − | + | ++ | +++ | Z-value | P-value |
|---|
| Control | 12 | 12 | 0 | 0 | 0 | −2.331 | 0.02a |
| GCB | 19 | 12 | 4 | 2 | 1 | −2.053 | 0.04b |
| N-GCB | 36 | 13 | 10 | 5 | 8 | −3.576 |
<0.01c |
Spearman correlation analysis suggested that there
was a positive correlation between miR-21 and Bcl-2 expression
levels in both GCB-DLBCL (rs=0.528, P=0.02) and
non-GCBDLBCL tissues (rs=0.708, P<0.01), whereas no
significant correlation between miR-21 level and Bcl-2 protein
level was detected in normal tissues.
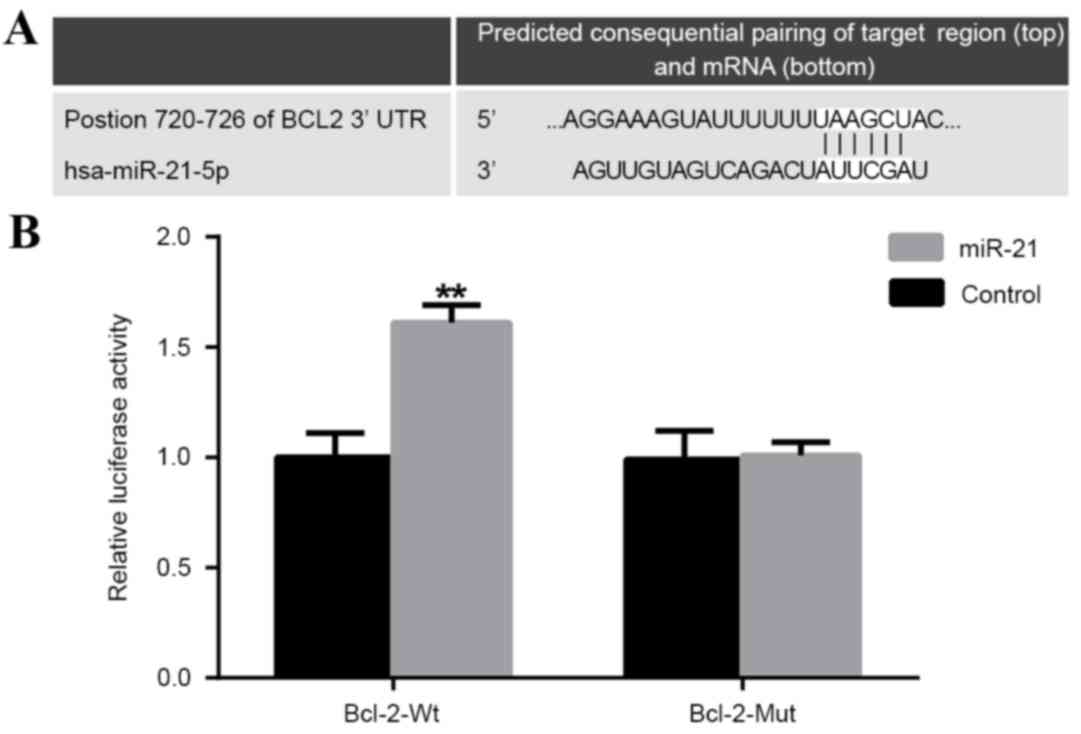
Targeting Bcl-2 by miR-21
A putative conserved binding site for miR-21 at
nucleotide position 720–726 of human Bcl-23′UTR was predicted using
TargetScan. Perfect base pairing was observed between the seed
sequence of mature miR-21 and the 3′UTR of Bcl-2 mRNA (Fig. 3A). Dual luciferase reporter gene
assays revealed that miR-21 significantly promoted the luciferase
activity of Bcl-2 wild-type with an upregulation of 61%
(P<0.01); however, there was no significant effect on the
luciferase activity of Bcl-2 mutant-type 3′UTR (Fig. 3B). Collectively, these findings
indicated that Bcl-2 was likely to be a direct target for
miR-21.
