Introduction
Ankylosing spondylitis (AS) is a kind of chronic
inflammatory disease which occurs in enthuses and spine with 2
basic characteristics, osteoporosis and ankylosis of axial joints
(1). Studies show that elevated
levels of inflammatory cytokines and matrix metalloproteinases
(MMPs) are observed in AS patients, such as tumour necrosis
factors, interleukins, MMP2, MMP3 and MMP8 (2–4).
Recently, signal pathway of receptor activator of nuclear factor-κB
ligand (RANKL)/osteoprotegerin (OPG) attracted scholars' attention.
It was reported that increased RANKL could mediate
osteoclastogenesis in AS patients or animal models (3). Moreover, bone resorption of skeleton
could be protected by secreting OPG via osteoblasts. As reported,
the balance of OPG and RANKL, as known as OPG/RANKL ratio, is
important for maintaining normal bone metabolism. It is widely
known that the OPG/RANKL ratio increases during the differentiation
process of osteoblasts. In addition, upregulation of OPG/RANKL
ratio has been well detected in AS patients (4–6).
Bromodomain and extra-terminal domain (BET) proteins
is a ‘histone reading protein’ class and epigenetic-related
proteins (3–5,7). It was
reported that BET proteins played important roles in inflammatory
bone resorption and osteoclastogenesis (3–5). In
addition, BET bromodomain inhibitor could reduce osteosarcoma cell
proliferation and osteoblastic differentiation (8–10). It
was also reported that I-BET151, a BET bromodomain inhibitor, could
regulate inflammatory factors in process of inflammatory diseases
(7). However, there was no report
focusing on the effects of BET inhibitors on the expression of
OPG/RANKL system related factors in AS.
This study was designed to investigate the effect of
I-BET151, a BET inhibitor, on the process of AS both in vivo
and in vitro by AS cell model and animal model. The
expression of RANKL, OPG, and MMPs including MMP3 and MMP9 in AS
patients and animal serum as well as cells were detected.
Materials and methods
AS patients
A total of 38 AS Chinese patients were recruited
from outpatient clinics at the Department of Orthopedics, Changhai
Hospital, Second Military Medical University (Shanghai, China). All
patients fulfilled the modified AS criteria of New York
classification (4) and another 38
sex- and age-matched healthy people were included In the present
study as a healthy control. The Bath AS Function Index (BASFI,
0=none and 10=worst) and the Bath AS Disease Activity Index
(BASDAI, 0=none and 10=worst) of AS patients were assessed
(11,12). The participants demographic and
disease characteristics were showed in Table I. Two inflammation markers including
erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)
levels in serum from participants were detected. All the
experiments performed In the present study followed the approval of
the Ethics Ccommittee of Changhai Hospital and written informed
consents were obtained from all participants.
 | Table I.The participants demographic and
disease characteristics of the study subjects. |
Table I.
The participants demographic and
disease characteristics of the study subjects.
| Characteristics | AS patients | Healthy controls |
|---|
| Male/female, no.
(%) | 30/8 (78.9) | 30/8 (78.9) |
| Age, years | 31.4±6.0 | 32.3±5.6 |
| Symptom duration,
years |
3.8±5.4 | NA |
| HLA-B27 positive, no.
(%) | 33
(86.8) | NA |
| BASFI |
3.0±3.1 | NA |
| BASDAI |
3.8±3.5 | NA |
| CRP, mg/l |
22.0±21.8 | 1.95±0.84 |
| ESR, mm/h |
23.9±18.6 | 4.63±1.25 |
Cells and treatment
A density of 2×105 cells/ml of human MG63
osteoblasts (American Type Culture Collection, Rockville, MD, USA)
were incubated in 6-well plates with DMEM (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 1%
penicillin-streptomycin and 10% FBS (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2
for 24 h. Then cells were subjected into 250 µl of serum from AS
patients in DMEM for another 48 h to induce AS cell model (4). For inhibition of RANKL/OPG system, MG63
cells were pretreated with 50, 100 and 200 ng/ml BET inhibitor
I-BET151 (GlaxoSmithKline, Brentford, UK) for 2 h before AS serum
addition (13,14). Each experiment was performed in
triplicate.
