Introduction
Hyperoxic treatment (hyperoxia) is an indispensable
therapeutic measure in some neonatal critical care conditions. For
instance, supplemental oxygen used to treat infants born
prematurely disrupts angiogenesis (1). An appropriate level of hyperoxia can
induce an antioxidant response (2).
However, hyperoxia over a prolonged period of time may have a
serious toxic effect on bodily organs. A previous study indicated
that the extensive clinical use of hyperoxia is hindered by
potential organ injury that may arise by increasing the production
of reactive oxygen species (ROS) (3). ROS are derivatives of cellular
metabolic reactions that modulate the fundamental physiological
functions of aerobic life (4). ROS,
which may be produced in the mitochondria, have traditionally been
regarded as by-products of aerobic metabolism, and primarily
include oxygen ions and hydrogen peroxide
(H2O2) (5,6). ROS has
been demonstrated to affect cell viability, proliferation,
differentiation, aging, apoptosis and various other physiological
and pathological processes (5).
Mitochondrial ROS are critical for the functional stimulation of
inflammation (7). At low levels, ROS
may act as signaling molecules within cells (8). Under normal conditions, the generation
and removal of ROS are in dynamic balance in vivo and are
beneficial to the organism. Furthermore, previous results have
indicated that ROS have a critical function upstream and downstream
of nuclear factor (NF-κB) and tumor necrosis factor pathways
(9). However, excessive generation
of ROS is harmful, with the hydroxyl radical considered the most
harmful (9). In particular,
H2O2 may significantly reduce the activity of
superoxide dismutase, glutathione peroxidase, catalase and lipase
(10). Furthermore,
H2O2 affects numerous intracellular signaling
pathways such as mitogen-activated protein kinase (MAPK) and c-Jun
N-terminal kinase, modify the activity of key signaling proteins
including catalase, glutathione peroxidase and peroxiredoxin and
promotes tyrosine phosphorylation by activating protein tyrosine
kinases (11). These activities
demonstrate that hyperoxia may inhibit cell proliferation and
stimulate cell mortality (12).
Immunoglobulin A (IgA) is the first line of defense
in mucosal immunology (13).
Secretory immunoglobulin A (SIgA) is composed of IgA, secretory
component (SC) protein and J chain protein (14). SC is the extracellular component of
the polymeric immunoglobulin receptor (pIgR), which is located on
the basolateral surface of mucosal epithelial cells (15,16).
pIgR has been identified as the precursor of SC protein (17) and the membrane SC has been termed
pIgR (15). pIgR transports IgA
antibodies across intestinal epithelial cells (18), and has acritical role in mucosal
immune systems and intestinal defense (13,19). SC
participates in innate protection against mucosal pathogens
(20) and protects SIgA from
proteolytic degradation (21).
Long-term hyperoxic treatment may have serious toxic
effects on intestinal epithelial cells, in vitro and in
vivo (22–25). Our previous results demonstrated that
SIgA and SC were markedly increased in neonatal rats under
hyperoxic conditions (25). SC has
also been demonstrated to have an important role in preventing
pathogen adhesion to host cells (26). Therefore, although it is
well-established that SC is crucial for normal bodily functions,
the influence of hyperoxia on SC remains clear.
In the present study, investigations into intestinal
injuries induced by ROS under hyperoxic conditions were performed.
The influence of ROS on the SC in the intestinal epithelium and
whether ROS was a predominant factor in causing intestinal injury
during hyperoxia were also explored.
Materials and methods
Cell culture
A human colon adenocarcinoma cell line, Caco-2, was
obtained from the Cell Biological Institute of Shanghai, Chinese
Academy of Sciences (Shanghai, China). Cells were cultured at 37°C
in an atmosphere containing 95% air and 5% CO2 in high
glucose Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine
serum (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing,
China), 1% L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin
and 0.25 mg/ml amphotericin. Culture medium was changed every 2 to
3 days. Prior to treatment, the cells (1×106 cells/ml)
were plated with fresh DMEM and cultured for 2 days. The cells were
then treated with different concentrations of
H2O2 (100, 200 or 400 µM), or exposed to 85%
O2 (hyperoxia) at 37°C for 24 h. Control cells did not
receive treatment. Subsequently, the cultured cells were harvested
for RNA and protein extraction. All experiments were repeated 6–10
times.
Annexin V(AV)/propidium iodide (PI)
double staining assay
Apoptotic cells were quantified using an
AV-fluorescein isothiocyanate (FITC)/PI kit (Nanjing Kaiji
Materials Co., Ltd., Nanjing, China) according to the
manufacturer's instructions, detected using a flowcytometer
(FACSCalibur; BD Biosciences, San Jose, CA, USA) and analyzed with
CellQuest Pro software (BD Biosciences, San Jose, CA, USA). Caco-2
cells were plated at the same cell density (1×106
cells/ml) at each passage. Untreated cells were used as the control
group. Caco-2 cells were pretreated with either 100, 200 or 400 µM
H2O2 or 85% O2 (hyperoxia) for 6,
12, and 24 h. Cells were harvested and resuspended in binding
buffer (pH 7.5, 10 mM HEPES, 2.5 mM CaCl2 and 140 mM
NaCl) and incubated with AV-FITC and PI at room temperature for 10
min in the dark prior to flow cytometric analysis. AV-positive
cells were considered to be in the early stages of apoptosis,
whereas AV and PI double-positive cells were considered to be in
the late stages of apoptosis (27).
Apoptotic cells were stained with FITC/PI and detected by flow
cytometry. The positive area of PI indicated necrotic cells, the
positive area of AV and double positive area indicated apoptosis
cells. The apoptotic rate of cells was evaluated as follows:
Percentage of apoptotic cells = number of apoptotic cells / (number
of apoptotic cells + number of viable cells) × 100%. The mortality
rate of cells was evaluated as follows: Percentage of necrotic
cells = number of necrotic cells / (number of necrotic cells +
number of viable cells) × 100%.
Immunohistochemistry analysis
Caco-2 cells that had received treatment with 100,
200 or 400 µM H2O2, or hyperoxia at 37°C for
24 h were cultured on coverslips and fixed with 4% paraformaldehyde
at 37°C for 30 min. Fixed cells were subsequently treated with 10%
goat serum at 37°C for 30 min, and incubated with mouse anti-human
SC (1:1,000; catalogueno. I6635, Sigma-Aldrich; Merck KGaA) at 4°C
overnight, and was washed with PBS for 5 min (repeated three
times), and incubated with the working solution of secondary
antibody (biotin-labeled goat anti-mouse IgG, catalogue no.