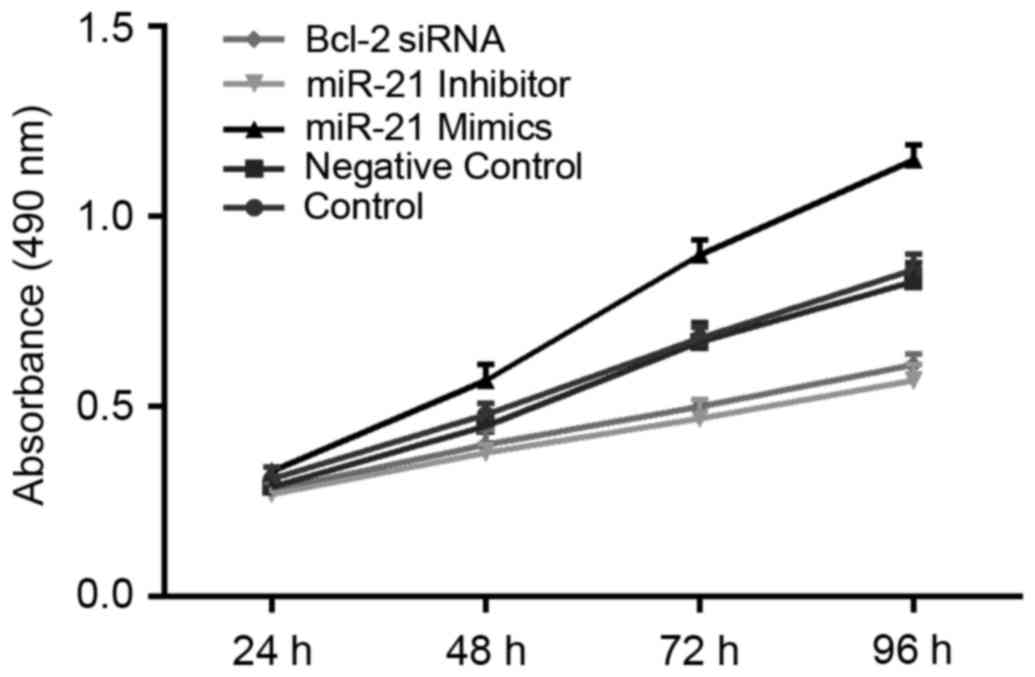
Proliferation of DLBCL cells
Results of MTT assays suggested that decreased
proliferation of OCI-LY3 cells was observed in groups transfected
with miR-21 inhibitor and Bcl-2 siRNA for 48 h when compared with
the control and NC groups (all P<0.05). This trend was more
pronounced as the length of time extended. miR-21 inhibitor
appeared to have a stronger ability than Bcl-2 siRNA with respect
to the inhibition of cell proliferation; however, this distinction
did not reach statistical significance. The cell number of the
control group at each time point was not significantly different
from that of the NC group. Compared with the other four groups, the
proliferation capacity of OCI-LY3 cells significantly increased
after the transfection of miR-21 mimics for 48 h (all P<0.05;
Fig. 4).
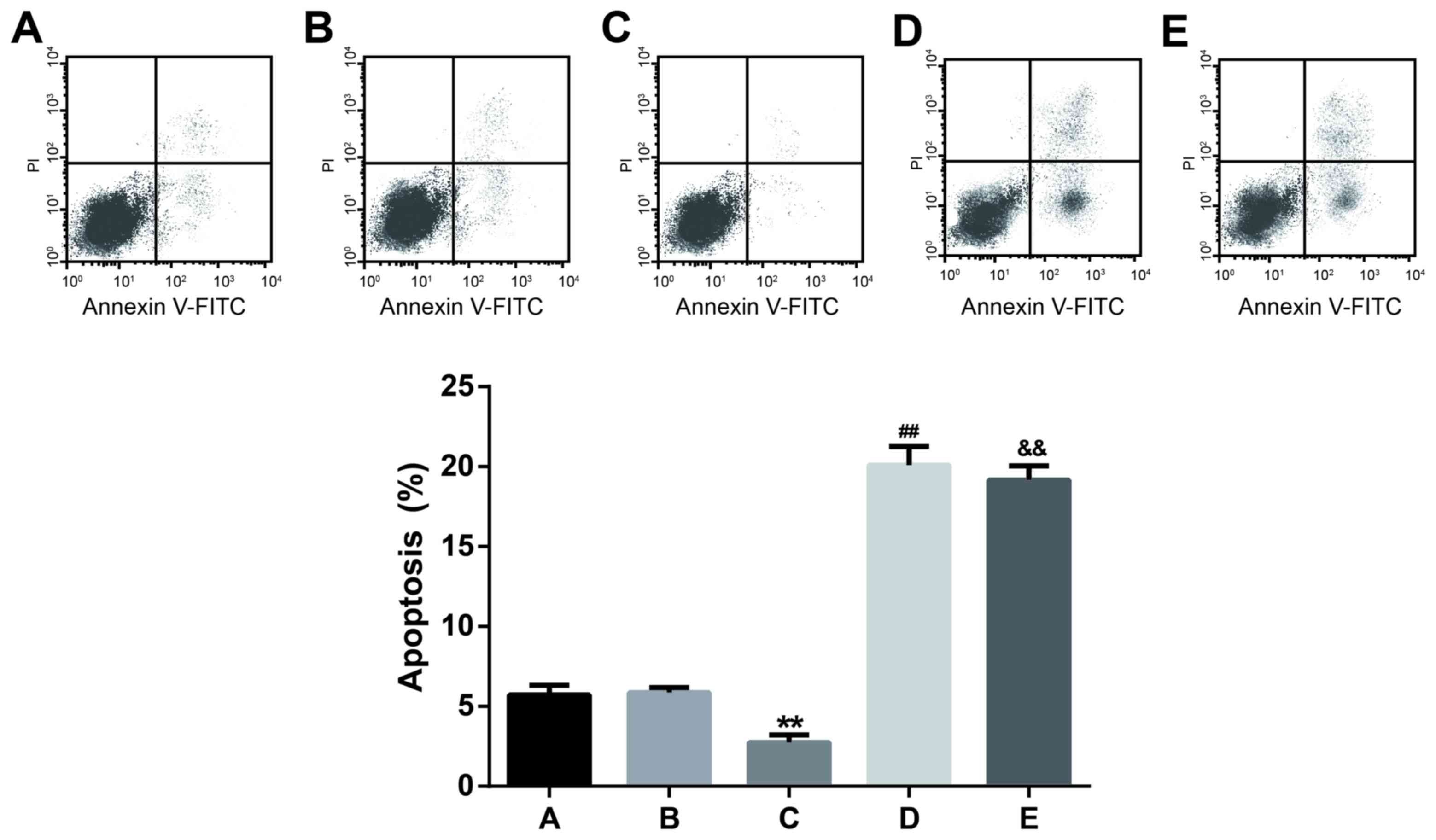
Apoptosis assay
Results of flow cytometry analysis indicated that
the apoptosis percentages of cells transfected with miR-21
inhibitor and Bcl-2 siRNA were 20.10±1.16 and 19.15±0.91%, with no
significant difference observed. However, the apoptosis percentages
of miR-21 inhibitor and Bcl-2 siRNA groups were significantly
