Introduction
Lung cancer is the most commonly diagnosed cancer
and the leading cause of cancer-associated mortality worldwide
(1). Non-small cell lung cancer
(NSCLC) is the predominant form of lung cancer, accounting for
>80% of all lung cancer cases (2). There have been numerous improvements in
surgical treatment and chemotherapy; however there has no
corresponding improvement in the survival rate of NSCLC patients
over the past 10 years (3).
Therefore, studies into novel therapeutic targets and strategies to
treat patients with NSCLC are warranted. The 14-3-3 protein family
consists of at least seven isoforms, namely β, ε, γ, η, σ, τ/θ and
ξ, which are found in mammalian cells (4). A number of important signaling events,
including cell proliferation and survival are regulated by 14-3-3
proteins (5,6). This is important, as enhanced cell
proliferation and survival are key characteristics of cancer cells
(7). Overexpression of 14-3-3
proteins has been detected in many types of human cancer such as
pancreatic cancer (8), lung
adenocarcinoma (9), breast tumor
(10) and is correlated with more
aggressive tumors and poor prognosis (10,11). In
lung cancer, it has been demonstrated that the 14-3-3 isoforms β,
γ, σ and θ are overexpressed in tumor tissue compared with normal
tissues (4,12). Among these, 14-3-3σ has been
indicated to be elevated in NSCLC as a result of 14-3-3σ DNA
hypomethylation (13). The results
of a recent study also suggested that 14-3-3σ expression was
correlated with cisplatin resistance in NSCLC cells (14).
Long non-coding RNAs (lncRNAs) serve key regulatory
roles in cellular and biochemical processes (15). Regarding lung cancer, the lncRNA HOX
transcript antisense RNA (HOTAIR) has been demonstrated to repress
gene expression and promote proliferation, survival, metastasis,
invasion and drug resistance in lung cancer cells (15). The expression of HOTAIR may also be
elevated in lung cancer and correlate with cancer metastasis and
poor patient prognosis (16).
Furthermore, a recent study observed that HOTAIR was overexpressed
in metastatic lung cancer tissue, suggesting that HOTAIR may be
associated with the invasion and progression of lung cancer
(17).
Collectively, the results from these previous
studies suggest that both 14-3-3σ and HOTAIR are involved in the
tumorigenesis and progression of lung cancer. Therefore, the
current study, investigated the association between 14-3-3σ and
HOTAIR in NSCLC.
Materials and methods
Tissue samples
Non-cancerous lung and NSCLC tissues were collected
from 54 NSCLC patients (mean age, 59.46±10.05) undergoing surgical
treatment at the Second Xiangya Hospital of Central South
University (Changsha, China) from June 2010 to April 2014. All
patients had been diagnosed with NSCLC (stage I, II and III) based
on histopathological evaluation (18). Clinicopathological characteristics
including tumor-node-metastasis (TNM) staging were collected
(19). No local or systemic
treatment was conducted in these patients prior to surgery. All
tissue samples were immediately frozen in liquid nitrogen prior to
use in the current experiments. The clinicopathological
characteristics of patients in the current study are presented in
Table I. The present study was
approved by the Ethics Committee of the Second Xiangya Hospital and
all patients provided written informed consent.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Number | % |
|---|
| Sex |
| Male | 44 | 81.5 |
|
Female | 10 | 18.5 |
| Smoker |
| Yes | 34 | 63.0 |
| No | 20 | 37.0 |
| Cancer type |
|
Adenocarcinoma | 21 | 38.9 |
| Squamous
cell carcinoma | 33 | 61.1 |
| Differentiation |
|
Low-Moderate | 49 | 90.7 |
| High | 5 | 9.3 |
| Tumor T stage |
| T1 | 5 | 9.3 |
| T2 | 45 | 83.3 |
| T3 | 4 | 7.4 |
| Tumor N stage |
| N0 | 15 | 27.8 |
|
N1-N3 | 39 | 72.2 |
| Clinical stage |
| I–II | 33 | 61.1 |
| III | 21 | 38.9 |
Cell culture
The human NSCLC cell line PC9 was purchased from the
Chinese Academy of Sciences (Shanghai, China). PC9 cells were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
(Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA). Cell cultures were
incubated in a humidified atmosphere of 5% CO2 at 37°C.