SP-9002; ZSGB-BIO, Beijing, China) for 40 min at 37°C, according to
manufacturers instructions. The cells were stained with using a
diaminobenzidine kit (1:50; catalogue no. ZLI-9018; ZSGB-BIO, Inc.)
at room temperature for 1 min, counterstained with hematoxylin and
observed using a digital camera (Olympus Corp., Tokyo, Japan)
attached to a light microscope at a magnification of ×400. Primary
antibody was replaced with PBS as a negative control. Mean
absorbance values for SC protein were determined using Prism Graph
version 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA)
following scanning.
Western blot analysis
Proteins were extracted from Caco-2 cells according
to the manufacturer's instructions as follows: Caco-2 cells were
lysed in RIPA buffer (Beyotime Institute of Biotechnology,
Shanghai, China), then centrifuged at 14 × g and 4°C for 30 min.
Subsequently a BCA Protein Assay Kit (P0013C, Beyotime Institute of
Biotechnology) was used to determine the protein concentration
according to the manufacturer's instructions. Proteins (40 µg) were
loaded and separated by 8% SDS-PAGE (P0012A, Beyotime Institute of
Biotechnology) and transferred onto polyvinylidene fluoride
membranes. Membranes were incubated with Tris-buffer containing 5%
non-fat milk at room temperature for 2 h and probed with mouse
anti-human SC (1:1,000) or anti-GAPDH (1:10,000; catalogue no.
KC-5G5, Kangchen Bioengineering, Co., Ltd., Shanghai, China) at 4°C
overnight. The membranes were then washed by a Tris Buffered Saline
containing Tween-20 (TBST) at room temperature for 15 min (repeated