higher than the miR-21 mimic, control and NC groups (P<0.01;
Fig. 5). Furthermore, there was no
significant difference observed in the apoptosis percentage between
the control (5.71±0.62%) and NC (5.86±0.32%) groups. The apoptosis
rate (mean ± standard deviation) of the miR-21 mimic group was
2.73±0.48%, which was significantly lower than those of the other
four groups (P<0.01; Fig. 5).
These findings indicated that miR-21 may inhibit the apoptosis of
OCI-LY3 cells and the downregulation of miR-21 or Bcl-2 may
increase the apoptotic ability of OCI-LY3 cells.
Caspase-3 activity assay
The caspase-3 activity in cells transfected with
miR-21 mimics was 0.47±0.05, indicating a significant difference
when compared with the other four groups (all P<0.01; Fig. 6). Caspase-3 activity levels for cells
transfected with miR-21 inhibitor and Bcl-2 siRNA were 2.6±0.24 and
2.47±0.15, with no significant difference. However, the caspase-3
activities in miR-21 inhibitor and Bcl-2 siRNA groups were
significantly increased compared with the control, NC and miR-21
mimic groups (P<0.01; Fig. 6).
These results suggest that miR-21 may restrain the activity of
caspase-3 and the downregulation of miR-21 or Bcl-2 may increase
the caspase-3 activity.