For transfection experiments, PC9 cells were seeded
1×104 per well in 6-wells plate.
Plasmids and transient
transfection
The plasmids were described in our previous paper
(20). Briefly, the HOTAIR
expression plasmid pLZRS-HOTAIR was provided by Dr Howard Chang
(Stanford University, Stanford, CA, USA) (16) and the HOTAIR coding region was
subcloned into the retroviral vector pLVX-EF1α-IRES-Puro (pLZRS;
Clontech Laboratories, Inc., Mountainview, CA, USA). HOTAIR shRNA
vectors (GV248; HOTAIR-shRNA 1/2) and control shRNA (NC-sh) were
purchased from GeneChem Co., Ltd., Shanghai, China. Transfection of
plasmids was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Stable shRNA expressing
colonies were selected using puromycin. The experiments included 5
groups: Overexpressed control (pLZRS), HOTAIR (pLZRS-HOTAIR),
downregulated control (NC-shRNA), shHOT-1 and shHOT-2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from PC9 cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.). A total of 1 µg mRNA was
reverse transcribed into cDNA using SuperScript II reverse
transcriptase (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, and the reaction product was treated with
ezDNase (Thermo Fisher Scientific, Inc.). qPCR was performed on an
Abi-Prism 7700 Sequence Detection System using the SYBR-Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) to
determine the relative expression levels of target genes, according
to the manufacturer's protocol. β-actin was used as the reference
and normalization control. The following cycling conditions were
performed: Holding stage, 1 cycle of 50°C for 2 min and 95°C for 10
min; cycling stage, 40 cycles of 95°C for 15 sec and 60°C for 60
sec; and melt curve stage, 95°C for 15 sec, 60°C for 60 sec, 95°C
for 30 sec and 60°C for 15 sec. The primers used were as follows:
HOTAIR, forward 5′-GCAGTGGAATGGAACGGATT-3′ and reverse
5′-CGTGGCATTTCTGGTCTTGTA-3′; 14-3-3σ, forward
5′-TCCGTCTTCCACTACGAGAT-3′ and reverse 5′-TGATGAGGGTGCTGTCTTTG-3′;
and β-actin, forward 5′-GCACCACACCTTCTACAATGAG-3′ and reverse
5′-GATAGCACAGCCTGGATAGCA-3′. Levels of target mRNA were quantified
using the ΔΔCq method (21) and normalized against that of β-actin
in the same sample. RT-qPCR was repeated in duplicate in three
independent experiments.
Western blot analysis
PC9 cells were lysed with radioimmunoprecipitation
assay buffer (Beyotime Biotechnology, Haimen, China) added with