AS animal model
HLA-B27/β2 m transgenic AS Lewis rat model was
constructed as previously described (15,16). A
total of 20 AS rats were constructed and all animals (including
normal Lewis rats, n=10) were housed in standard conditions under a
12-h light/dark cycle with free access to food and water. For
I-BET151 treatment, 20 transgenic rats were intraperitoneally
administrated with 30 mg/kg of I-BET151 (n=10; GlaxoSmithKline) and
equal volume normal saline (n=10) once per day for 5 weeks
(10). At the end of 5 weeks, all
animals were anesthetized and 0.5 ml of blood samples were
collected before sacrifice. Animal procedures were performed
according to Institutional Standards for Human Care and Use of
Laboratory Animals.
ELISA assay
Blood samples collected from patients and animals
were put into tubes and centrifuged at 1000 × g for 10 min and
supernatants were collected for ELISA assay for RANKL, OPG, MMP3,
and MMP9 (R&D Systems, Inc., Minneapolis, MN, USA). ELISA
reader was used for absorption at 450 nm with batched controls.
Cellular RNA isolation and qPCR
Cellular RNA was extracted and the first-strand cDNA
was synthesized for mRNA expression levels detection using ABI 7500
Real-Time PCR software (Applied Biosystems, Carlsbad, CA, USA) by a
SYBR-Green PCR Master mix Kit (Applied Biosystems) according:
denaturation at 94°C for 4 min, 40 cycles of desaturating at 94°C
for 40 sec, annealing at 60°C for 30 sec and elongation at 70°C for
20 sec. Relative mRNA expression levels were calculated to
reference GAPDH gene using the 2−ΔΔCq method.
Western immunoblotting analysis
Cell lysates were prepared and separated using
SDS-PAGE (10%). Then proteins were transferred to PVDF membrane.
After being blocked with BSA, membranes were then incubated with