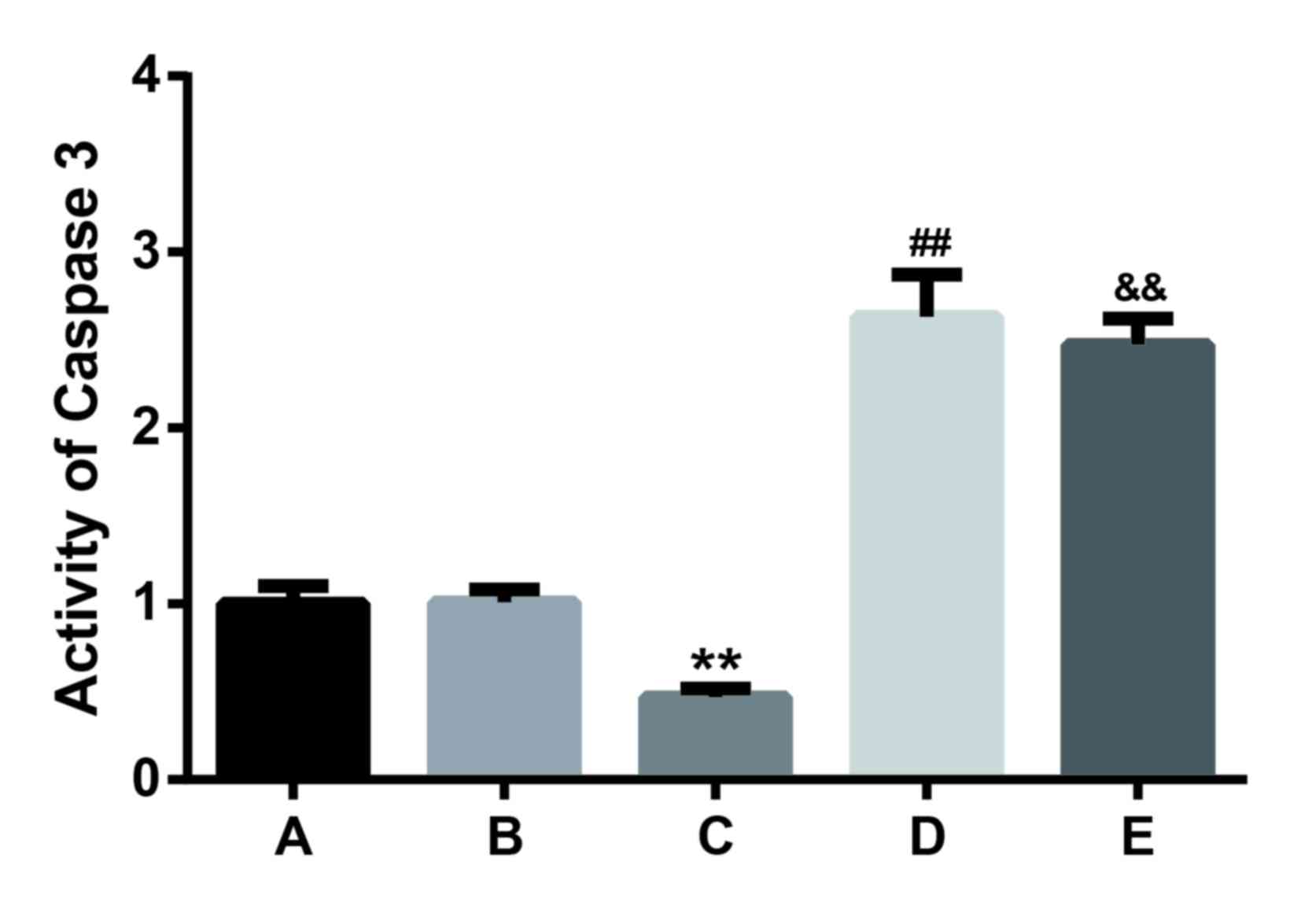 | Figure 6.Caspase-3 activity in cells analyzed
after transfection with the control, negative control, miR-21
mimics, miR-21 inhibitor, or Bcl-2 siRNA for 48 h. Results
presented are the mean + standard deviation of three independent
experiments. **P<0.01 vs. the control, negative control, miR-21
inhibitor and Bcl-2 siRNA groups; ##P<0.01 vs. the
control, negative control and Bcl-2 siRNA groups;
&&P<0.01 vs. the control and negative control
groups. Bcl-2, B-cell lymphoma; miR21, microRNA-21; siRNA, small
interfering RNA. |
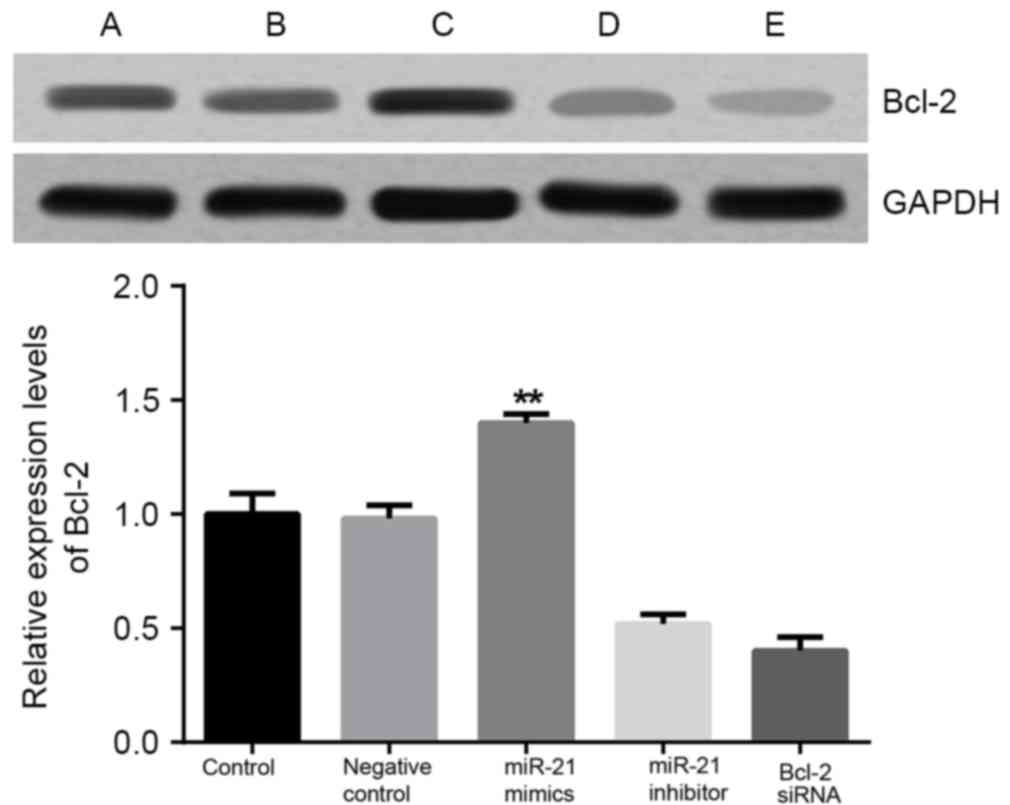
Bcl-2 mRNA and Bcl-2 protein
expression levels in DLBCL cells
Results from RT-qPCR and western blotting
demonstrated that the expression levels of Bcl-2 mRNA and Bcl-2
protein were significantly decreased when compared to the control
and NC groups after 48 h transfection with the Bcl-2 siRNA or
miR-21 inhibitor (P<0.01; Figs. 7
and 8, respectively). No significant
difference in Bcl-2 mRNA and Bcl-2 protein expression levels were
indicated between the control and NC group (P>0.05). Bcl-2
expression levels in cells transfected with miR-21 mimics were
significantly upregulated when compared with the other four groups
(P<0.01). These findings indicated that miR-21 may target and
regulate the expression of the Bcl-2 gene in OCI-LY3 cells.
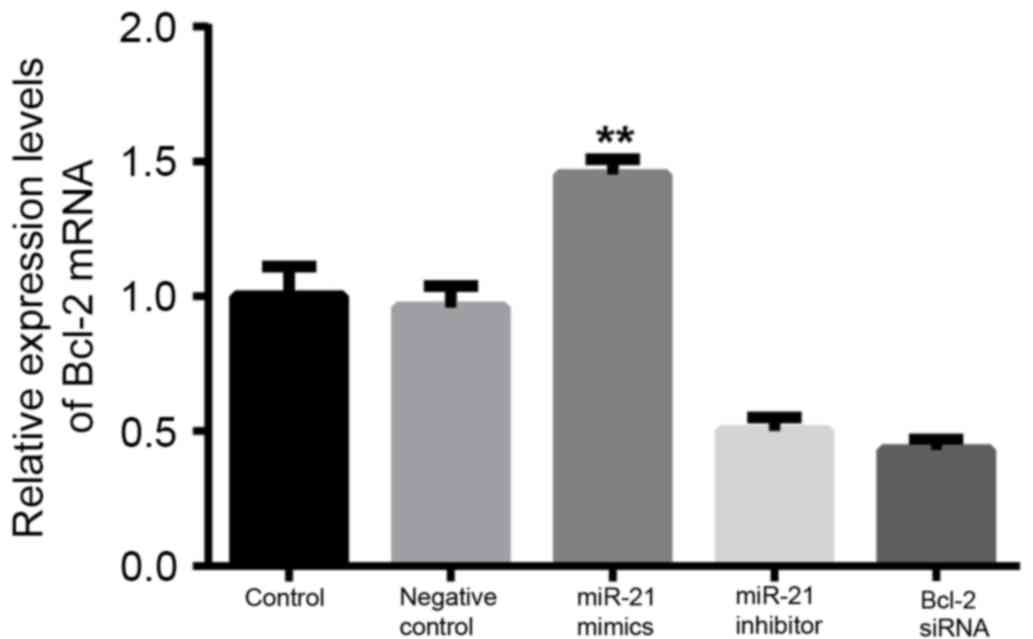 | Figure 7.miR-21 increases Bcl-2 mRNA
expression. OCI-LY3 cells were transfected with the control,
negative control, miR-21 mimics, miR-21inhibitor, or Bcl-2 siRNA
for 48 h. Bcl-2 mRNA expression was detected by reverse
transcription-quantitative polymerase chain reaction. Results
presented are the mean + standard deviation of three independent
experiments. **P<0.01, vs. the control, negative control, miR-21
inhibitor and Bcl-2 siRNA groups. Bcl-2, B-cell lymphoma; miR21,
microRNA-21; siRNA, small interfering RNA. |