three times) and were incubated with a peroxidase-conjugated
secondary antibody (1:10,000; catalogue no. ZB-5305; ZSGB-BIO,
Inc.) at room temperature for 2 h. Then the membranes were washed
by TBST at room temperature for 15 min (repeated three times).
Subsequently, the membranes were incubated with an enhanced
chemiluminescent substrate (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and images were captured using a C300 gel imaging
system (Azure Biosystems, Inc., Dublin, CA, USA). Tanon 2500 Fully
Automatic Digital Gel Imaging system (Tanon Science &
Technology Co., Ltd., Shanghai, China) was used to scan images and
analyze densitometry values of SC protein normalized to GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from Caco-2 cells using
TRIzol Reagent (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's instructions. Total RNA (100 ng)
was reverse transcribed into cDNA using a SYBR Premix Ex
Taqkit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Levels of individual RNA transcripts
were quantified using qPCR. The following primers were used: SC,
forward 5′-GCTTGTCTCCCTGACCCTG-3′ and reverse
5′-AATGGCTTTGTTCTCAATCTC-3′; and GAPDH, forward
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′.
Primers and fluorescence probes for SC, and the internal reference
(GAPDH), were purchased from Takara Biotechnology Co., Ltd. PCR
conditions were as follows: Initial denaturation at 95°C for 10
sec, followed by 45 cycles of 95°C for 5 sec and 60°C for 20 sec,
followed by 1 min at 60°C and 5 sec at 95°C. The efficiency of
amplification for each target gene (GAPDH) was confirmed as 100% in