protease inhibitor cocktail (cOmplete ULTRA tablets; Roche
Diagnostics GmbH, Mannheim, Germany) then incubated on ice for 30
min prior to removal of cell debris by centrifugation at 2,000 × g
for 15 min at 4°C. The protein concentration of resulting lysates
was determined using a BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Equal amounts of protein (20 µg) from each sample were loaded and
separated using 10% SDS-PAGE and blotted onto polyvinylidene
difluoride microporous membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk in TBS
Tween-20 (TBST; 25 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Tween-20)
for 2 h at room temperature, then incubated for overnight at 4°C
with a 1:500 dilution of mouse anti-human 14-3-3σ monoclonal
antibody (cat. no. ab76532; Abcam, Cambridge, MA, USA) and 1:5,000
dilution of mouse anti-human β-actin (cat. no. 60008-1-Ig; Wuhan
Sanying Biotechnology, Wuhan, China). After washing three times
(each time for 10 min) with TBST, membranes were then incubated
with a 1:5,000 dilution of bovine anti-mouse secondary antibody
conjugated to horseradish peroxidase (cat. no. sc-2371; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
Peroxidase labeling was detected using a GE Healthcare enhanced
chemiluminescence kit (GE Healthcare Life Sciences, Shanghai,
China) and quantified by using the Moecular Imager ChemiDoc XRS
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin was
used for loading control. The band intensities were semiquantified
by densitometry using Quantity-One software v4.62 (Bio-Rad
Laboratories, Inc.) and a ChemiDoc XRS System. Three independent
experiments were performed.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Student's t-tests were used to evaluate significant difference
between any two groups of data. All data are represented as mean ±
standard deviation. Correlation analyses were performed with
Spearman's correlation analysis test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HOTAIR and 14-3-3σ are upregulated in
NSCLC tissues
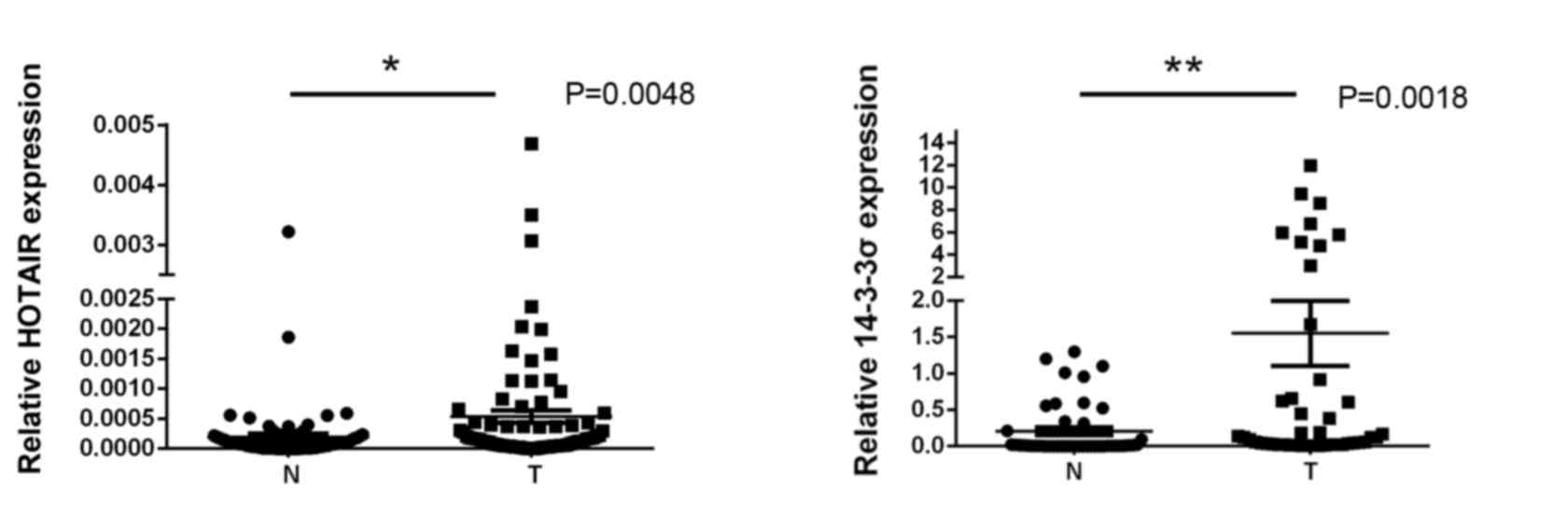
NSCLC tumor and adjacent non-cancerous lung tissues
were isolated from 54 NSCLC patients, and levels of HOTAIR and
14-3-3σ expression were measured using RT-qPCR. Expression of
HOTAIR and 14-3-3σ in NSCLC tissues was significantly higher than
in adjacent non-cancerous lung tissues (0.0047±0.0011 vs.