Discussion
A number of studies have demonstrated that miRNA
exhibited differential expression levels in various tumors, and
they may interplay with tumor suppressor genes or oncogenes to
affect tumorigenesis (18). Indeed,
significant overexpression of miR-21 has been reported in glioma
(19), pancreatic cancer (20), lung cancer (21), leukemia (22), and lymphoma (23). In the present study, we examined
miR-21 expression levels in both DLBCL and normal lymph node
tissues. The present results suggest that miR-21 was upregulated in
DLBCL tissues and its expression in non-GCB tissues was higher when
compared with GCB tissues. These findings were consistent with the
results from previous studies (24,25).
Bcl-2 directly participates in cell apoptosis and
functions as an anti-apoptotic substance (26); furthermore, it has been indicated
that Bcl-2 exhibited low expression levels in apoptotic cells
(27). Moreover, Bcl-2 expression is
associated with the development of various types of cancer,
including breast cancer (28),
non-small cell lung cancer (29),
nasopharyngeal cancer (30), gastric
cancer (31) and non-Hodgkin B-cell
lymphoma (32–34). Immunohistochemical results from the
present study revealed that the Bcl-2 protein was overexpressed in
DLBCL tissues and that Bcl-2 protein expression levels exhibited a
significant difference between GCB-DLBCL and non-GCBDLBCL tissues.
Therefore, we hypothesized that Bcl-2 was heterogeneously expressed
in different histological subtypes.
Additionally, a positive correlation was determined
between miR-21 and Bcl-2 expression levels in both GCB-DLBCL and
non-GCBDLBCL tissues. The dual-luciferase reporter assay was
conducted to illuminate the potential mechanisms. The results
indicated that Bcl-2 was a target gene of miR-21 and the Bcl-2
expression levels may be increased through the direct binding of
miR-21 to the 3′UTR of Bcl-2 mRNA. However, further comprehensive