primary antibodies anti-RANKL (1:1,000; BD Transduction
Laboratories, San Jose, CA, USA), -OPG (1:1,000; Millipore Corp.,
Billerica, MA, USA), MMP3 (1:1,500; Millipore Corp.), MMP9
(1:1,000; BD Transduction Laboratories), GAPDH (1:2,000; BD
Transduction Laboratories) at 4°C overnight followed by incubation
with secondary antibodies for 1 h and subjection to ECL reagents
(Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) for
visualization of western blots.
Statistical analysis
Each experiment was performed in triplicate, and all
data are showed as the mean ± standard deviation. Differences
between groups and among groups were assessed using SPSS by
analysis of Student's t-test or ANOVA, respectively. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of RANKL, OPG, MMP3, MMP9
in AS patients
The demographic and disease characteristics of the
76 participants are listed in Table
I. Data showed that there was a higher AS accordance in man
than that of women, and a 33 AS patients among the 38 patients were
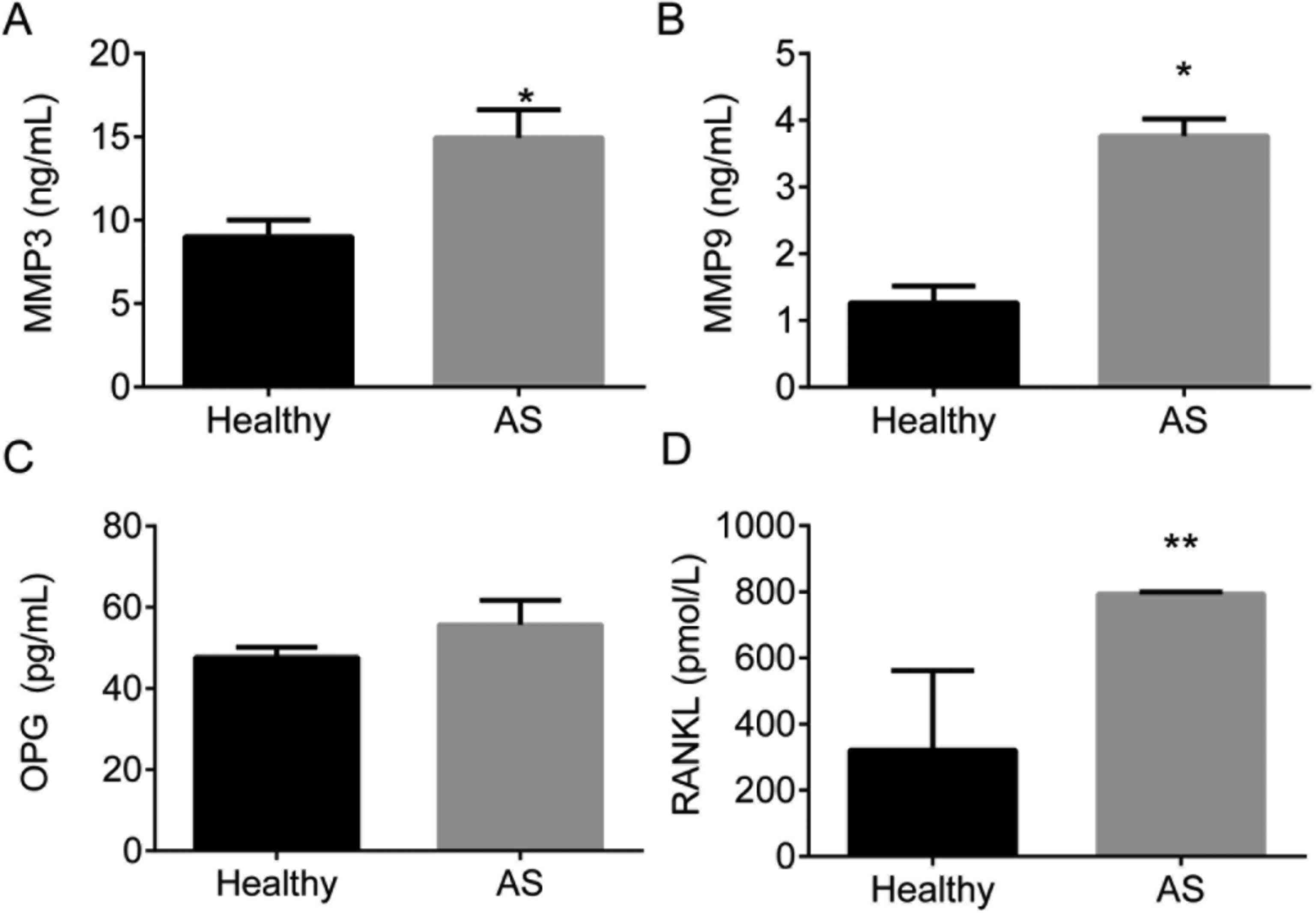
HLA-B27 positive (86.8%). Expression of RANKL, OPG, MMP3, and MMP9
in different groups was detected by ELISA and showed in Fig. 1. ELISA assay showed that the
expression of RANKL, MMP3, and MMP9 was significantly upregulated
in AS serum than those in healthy subjects, P<0.05. However, no
significant difference was observed in OPG in AS patients compared
with the healthy control, P<0.05.
I-BET151 inhibited AS serum induced
expression of RANKL, OPG, MMP3, MMP9 in MG63 cell model
We treated MG63 cells with AS serum to induce the AS
cell model in vitro. The expression of RANKL/OPG system
factors RANKL, OPG, MMP3, and MMP9 mRNA and proteins were detected.