the exponential phase of PCR. The value of the relative mRNA
quantity for the control group was arbitrarily set to one for
normalization and the data were analyzed using the comparative Cq
method (2−ΔΔCq) (26).
The levels of mRNA in Caco-2 cells exposed to hyperoxia and
H2O2 were compared with that of the control
group.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). All statistical analyses were performed using SPSS version
20.0 software (IBM Corp., Armonk, NY, USA). A Student's t-test was
used to determine significant differences between treatment groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell apoptosis
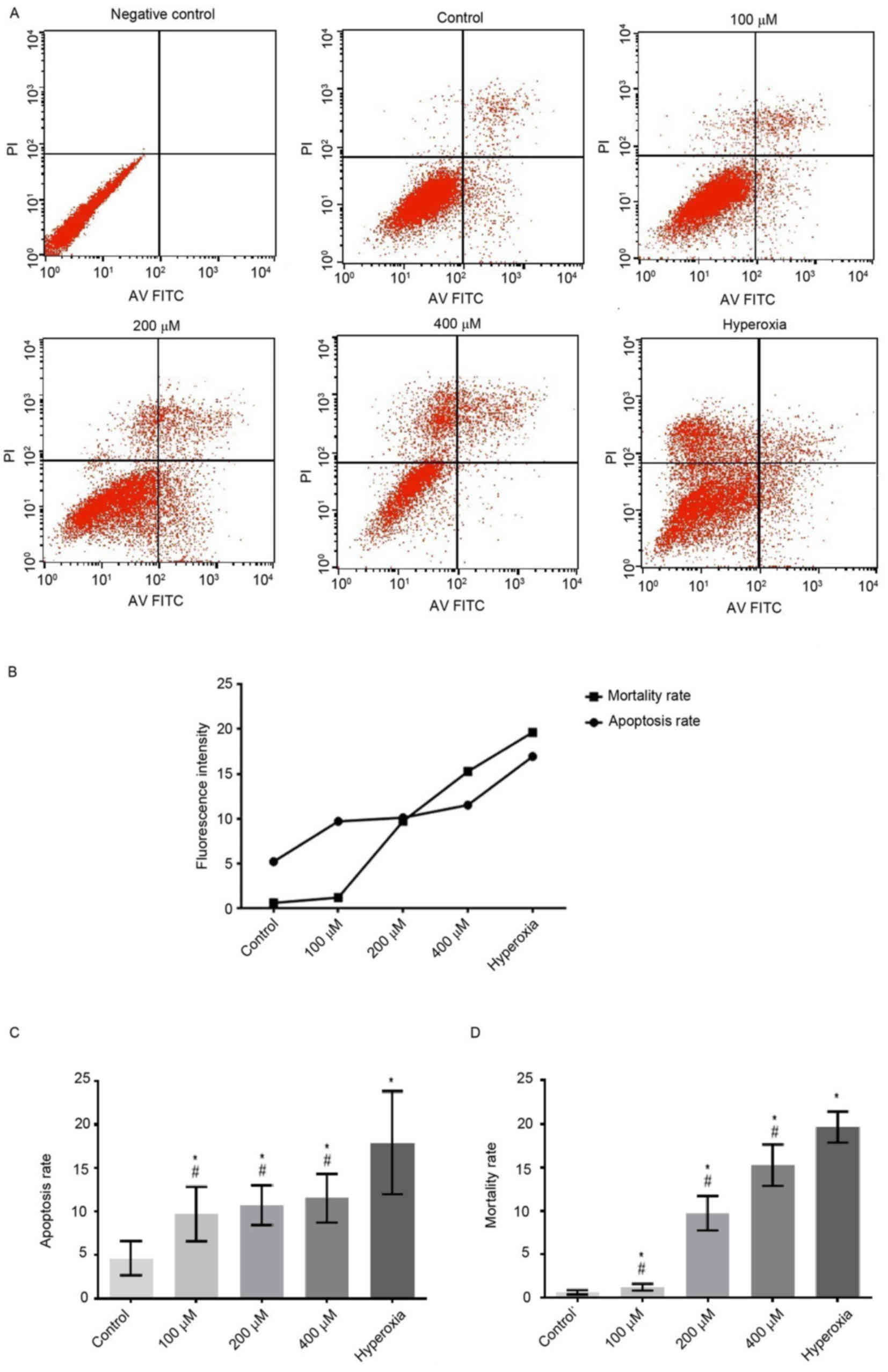
Cytotoxic effects of H2O2 are
typically attributed to the induction of cell apoptosis (5). The cytotoxicity of
H2O2 on Caco-2 cells was investigated in the
present study. Following treatment with H2O2
for 6 h, the apoptosis rates of cells exposed to 100, 200 and 400
µM H2O2 and hyperoxia were significantly
increased compared with that of the control group (P<0.05;
Fig. 1). Apoptotic rates were
increased in a H2O2 concentration-dependent
manner. In the hyperoxia group, the apoptotic rate was
significantly increased compared with those of the
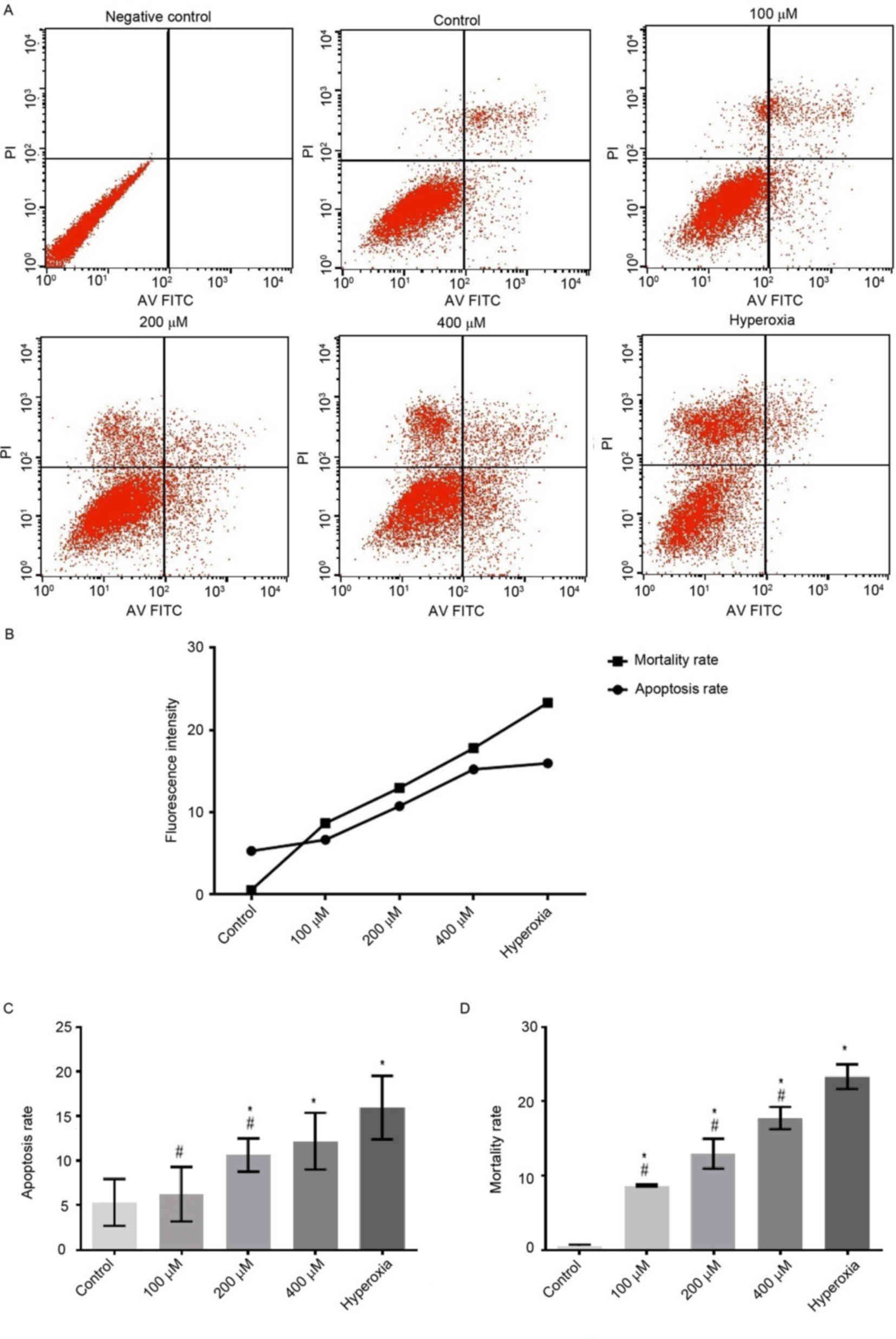
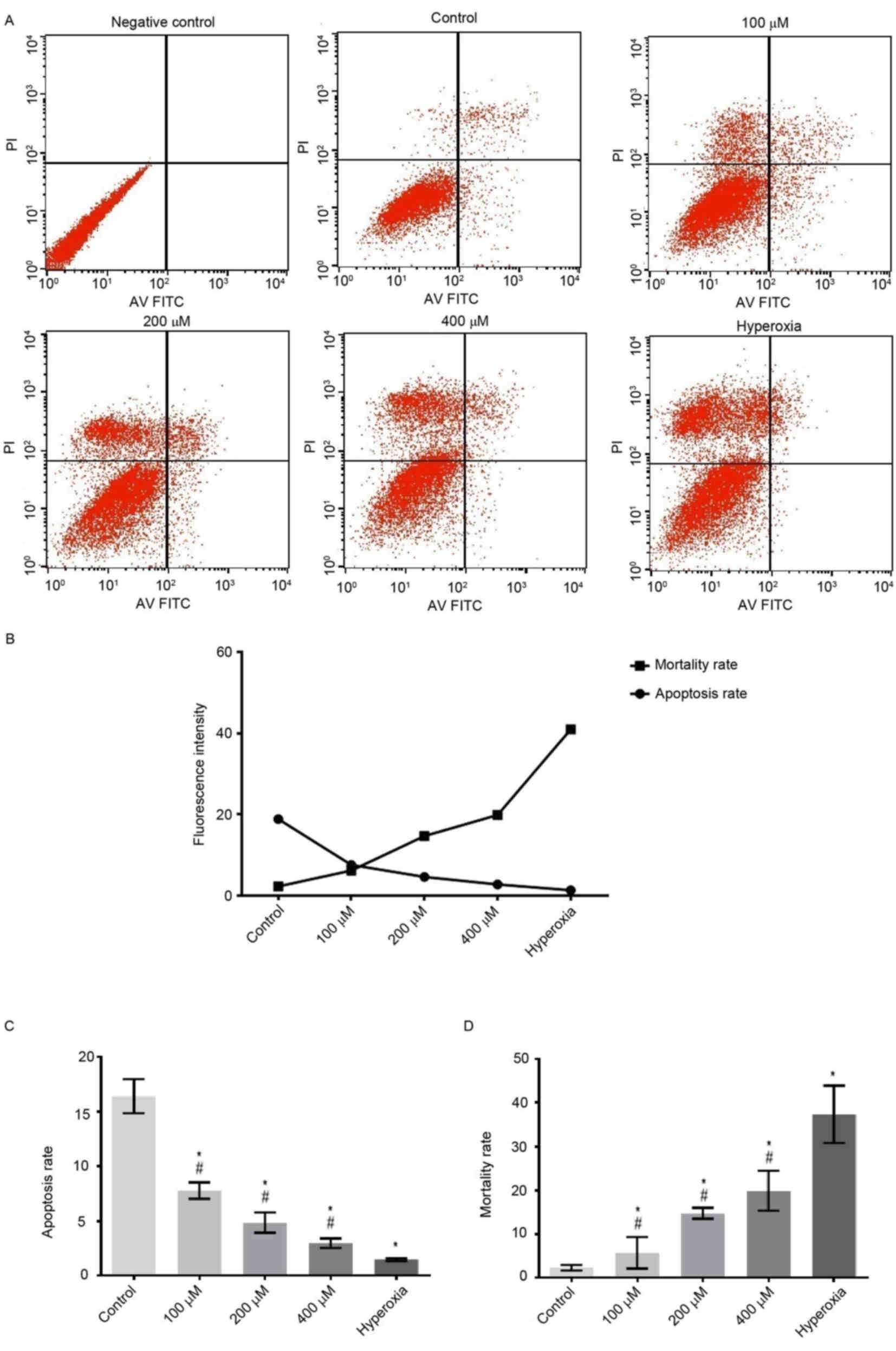
H2O2 groups (P<0.05; Figs. 1 and 2). However, the apoptotic rates of cells
exposed to 100, 200 and 400 µM H2O2 and
hyperoxia for 24 h were significantly decreased compared with the
control group (P<0.05; Fig.
3).
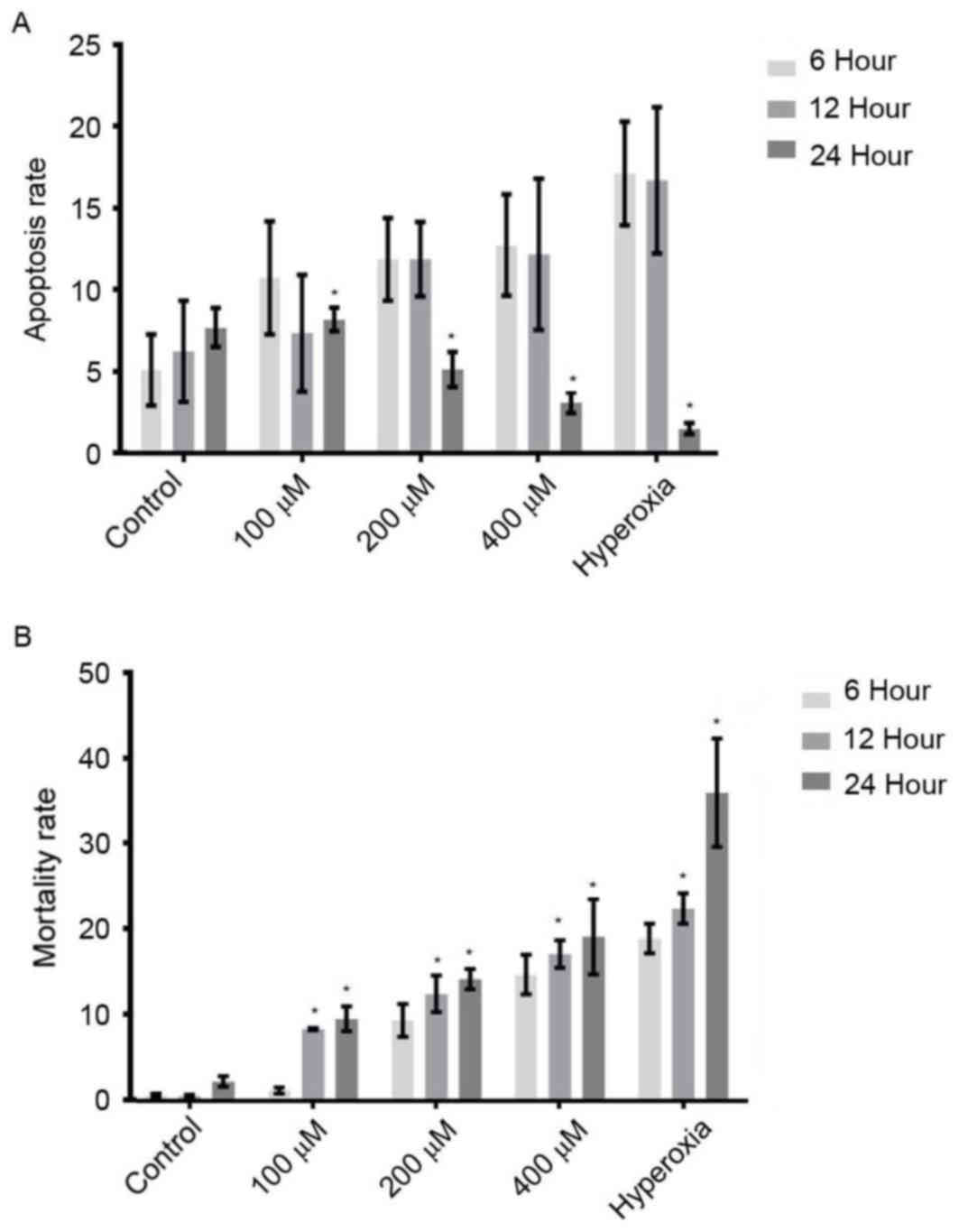
As indicated in Fig.