0.00018±0.00003 and 1.32±0.56 vs. 0.12±0.0035, respectively; both
P<0.05; Fig. 1). Spearman's
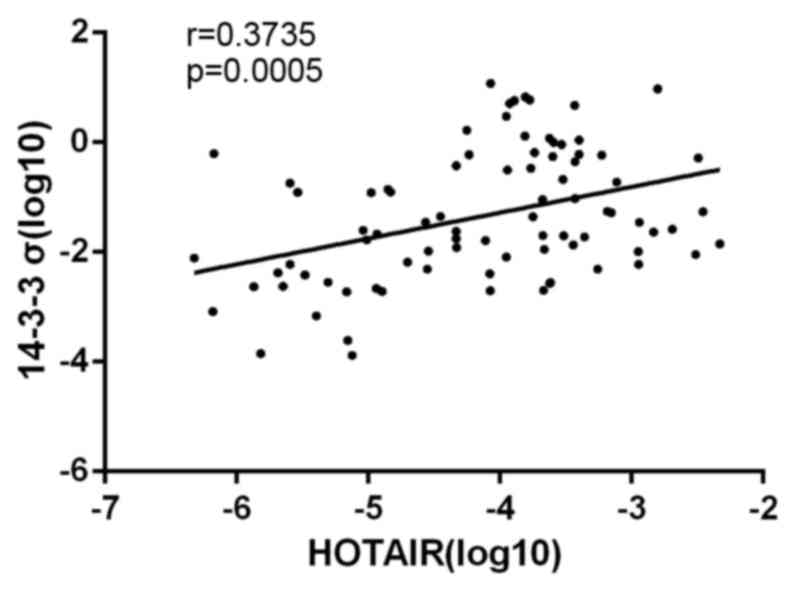
correlation analysis also demonstrated that levels of HOTAIR and
14-3-3σ in NSCLC tissues were significantly correlated (r=0.3735;
P=0.0005; Fig. 2), indicating a
regulatory relationship between HOTAIR and 14-3-3σ expression.
HOTAIR promotes the expression of
14-3-3σ in NSCLC cells
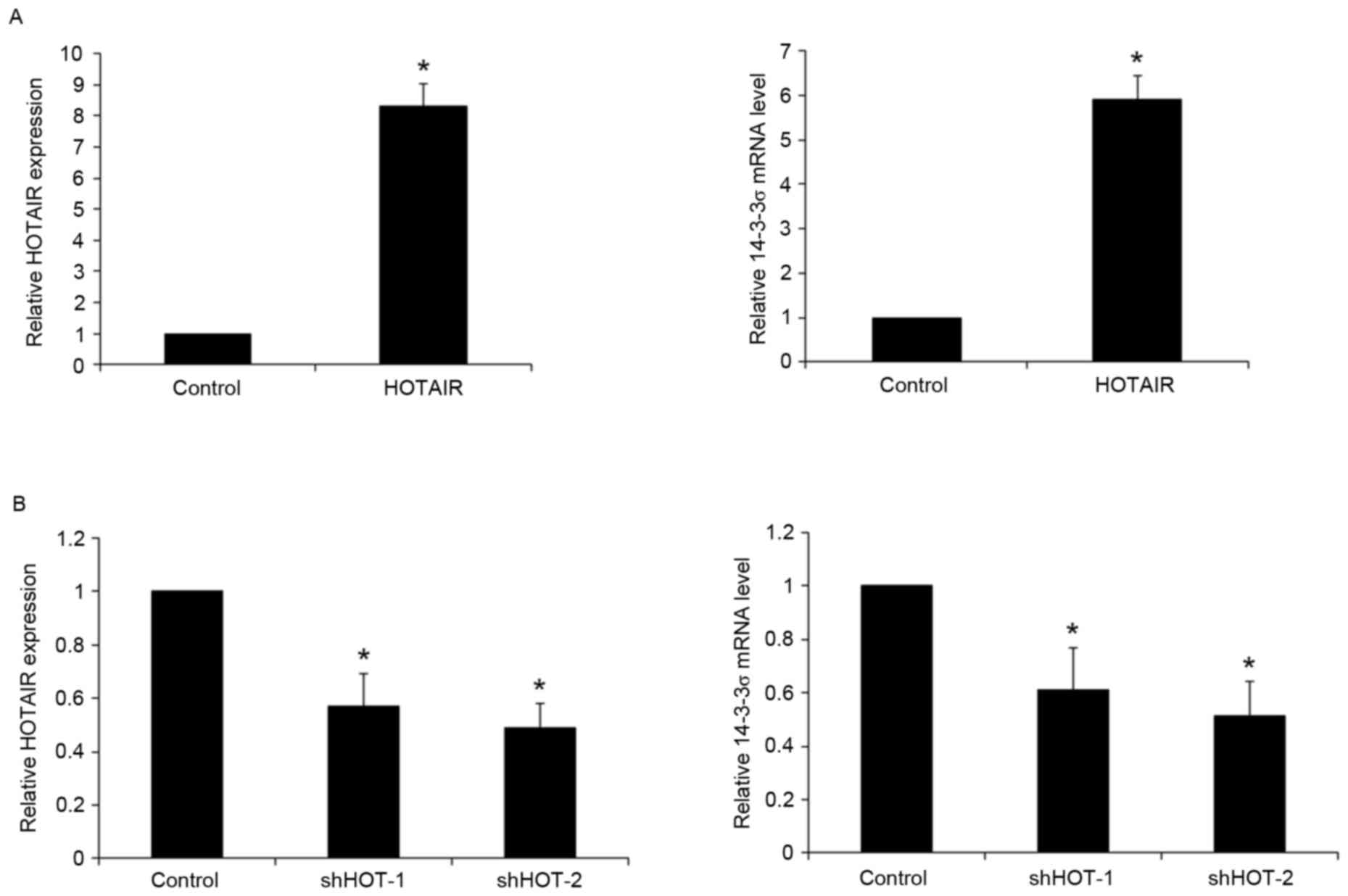
To determine the potential regulatory effects of
HOTAIR on 14-3-3σ expression, HOTAIR was overexpressed and
knocked-down in human NSCLC PC9 cells. Transfection of PC9 cells
with a HOTAIR expression vector significantly increased the
expression of HOTAIR by >8-fold, relative to blank plasmid
control (P<0.05). This overexpression of HOTAIR led to a
significant 5.9-fold increase in 14-3-3σ mRNA expression in PC9
cells (P<0.05; Fig. 3A). Compared
with shRNA control, transfection of PC9 cells with two HOTAIR shRNA
expression vectors (HOTAIR-shRNA 1/2) significantly decreased the
expression of HOTAIR by 0.43 and 0.51-fold respectively (both
P<0.05), which led to a 0.39- and 0.48-fold significant decrease
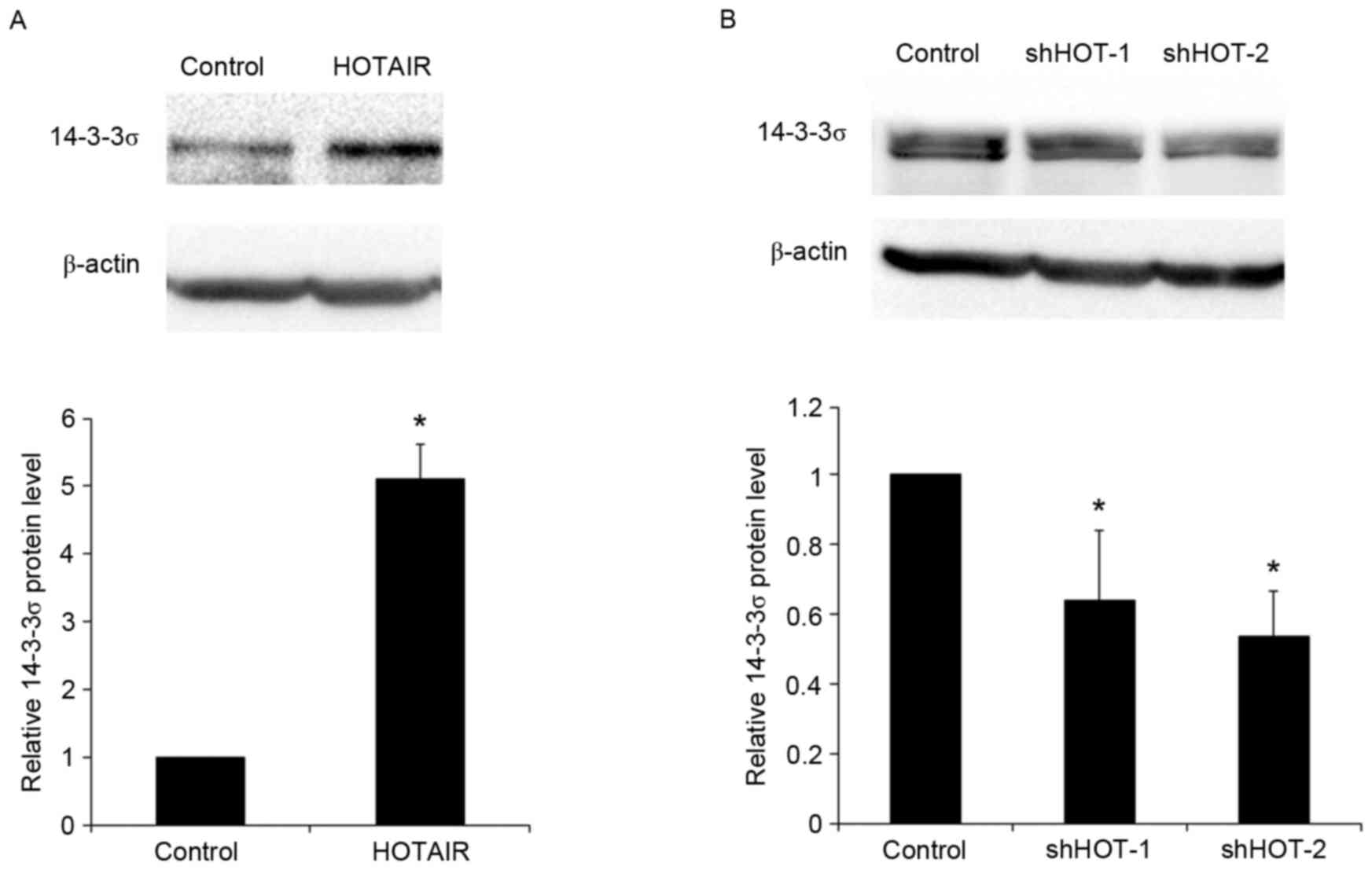
in 14-3-3σ mRNA expression (both P<0.05; Fig. 3B). These results were confirmed by