The results showed that both the mRNA and proteins of RANKL, OPG,
MMP3, and MMP9 were significantly upregulated in AS serum induced
MG63 cells compared with the control, P<0.01 (Fig. 2A-D). However, MG63 cells pretreated
with I-BET151 showed significantly inhibitory effects on expression
of RANKL, OPG, MMP3, and MMP9 in both mRNAs and proteins levels
compared with MG63 AS model, with an dose-dependent manner. The
expression of RANKL, OPG, MMP3, and MMP9 mRNAs and proteins showed
the lowest expression levels in MG63 cells pretreated with 200
ng/ml I-BET151 than those of 50 and 100 ng/ml I-BET151, P<0.05.
These results demonstrated that I-BET151 suppressed the expression
of RANKL/OPG system.
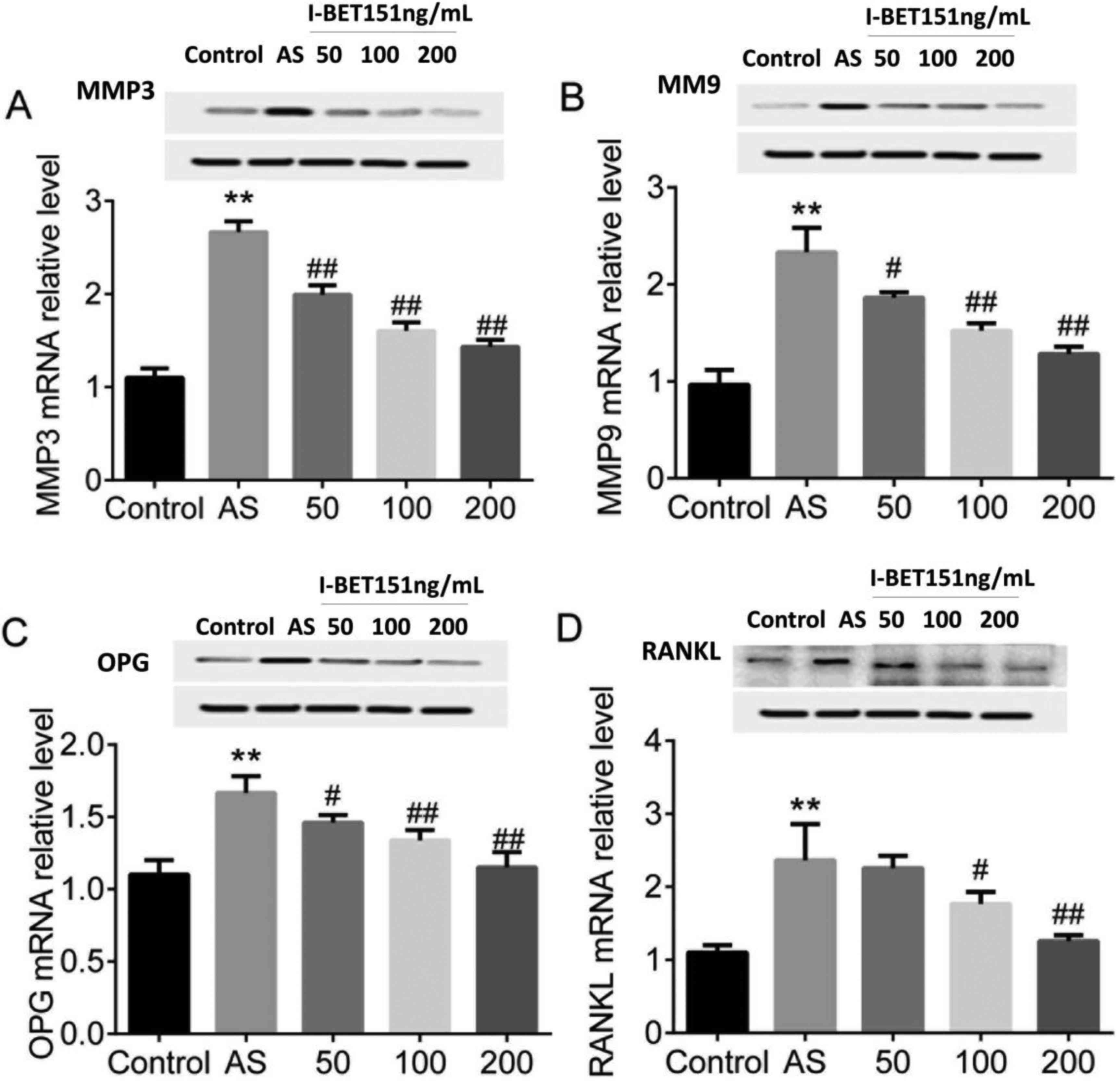 | Figure 2.Expression of MMP3, MMP9, OPG and
RANKL in AS MG63 cells. MG63 treated with AS serum were pretreated
with 50, 100 and 200 ng/ml I-BET151. mRNA and protein expression of
(A) MMP3, (B) MMP9, (C) OPG and (D) RANKL were detected using
quantitative polymerase chain reaction and western blot assay. **,
indicates difference at P<0.01, AS vs. control. # and
##, indicates difference at P<0.05 and P<0.01;