4A, the apoptosis rates of cells treated with various
concentrations of H2O2 for 6 h were
significantly increased when compared with those for 24 h
(P<0.05). Compared with cells treated for 6 h, the apoptotic
rates of H2O2- and hyperoxia-treated cells
for 12 h were not significantly different. As shown in Fig. 4B, with increasing ROS concentration
and duration of incubation, the mortality rates were gradually
increased. The mortality rates of the cells treated with
H2O2 and hyperoxia for 12 and 24 h were
significantly increased, as compared with 6 h (P<0.05; Fig. 4B). These results suggested that
increased ROS accelerated cell apoptosis in the early phase of
hyperoxia and accelerated cell death in the late phase of hyperoxia
(Fig 4B). These findings implied
that ROS might be responsible for cell injury during hyperoxia.
Immunohistochemical staining of
SC
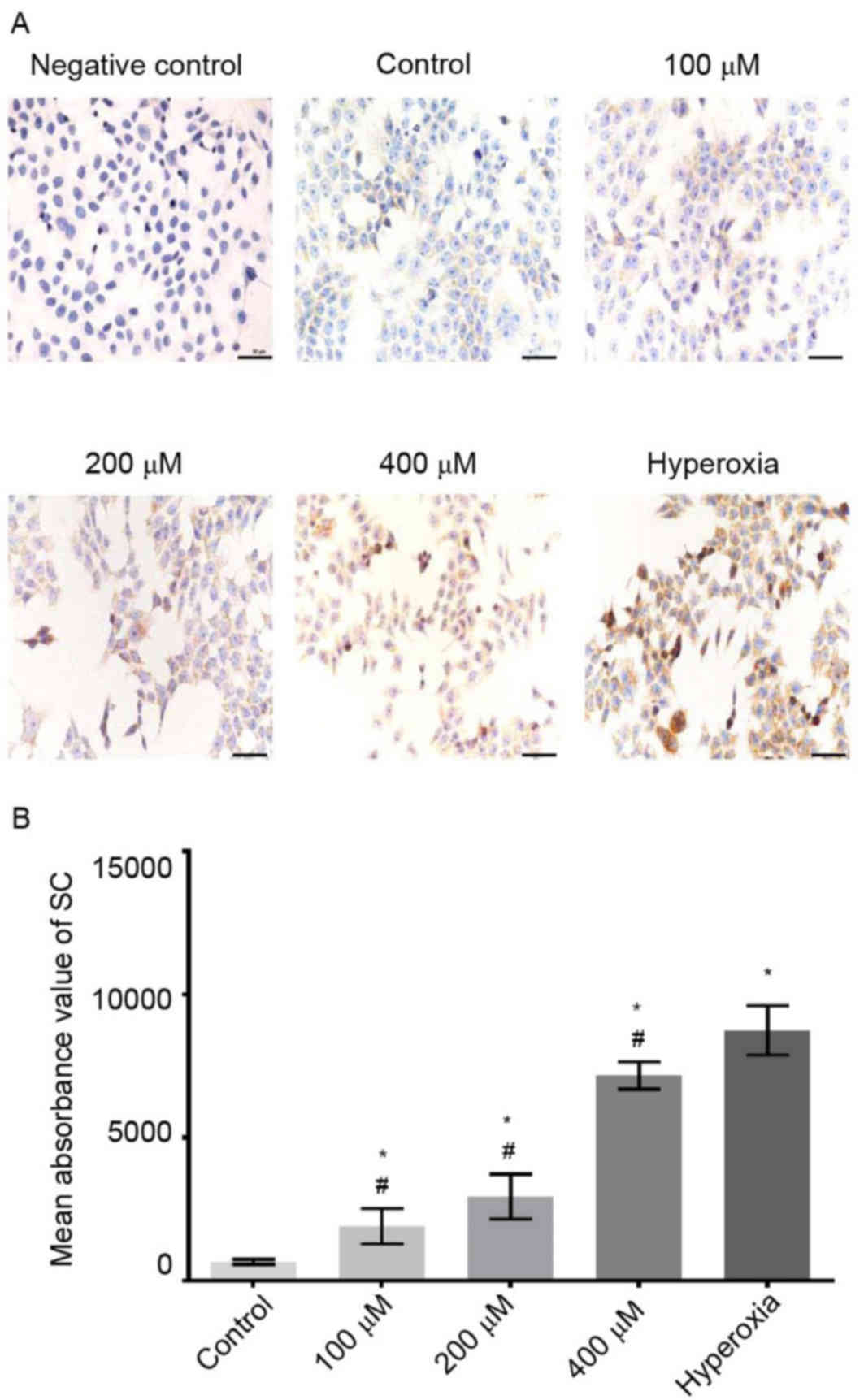
Immunostaining indicated that SC was mainly
localized on the cell membrane and in cytoplasm (Fig. 5A). Compared with the control group,
the SC expressions of cells exposed to 100, 200 and 400 µM
H2O2 were significantly increased. In the
hyperoxia group, the morphology of cells was changed, and the
expression of SC was significantly increased compared with those of
the control and H2O2 groups (P<0.05;
Fig. 5B).