western blot analysis. Overexpression of HOTAIR significantly
increased the levels of 14-3-3σ protein by 5.1-fold in PC9 cells
(P<0.05), while knockdown of HOTAIR with HOTAIR shRNA 1/2
vectors significantly reduced 14-3-3σ levels by approximately 0.36-
and 0.46-fold, respectively (both P<0.05; Fig. 4). The results indicate that HOTAIR
promotes the expression of 14-3-3σ mRNA and protein in NSCLC
cells.
Discussion
The present study provided the first evidence to
suggest that HOTAIR promotes the expression of 14-3-3σ in NSCLC.
14-3-3 proteins bind to numerous phospho-client proteins within
cells, thus regulating key signaling events. In particular, 14-3-3
proteins regulate the Raf mitogen-activated protein kinase and
phosphoinositide-3-kinase-Akt signaling pathways (5,6), which
are considered to be critical mediators of tumorigenesis and cancer
progression (7). Recent studies have
demonstrated that 14-3-3σ is overexpressed in lung cancer compared
with adjacent normal lung tissue, and may be involved in
determining the malignancy of lung cancer (12,13).
Analogous to previous results, the present study detected that
expression of 14-3-3σ in NSCLC tissues was significantly higher
than in adjacent normal lung tissues. Previous studies have
demonstrated that HOTAIR promotes proliferation, survival,