I-BET151 vs. AS. AS, ankylosing spondylitis; MMP, matrix
metalloproteinase; OPG, osteoprotegerin; RANKL, receptor activator
of nuclear factor-κB ligand. |
I-BET151 suppressed expression of
RANKL, OPG, MMP3, and MMP9 in AS rat model
We established the rat AS model using HLA-B27/β2 m
transgenic Lewis rats. Levels of RANKL, OPG, MMP3, and MMP9 in rat
serum were determines. The results showed that RANKL, OPG, MMP3,
and MMP9 were upregulated in AS rats compared with the control
rats, P<0.05 (Fig. 3). On the
contrary, AS rats treated with 30 mg/kg of I-BET151 for 5 weeks
showed significant inhibitory effects on levels of RANKL, OPG,
MMP3, and MMP9 compared with the AS model, P<0.05. These data
demonstrated that I-BET151 could inhibit AS induced expression of
RANKL, OPG, MMP3, and MMP9 in AS rats.
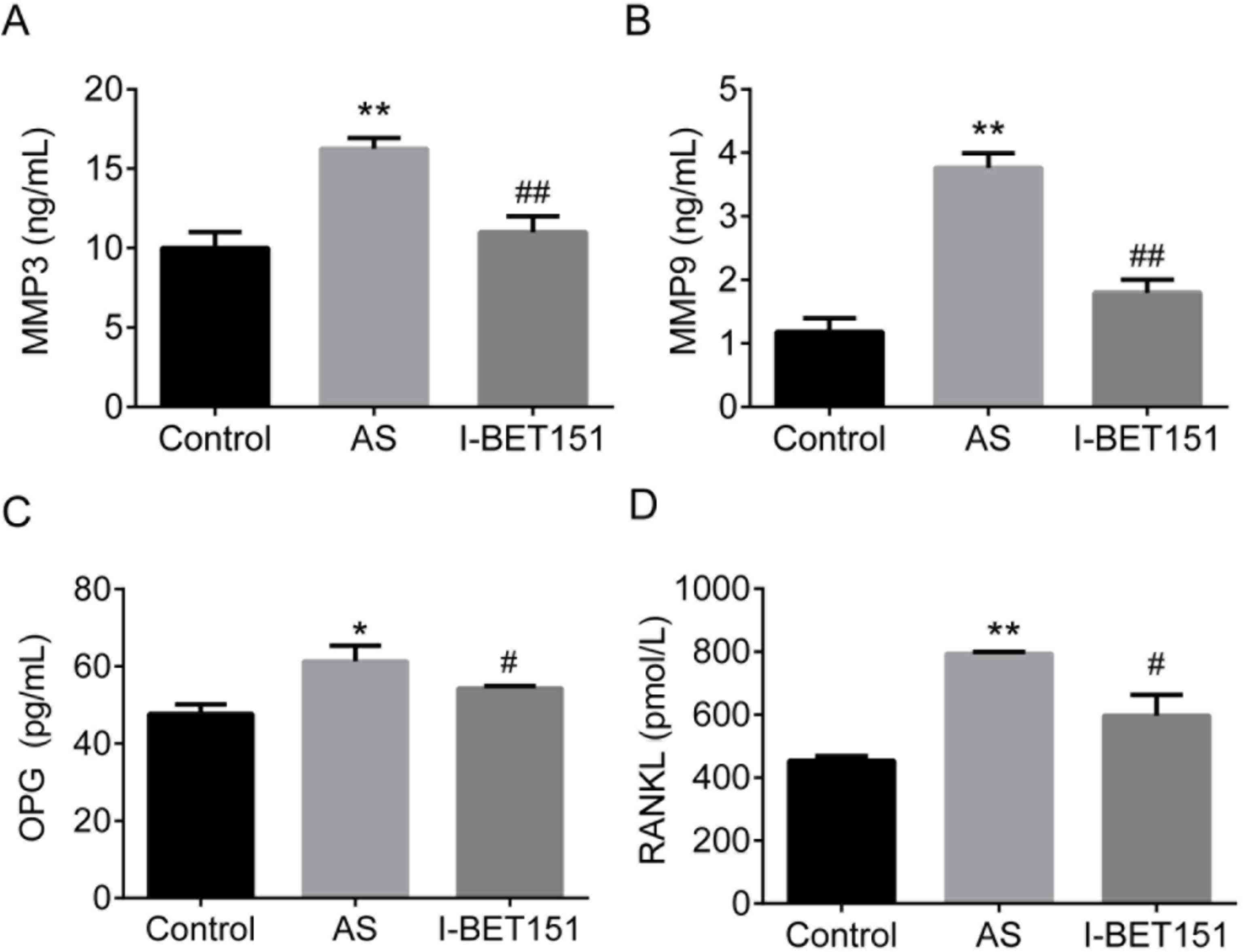 | Figure 3.Expression of (A) MMP3, (B) MMP9, (C)
OPG and (D) RANKL in AS rats. HLA-B27/β2 m transgenic Lewis rats
were treated with 30 mg/kg of I-BET151 for 5 weeks, and serum were
pretreated for ELISA. * and **, indicates difference at P<0.05
and P<0.01; AS vs. control. # and ##,
indicates difference at P<0.05, and P<0.01; I-BET151 vs. AS.
AS, ankylosing spondylitis; MMP, matrix metalloproteinase; OPG,
osteoprotegerin; RANKL, receptor activator of nuclear factor-κB
ligand. |
Discussion
Previous reports have demonstrated the upregulation
of MMPs, OPG, and RANKL in AS patients (2,3,6). However, to our best knowledge, there is
no study focusing on effects of BET and its inhibitors in AS up to
now. In the present study, we demonstrated the inhibitory effects
of a BET inhibitor, I-BET151, on expression of MMPs, OPG, and RANKL
in AS both in vivo and in vitro.
First we detected the expression of MMPs, OPG, and
RANKL in AS patients as well as healthy control. Results showed
that in AS patients all levels of above proteins increased. This
result was in consistent with other studies. It was reported that
RANKL and MMPs are inflammatory factors related to arthritis
category (6). Association between
RNKL expression and bone destruction was confirmed in inflammatory
joints using animal model and the inflammatory cytokines of
RANKL/OPG axis showed essential roles in AS process (4,17). In
addition, MMPs upregulation, especially for MMP3, has been detected
in AS serum and thus could be used as biomarkers for AS diagnosis
(6,18).