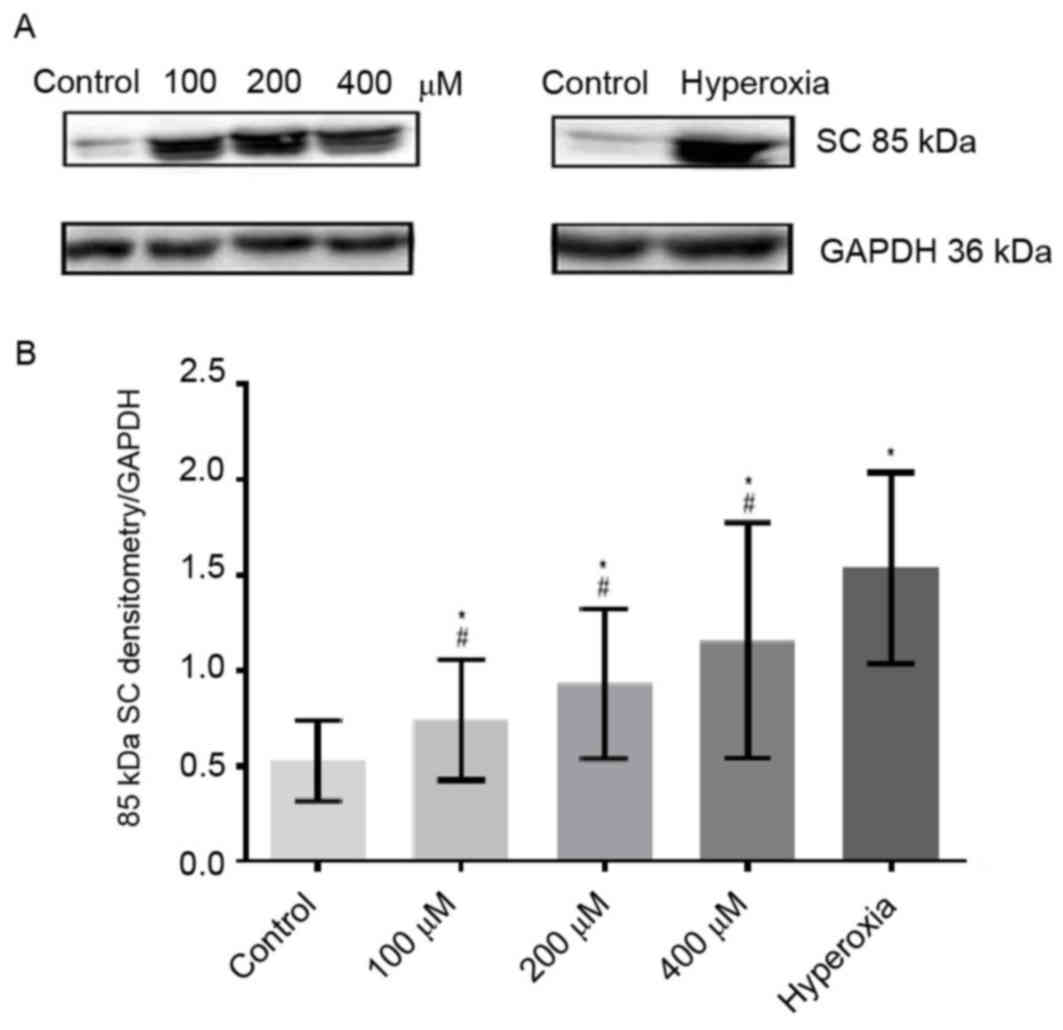
Protein expression levels of SC
Expression levels of SC protein (85 kDa; Fig. 6A) increased in a
H2O2 concentration-dependent manner. Notably,
the expression levels of SC were markedly increased in
hyperoxia-treated cells, as compared with the control (Fig. 6A). Densitometry analysis revealed
that SC levels in hyperoxia-treated cells were significantly
increased compared with those in the control (P<0.05) and
H2O2-treated cells (P<0.05; Fig. 6B).
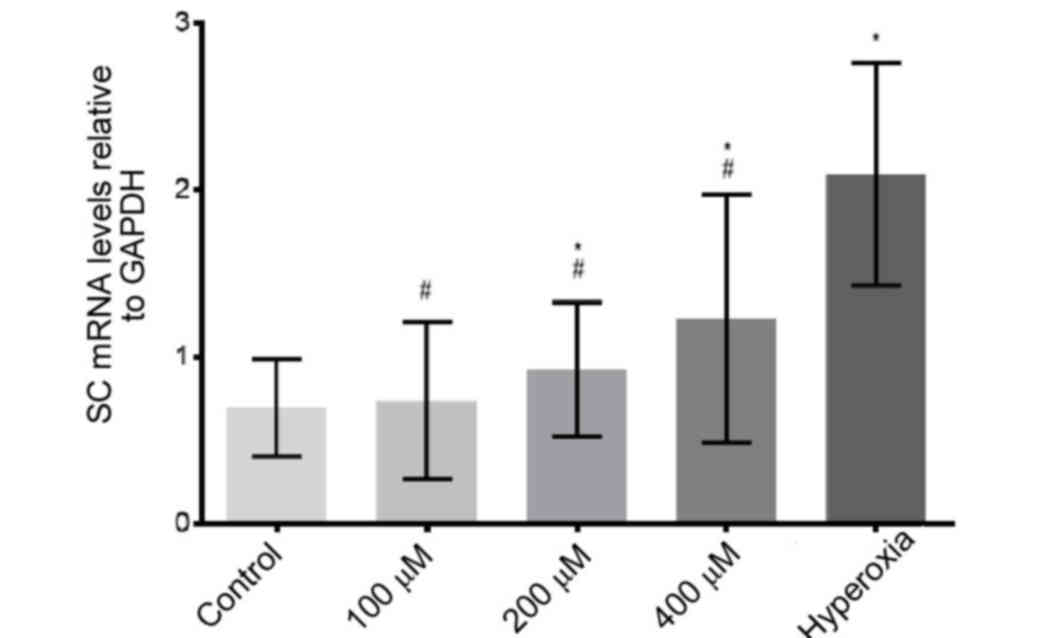
SC mRNA expression levels
Compared with the control group, the expression
levels of SC mRNA were significantly increased in the 200 µM, 400
µM H2O2 and hyperoxia groups (P<0.05;
Fig. 7). The mRNA expression levels
of SC increased in a H2O2 dose-dependent
manner. In hyperoxia-treated cells, SC mRNA expression levels were
significantly increased compared with
H2O2-treated cells (P<0.05; Fig. 7).