research is required to confirm these findings.
miR-21 is believed to be a multi-functional miRNA
involved in the proliferation, differentiation and anti-apoptosis
of cancer cells (35). A previous
study has reported that miR-21 was able to increase cell growth and
inhibit apoptosis in DLBCL (36).
Our findings revealed that upregulation of miR-21 not only
exacerbated cell proliferation, but also suppressed the apoptosis
of DLBCL cells. However, the inhibition of miR-21 expression may
inhibit the proliferation of DLBCL cells and promote the apoptosis
of DLBCL cells. Consequently, the present study provides evidence
that miR-21 may regulate cell viability and apoptosis in DLBCL and
miR-21 mimics may increase Bcl-2 expression levels in DLBCL OCI-LY3
cells. Furthermore, downregulation of miR-21 may contribute to the
significant decrease in Bcl-2 expression levels observed in both
mRNA and protein levels in OCI-LY3 cells. Our data indicates that
the expression of Bcl-2 was modulated by miR-21, which may decrease
cell apoptosis through its anti-apoptosis effects via upregulating
Bcl-2 gene expression.
Caspase-3 is a member of the caspase protease family
and functions as a pivotal participant in cellular apoptosis
(37). The activation of caspase-3
has been identified in various types of cells observed with
apoptosis (38). Caspase-3 was
previously revealed as a downstream molecule of the Bcl-2 family
(37). The present study findings
were consistent with these previous reports and suggested that
higher caspase-3 activity along with increased apoptosis was
reflected in cells transfected with Bcl-2 siRNA, whereas lower
caspase-3 activity accompanied by decreased apoptosis was observed
in cells transfected with miR-21 mimics. Therefore, our data
provided evidence that caspase-3 may participate in cellular
apoptosis and an underlying interaction may exist between Bcl-2 and
caspase-3.