Then we used AS serum to induce AS model in
vitro and established AS rats model in vivo. Then we
determined expression of MMPs, OPG, and RANKL both in vitro
and in vivo. Results showed that all OPG, RANKL, MMP3 and
MMP9, were significantly upregulated in AS serum treated MG63 cells
as well as in HLA-B27/β2 m transgenic Lewis rats. However, when
treated with I-BET-151, all these effects induced by AS were
significantly inhibited.
The upregulation of RANKL and conflicting
dysregulation of OPG in AS serum have been reported in numerous
studies (6). Both the up-, down-,
and no-regulation of OPG had been reported in AS studies. However,
effect of BET inhibitor in AS is unknown.
The important role of BET bromodomain signaling in
osteosarcoma is widely accepted, and the inhibition of it can
suppresses growth of osteosarcoma tumour (8). Moreover, inhibition of BET protein
suppresses osteoblastogenesis as well as osteoclast differentiation
(19–21).
In the present study, we firstly demonstrated that
I-BET151, a BET inhibitor, could suppress the upregulation of
RANKL, OPG, MMP3, and MMP9 in AS serum treated MG63 cells and AS
rats, suggesting the potential of using I-BET151 as a therapeutic
strategy for AS both in vitro and in vivo.
In conclusion, In summary, we demonstrated the
upregulation of RANKL, OPG, MMP3, and MMP9 in AS in vitro
and in vivo. The administration of BET inhibitor I-BET151
suppressed the expression of RANKL, OPG, MMP3, and MMP9 in AS in a
dose-dependent manner. We concluded that I-BET151 might be used as
a potential therapeutic strategy for AS patients after more future
clinical studies.
References
|
1
|
Stupphann D, Rauner M, Krenbek D, Patsch
J, Pirker T, Muschitz C, Resch H and Pietschmann P: Intracellular
and surface RANKL are differentially regulated in patients with
ankylosing spondylitis. Rheumatol Int. 28:987–993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattey DL, Packham JC, Nixon NB, Coates L,
Creamer P, Hailwood S, Taylor GJ and Bhalla AK: Association of
cytokine and matrix metalloproteinase profiles with disease
activity and function in ankylosing spondylitis. Arthritis Res
Ther. 14:R1272012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siebuhr AS, Wang J, Karsdal M and
Bay-Jensen ACYJQZ: Matrix metalloproteinase-dependent turnover of
cartilage, synovial membrane, and connective tissue is elevated in
rats with collagen induced arthritis. J Transl Med. 10:1952012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Z, Lin D, Qi J, Qiu M, Lv Q, Li Q, Lin
Z, Liao Z, Pan Y, Jin O, et al: Serum from patients with ankylosing
spondylitis can increase PPARD, fra-1, MMP7, OPG and RANKL
expression in MG63 cells. Clinics (Sao Paulo). 70:738–742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raju R, Balakrishnan L, Nanjappa V,
Bhattacharjee M, Getnet D, Muthusamy B, Thomas Kurian J, Sharma J,
Rahiman BA, Harsha HC, et al: A comprehensive manually curated
reaction map of RANKL/RANK-signaling pathway. Database (Oxford).