Discussion
The intracellular redox state is a key determinant
of cell fate (28). Oxidative stress
is a specific cellular stress that creates reactive species,
including free oxygen radicals (4).
ROS are generated in the mitochondria (29) and have a critical role in determining
the responsiveness of the cell to stress (30). ROS are important in facilitating
signal transduction processes within the cell (31) and are able to control cellular growth
and death (5). Maintaining balanced
intracellular ROS levels is a key factor in preventing
pathophysiological conditions (32).
ROS, as an important group of free radicals, exerts notable
oxidative and biological effects (33). During normal metabolism, ROS signals
cells to stimulate proliferation or to induce cellular damage,
depending on the concentration (34). Excessively generated ROS are
typically counteracted by ubiquitously expressed antioxidant
proteins (35). ROS are toxic to the
cell and are secondary messengers in intracellular signal
transduction (30). Excess ROS
production disrupts the redox balance and amplifies the
inflammatory responses via NF-κB (9).
H2O2 is the primary byproduct
of oxidative metabolism (36). A
small proportion of mitochondrial H2O2 is
produced by intracellular oxidative stress and has been associated
with accelerated ageing (5).
H2O2 is produced and released to impair redox
homeostasis during oxidative stress (35) and significantly increases the number
of free radicals in cells, which results in critical DNA damage
(10). As the levels of ROS
increase, H2O2-induced oxidative stress and
the rate of apoptosis gradually increases (37,38).
Therefore, we hypothesized that an increase in the concentration of
H2O2 affects cell apoptosis.
A previous study reported that ROS was associated
with apoptosis of cells (39). In
the present study, Caco-2 cells incubated for 6 and 12 h in the
presence of hyperoxia and at higher doses of
H2O2 exhibited a significantly increased
number of apoptotic cells. Compared with cells treated for 6 h,
apoptosis and mortality rates were significantly increased at 12 h.
Furthermore, the mortality rates of cells were significantly
increased during hyperoxia and at higher doses of
H2O2 for 24 h, as compared with cells treated
for 6 and 12 h. These findings suggested that the large number of
ROS produced might result in cell necrosis during prolonged
hyperoxia. In addition, increased ROS concentration and longer
incubation times resulted in increased apoptotic and mortality
rates. We therefore concluded that excessive ROS leads to cell
mortality.
pIgR is essential in intestinal defense against
pathogenic microbes (19). A
previous study indicated that upregulation of pIgR expression
increased the capacity of mucosal epithelial cells to transport
dimeric IgA (21). Additionally,
SIgA, IgA and SC induced by hyperoxia reflect local immunity in the
respiratory tract (14). Therefore,
the expression of SC protein under hyperoxia conditions or
treatment with H2O2 for 24 h was determined.
Increased oxidative stress induced by hyperoxia and
H2O2 resulted in a significant increase in
mRNA and protein expression levels of SC. Previous studies have
indicated that ROS induces cytokines during inflammation (40), and the increased expression of these
cytokines may contribute to the expression of IgA and SC (14). The observed increase in SC in the
present study suggested that intestinal inflammation might occur in
hyperoxia and that ROS might have an important role in such
inflammation.
In conclusion, ROS was indicated to cause the injury
of intestinal epithelial cells during hyperoxia. Future research on
the intestinal mechanisms of ROS-induced injury may demonstrate an
even greater role for ROS during intestinal inflammation. However,
novel strategies for treating hyperoxia-induced intestinal injury
require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30871158 and
81170604), the Education Department Foundation of Liaoning Province
(grant no. LK201620) and the Outstanding Scientific Fund of
Shengjing Hospital (grant no. MA66).
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
H2O2
|
hydrogen peroxide
|
|
IgA
|
immunoglobulin A
|
|
SC
|
secretory component
|
|
SIgA
|
secretory immunoglobulin A
|
|
pIgR
|
polymeric immunoglobulin receptor
|
References
|
1
|
Yee M, Buczynski BW and O'Reilly MA:
Neonatal hyperoxia stimulates the expansion of alveolar epithelial
type II cells. Am J Respir Cell Mol Biol. 50:757–766. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steer JH, Mann TS, Lo SZ, Inglis JJ, Yap
HS, Henry PJ and Joyce DA: Early induction of uncoupling protein-2
in pulmonary macrophages in hyperoxia-associated lung injury. Inhal
Toxicol. 25:544–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong Y, Sun LI, Sun R, Chen H, Yu Y and
Xie K: Combination therapy of molecular hydrogen and hyperoxia
improves survival rate and organ damage in a zymosan-induced
generalized inflammation model. Exp Ther Med. 11:2590–2596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chowdhury Roy S, Sengupta S, Biswas S,
Sinha TK, Sen R, Basak RK, Adhikari B and Bhattacharyya A:
Bacterial fucose-rich polysaccharide stabilizes MAPK-mediated
Nrf2/Keap1 signaling by directly scavenging reactive oxygen species
during hydrogen peroxide-induced apoptosis of human lung fibroblast
cells. PLoS One. 9:e1136632014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carnesecchi S, Deffert C, Pagano A,
Garrido-Urbani S, Métrailler-Ruchonnet I, Schäppi M, Donati Y,
Matthay MA, Krause KH and Argiroffo Barazzone C: NADPH oxidase-1
plays a crucial role in hyperoxia-induced acute lung injury in
mice. Am J Respir Crit Care Med. 180:972–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crane DD, Bauler TJ, Wehrly TD and Bosio
CM: Mitochondrial ROS potentiates indirect activation of the AIM2
inflammasome. Front Microbiol. 5:4382014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oparka M, Walczak J, Malinska D, van Oppen
LM, Szczepanowska J, Koopman WJ and Wieckowski MR: Quantifying ROS
levels using CM-H2DCFDA and HyPer. Methods. 109:3–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishibashi T: Molecular hydrogen: New
antioxidant and anti-inflammatory therapy for rheumatoid arthritis
and related diseases. Curr Pharm Des. 19:6375–6381. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L,
Zhu S and Xu J: Pre-protective effect of lipoic acid on injury
induced by H2O2 in IPEC-J2 cells. Mol Cell Biochem. 378:73–81.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhee SG: Cell signaling. H2O2, a necessary
evil for cell signaling. Science. 312:1882–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Choo-Wing R, Sun H, Sureshbabu A,
Sakurai R, Rehan VK and Bhandari V: A potential role of the JNK
pathway in hyperoxia-induced cell death, myofibroblast
transdifferentiation and TGF-β1-mediated injury in the developing
murine lung. BMC Cell Biol. 12:542011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikami Y, Iwase T, Komiyama Y, Matsumoto
N, Oki H and Komiyama K: Secretory leukocyte protease inhibitor
inhibits expression of polymeric immunoglobulin receptor via the
NF-κB signaling pathway. Mol Immunol. 67:568–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu DY, Jiang T, Wang S and Cao X: Effect
of hyperoxia on pulmonary SIgA and its components, IgA and SC. J
Clin Immunol. 33:1009–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Macpherson AJ, McCoy KD, Johansen FE and
Brandtzaeg P: The immune geography of IgA induction and function.