The present study provides evidence that miR-21 may
exacerbate the viability of DLBCL cells and inhibit cell apoptosis
by positively regulating Bcl-2 expression in DLBCL. Since only one
DLBCL cell line (OCI-LY3) was used to detect interactive effects
between miR-21 and its target gene, Bcl-2, on tumor proliferation
and apoptosis in the present study, the molecular effect of miR-21
and Bcl-2 on tumorigenesis should be further studied.
In conclusion, miR-21 expression was upregulated in
DLBCL tissues and the expression of miR-21 was positively
correlated with Bcl-2 expression. These findings further
demonstrated that miR-21 regulates Bcl-2 expression in a direct
manner in DLBCL. Notably, the present study provided evidence that
miR-21 may exacerbate the viability of DLBCL cells and inhibit cell
apoptosis by targeting Bcl-2. Hence, both Bcl-2 and miR-21 are
likely to serve as effective targets for developing alternative
treatments for DLBCL.
References
|
1
|
Flowers CR, Sinha R and Vose JM: Improving
outcomes for patients with diffuse large B-cell lymphoma. CA Cancer
J Clin. 60:393–408. 2010.PubMed/NCBI
|
|
2
|
De Roos AJ, Davis S, Colt JS, Blair A,
Airola M, Severson RK, Cozen W, Cerhan JR, Hartge P, Nuckols JR and
Ward MH: Residential proximity to industrial facilities and risk of
non-Hodgkin lymphoma. Environ Res. 110:70–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang CM, Schroeder JC, Olshan AF, Dunphy
CH, Huang WY, Baric RS, Conway K, Cerhan JR, Lynch CF, Rothman N,
et al: A case-control study of tobacco use and other
non-occupational risk factors for lymphoma subtypes defined by
t(14; 18) translocations and bcl-2 expression. Cancer Causes
Control. 21:1147–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson CA, Habermann TM, Wang AH,
Vierkant RA, Folsom AR, Ross JA and Cerhan JR: Antioxidant intake
from fruits, vegetables and other sources and risk of non-Hodgkin's
lymphoma: The Iowa women's health study. Int J Cancer.
126:992–1003. 2010.PubMed/NCBI
|
|
5
|
Frankenfeld CL, Cerhan JR, Cozen W, Davis
S, Schenk M, Morton LM, Hartge P and Ward MH: Dietary flavonoid
intake and non-Hodgkin lymphoma risk. Am J Clin Nutr. 87:1439–1445.
2008.PubMed/NCBI
|
|
6
|
Fisher SG and Fisher RI: The epidemiology
of non-Hodgkin's lymphoma. Oncogene. 23:6524–6534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SS, Slager SL, Brennan P, Holly EA,
De Sanjose S, Bernstein L, Boffetta P, Cerhan JR, Maynadie M,
Spinelli JJ, et al: Family history of hematopoietic malignancies
and risk of non-Hodgkin lymphoma (NHL): A pooled analysis of 10 211
cases and 11 905 controls from the international lymphoma
epidemiology consortium (InterLymph). Blood. 109:3479–3488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazan-Mamczarz K and Gartenhaus RB: Role
of microRNA deregulation in the pathogenesis of diffuse large
B-cell lymphoma (DLBCL). Leuk Res. 37:1420–1428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Psathas JN, Doonan PJ, Raman P, Freedman
BD, Minn AJ and Thomas-Tikhonenko A: The Myc-miR-17-92 axis
amplifies B-cell receptor signaling via inhibition of ITIM
proteins: A novel lymphomagenic feed-forward loop. Blood.
122:4220–4229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tagawa H, Ikeda S and Sawada K: Role of
microRNA in the pathogenesis of malignant lymphoma. Cancer Sci.
104:801–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jardin F and Figeac M: MicroRNAs in
lymphoma, from diagnosis to targeted therapy. Curr Opin Oncol.
25:480–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai H, Wei J, Deng C, Yang X, Wang C and
Xu R: MicroRNA-21 regulates the sensitivity of diffuse large B-cell
lymphoma cells to the CHOP chemotherapy regimen. Int J Hematol.
97:223–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iqbal J, Neppalli VT, Wright G, Dave BJ,
Horsman DE, Rosenwald A, Lynch J, Hans CP, Weisenburger DD, Greiner
TC, et al: BCL2 expression is a prognostic marker for the activated
B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol.
24:961–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swerdllow SH, Campo E, Harris NL, Jaffe
ES, Pileri SA, Stein H, Thiele J and Vardiman JW: WHO
classification of tumours of haematopoietic and lymphoid tissues.