2011:bar0212011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mou YK, Zhang PP, Li QX, Lin ZM, Liao ZT,
Wei QJ and Gu JR: Changes of serum levels of MMP-3, sRANKL, and OPG
in juvenile-onset ankylosing spondylitis patients carrying
different HLA-B27 subtypes. Clin Rheumatol. 34:1085–1089. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barrett E, Brothers S, Wahlestedt C and
Beurel E: I-BET151 selectively regulates IL-6 production. Biochim
Biophys Acta. 1842:1549–1555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamoureux F, Baud'huin M, Calleja
Rodriguez L, Jacques C, Berreur M, Rédini F, Lecanda F, Bradner JE,
Heymann D and Ory B: Selective inhibition of BET bromodomain
epigenetic signalling interferes with the bone-associated tumour
vicious cycle. Nat Commun. 5:35112014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaidos A, Caputo V, Gouvedenou K, Liu B,
Marigo I, Chaudhry MS, Rotolo A, Tough DF, Smithers NN, Bassil AK,
et al: Potent antimyeloma activity of the novel bromodomain
inhibitors I-BET151 and I-BET762. Blood. 123:697–705. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parkmin KH, Lim E, Lee MJ, Park SH,
Giannopoulou E, Yarilina A, van der Meulen M, Zhao B, Smithers N,
Witherington J, et al: Inhibition of osteoclastogenesis and
inflammatory bone resorption by targeting BET proteins and
epigenetic regulation. Nat Commun. 5:54182013. View Article : Google Scholar
|
|
11
|
Zochling J and Braun J: Assessments in
ankylosing spondylitis. Best Pract Res Clin Rheumatol. 21:699–712.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin A, Garrett S, Whitelock H, Kennedy
LG, O'Hea J, Mallorie P and Jenkinson T: A new approach to defining
functional ability in ankylosing spondylitis: The development of
the Bath Ankylosing Spondylitis Functional Index. J Rheumatol.
21:2281–2285. 1994.PubMed/NCBI
|
|
13
|
Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou
M, Gu C, Zeng X, Yu L, et al: Sinomenine suppresses osteoclast
formation and Mycobacterium tuberculosis H37Ra-induced bone loss by
modulating RANKL signaling pathways. PLoS One. 8:e742742013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He L, Duan H and Li X, Wang S, Zhang Y,
Lei L, Xu J, Liu S and Li X: Sinomenine down-regulates TLR4/TRAF6
expression and attenuates lipopolysaccharide-induced
osteoclastogenesis and osteolysis. Eur J Pharmacol. 779:66–79.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin P, Bach M, Asquith M, Lee AY,
Akileswaran L, Stauffer P, Davin S, Pan Y, Cambronne ED, Dorris M,
et al: HLA-B27 and human β2-microglobulin affect the gut microbiota
of transgenic rats. PLoS One. 9:e1056842014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rysnik O, McHugh K, van Duivenvoorde L,
van Tok M, Taurog J, Kollnberger S, Baeten D and Bowness P: Data
showing non-conventional HLA-B27 expression in axial joints and gut
tissue from B27 transgenic rats, and in frozen and paraffin-fixed
synovial SpA tissue. Data Brief. 9:100–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atkinson SM, Bleil J, Maier R, Kühl AA,
Thorn M, Serikawa K, Fox B, Kruse K, Haase C, Skov S, et al:
Anti-RANKL treatment inhibits erosive joint destruction and lowers
inflammation but has no effect on bone formation in the
delayed-type hypersensitivity arthritis (DTHA) model. Arthritis Res
Ther. 18:282016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reveille JD: Biomarkers for diagnosis,
monitoring of progression, and treatment responses in ankylosing
spondylitis and axial spondyloarthritis. Clin Rheumatol.
34:1009–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baud'huin M, Lamoureux F, Jacques C,
Calleja Rodriguez L, Quillard T, Charrier C, Amiaud J, Berreur M,
Brounais-LeRoyer B, Owen R, et al: Inhibition of BET proteins and
epigenetic signaling as a potential treatment for osteoporosis.
Bone. 94:10–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shaw AT and Gravallese EM: Mediators of
inflammation and bone remodeling in rheumatic disease. Semin Cell
Dev Biol. 49:2–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Im CH, Kang EH, Ki JY, Shin DW, Choi HJ,
Chang EJ, Lee EY, Lee YJ, Lee EB, Kim HH and Song YW: Receptor
activator of nuclear factor kappa B ligand-mediated
osteoclastogenesis is elevated in ankylosing spondylitis. Clin Exp
Rheumatol. 27:620–625. 2009.PubMed/NCBI
|