Mucosal Immunol. 1:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mostov KE and Deitcher DL: Polymeric
immunoglobulin receptor expressed in MDCK cells transcytoses IgA.
Cell. 46:613–621. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen FE and Kaetzel CS: Regulation of
the polymeric immunoglobulin receptor and IgA transport: New
advances in environmental factors that stimulate pIgR expression
and its role in mucosal immunity. Mucosal Immunol. 4:598–602. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bruno ME, Frantz AL, Rogier EW, Johansen
FE and Kaetzel CS: Regulation of the polymeric immunoglobulin
receptor by the classical and alternative NF-κB pathways in
intestinal epithelial cells. Mucosal Immunol. 4:468–478. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davids BJ, Palm JE, Housley MP, Smith JR,
Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svärd SG,
Gillin FD and Eckmann L: Polymeric immunoglobulin receptor in
intestinal immune defense against the lumen-dwelling protozoan
parasite Giardia. J Immunol. 177:6281–6290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perrier C, Sprenger N and Corthesy B:
Glycans on secretory component participate in innate protection
against mucosal pathogens. J Biol Chem. 281:14280–14287. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaetzel CS: The polymeric immunoglobulin
receptor: Bridging innate and adaptive immune responses at mucosal
surfaces. Immunol Rev. 206:83–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baylor AE, Diebel LN, Liberati DM,
Dulchavsky SA, Diglio CA and Brown WJ: The effects of varying
oxygen conditions and immunoglobulin A on barrier defense to
bacterial invasion. Am Surg. 69:231–237. 2003.PubMed/NCBI
|
|
23
|
Diebel LN, Liberati DM, Brown WJ,
Dulchavsky SA, Painter TM, Diglio CA and Montgomery PC: Secretory
immunoglobulin A blocks hypoxia-augmented bacterial passage across
Madin-Darby canine kidney cell monolayers. J Trauma. 43:759–763.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torbati D, Tan GH, Smith S, Frazier KS,
Gelvez J, Fakioglu H and Totapally BR: Multiple-organ effect of
normobaric hyperoxia in neonatal rats. J Crit Care. 21:85–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu DY and Li JJ: Effect of hyperoxia on
the intestinal IgA secretory component in neonatal rats and on
intestinal epithelial cells in vitro. Braz J Med Biol Res.
43:1034–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corthesy B: Role of secretory
immunoglobulin A and secretory component in the protection of
mucosal surfaces. Future Microbiol. 5:817–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Tan X, Liang J, Wu S, Liu J, Zhang
Q and Zhu R: A reduction in reactive oxygen species contributes to
dihydromyricetin-induced apoptosis in human hepatocellular
carcinoma cells. Sci Rep. 4:70412014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–L1028. 2000.PubMed/NCBI
|
|
30
|
Matsuzawa A and Ichijo H: Redox control of
cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase
pathway in stress signaling. Biochim Biophys Acta. 1780:1325–1336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woolley JF, Stanicka J and Cotter TG:
Recent advances in reactive oxygen species measurement in
biological systems. Trends Biochem Sci. 38:556–565. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang KA, Lee HC, Lee JJ, Hong MN, Park MJ,
Lee YS, Choi HD, Kim N, Ko YG and Lee JS: Effects of combined
radiofrequency radiation exposure on levels of reactive oxygen
species in neuronal cells. J Radiat Res. 55:265–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen HM and Liu ZG: JNK signaling pathway
is a key modulator in cell death mediated by reactive oxygen and
nitrogen species. Free Radical Biol Med. 40:928–939. 2006.
View Article : Google Scholar
|
|
34
|
Kajino-Sakamoto R, Omori E, Nighot PK,
Blikslager AT, Matsumoto K and Ninomiya-Tsuji J: TGF-beta-activated
kinase 1 signaling maintains intestinal integrity by preventing
accumulation of reactive oxygen species in the intestinal
epithelium. J Immunol. 185:4729–4737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujisawa T, Takeda K and Ichijo H: ASK
family proteins in stress response and disease. Mol Biotechnol.
37:13–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao J, Deng J, Lv L, Kang Q, Ma P, Yan F,
Song X, Gao B, Zhang Y and Xu J: Hydrogen peroxide induce human
cytomegalovirus replication through the activation of p38-MAPK
signaling pathway. Viruses. 7:2816–2833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma Z, Moruzzi N, Catrina SB, Grill V and
Björklund A: Hyperoxia inhibits glucose-induced insulin secretion
and mitochondrial metabolism in rat pancreatic islets. Biochem
Biophys Res Commun. 443:223–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin S, Ray RM and Johnson LR:
TNF-alpha/cycloheximide-induced apoptosis in intestinal epithelial
cells requires Rac1-regulated reactive oxygen species. Am J Physiol
Gastrointest Liver Physiol. 294:G928–G937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee MK, Lu Y, Di LQ and Xu HQ: Protection
of Tong-Sai-Mai Decoction against Apoptosis Induced by H2O2 in PC12
Cells: Mechanisms via Bcl-2-Mitochondria-ROS-INOS Pathway. Evid
Based Complement Alternat Med. 2014:3714192014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kina S, Nakasone T, Takemoto H, Matayoshi
A, Makishi S, Sunagawa N, Liang F, Phonaphonh T and Sunakawa H:
Regulation of chemokine production via oxidative pathway in HeLa
cells. Mediators Inflamm. 2009:1837602009. View Article : Google Scholar : PubMed/NCBI
|