4th. 2. IARC; 2008
|
|
16
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi L, Chen J, Yang J, Pan T, Zhang S and
Wang Z: MiR-21 protected human glioblastoma U87MG cells from
chemotherapeutic drug temozolomide induced apoptosis by decreasing
Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 1352:255–264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giovannetti E, Funel N, Peters GJ, Del
Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A,
Falcone A, et al: MicroRNA-21 in pancreatic cancer: Correlation
with clinical outcome and pharmacologic aspects underlying its role
in the modulation of gemcitabine activity. Cancer Res.
70:4528–4538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Pei JH and Chen HM: Effects of
continuous positive airway pressure treatment on glycaemic control
and insulin sensitivity in patients with obstructive sleep apnoea
and type 2 diabetes: A meta-analysis. Arch Med Sci. 10:637–642.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao M, Guo L, Wang T, Zhu T, Jia L, Chen
L and Wen F: Interleukin-1B-31T/C promoter polymorphism and chronic
obstructive pulmonary disease risk: A meta-analysis. Arch Med Sci.
10:434–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawrie CH, Soneji S, Marafioti T, Cooper
CD, Palazzo S, Paterson JC, Cattan H, Enver T, Mager R, Boultwood
J, et al: MicroRNA expression distinguishes between germinal center
B cell-like and activated B cell-like subtypes of diffuse large B
cell lymphoma. Int J Cancer. 121:1156–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu N, Zheng Y, Zhu Y, Xiong S and Chu Y:
Selective impairment of CD4+CD25+Foxp3+ regulatory T cells by
paclitaxel is explained by Bcl-2/Bax mediated apoptosis. Int
Immunopharmacol. 11:212–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Q, Yang K and Li A: microRNA-21
protects against ischemia-reperfusion and
hypoxia-reperfusion-induced cardiocyte apoptosis via the
phosphatase and tensin homolog/Akt-dependent mechanism. Mol Med
Rep. 9:2213–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong Y, Ma XY, Zhang Z, Shao ZJ, Zhang YY
and Zhou LM: Apoptosis induced by β,β-dimethylacrylshikonin is
associated with Bcl-2 and NF-κB in human breast carcinoma MCF-7
cells. Oncol Lett. 6:1789–1793. 2013.PubMed/NCBI
|
|
29
|
Li Y, Zhang S, Geng JX and Hu XY: Curcumin
inhibits human non-small cell lung cancer A549 cell proliferation
through regulation of Bcl-2/Bax and cytochrome C. Asian Pac J
Cancer Prev. 14:4599–4602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Yan L, Zhang W, Wang H, Chen W, Hu N
and Ou H: miR-21 inhibitor suppresses proliferation and migration
of nasopharyngeal carcinoma cells through down-regulation of BCL2
expression. Int J Clin Exp Pathol. 7:3478–3487. 2014.PubMed/NCBI
|
|
31
|
Wei B, Song Y, Zhang Y and Hu M:
microRNA-449a functions as a tumor-suppressor in gastric
adenocarcinoma by targeting Bcl-2. Oncol Lett. 6:1713–1718.
2013.PubMed/NCBI
|
|
32
|
Mestre-Escorihuela C, Rubio-Moscardo F,
Richter JA, Siebert R, Climent J, Fresquet V, Beltran E, Agirre X,
Marugan I, Marín M, et al: Homozygous deletions localize novel
tumor suppressor genes in B-cell lymphomas. Blood. 109:271–280.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song B, Ji W, Guo S, Liu A, Jing W, Shao
C, Li G and Jin G: miR-545 inhibited Pancreatic ductal
adenocarcinoma growth by targeting RIG-I. FEBS Lett. 588:4375–4381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thompson RC, Vardinogiannis I and Gilmore
TD: The sensitivity of diffuse large B-cell lymphoma cell lines to
histone deacetylase inhibitor-induced apoptosis is modulated by
BCL-2 family protein activity. PLoS One. 8:e628222013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan X, Wang ZX and Wang R: MicroRNA-21: A
novel therapeutic target in human cancer. Cancer Biol Ther.
10:1224–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu L, Song G, Chen L, Nie Z, He B, Pan Y,
Xu Y, Li R, Gao T, Cho WC and Wang S: Inhibition of miR-21 induces
biological and behavioral alterations in diffuse large B-cell
lymphoma. Acta Haematol. 130:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tomek M, Akiyama T and Dass CR: Role of
Bcl-2 in tumour cell survival and implications for pharmacotherapy.
J Pharm Pharmacol. 64:1695–1702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|