invasion, metastasis and drug resistance in lung cancer cells
(15,17,20,22). In
addition, it has been determined that HOTAIR is elevated in lung
cancer (15). Similarly, the present
study observed that expression of HOTAIR in NSCLC was significantly
higher than in adjacent normal lung tissues.
As confirmation that both 14-3-3σ and HOTAIR were
elevated in NSCLC compared with adjacent normal lung tissues, the
present in vivo assays identified a correlation between
levels of 14-3-3σ and HOTAIR expression in NSCLC tissues,
indicating a regulatory relationship between HOTAIR and 14-3-3σ
expression in NSCLC. Analogous to these in vivo findings,
in vitro assays involving overexpression and knockdown of
HOTAIR in a human NSCLC cell line indicated that HOTAIR was a
potent upstream enhancer of 14-3-3σ expression in NSCLC cells.
HOTAIR represses gene expression through the recruitment of
chromatin modifiers, which may involve binding of HOTAIR to the
transcriptional co-repressor polycomb repressive complex 2 (PRC2)
and subsequent recruitment of PRC2, leading to the silencing of
target genes (23). The present
results indicate that HOTAIR promotes 14-3-3σ mRNA and protein
expression, thus it is unlikely that HOTAIR directly effects the
14-3-3σ gene but may act by inhibiting 14-3-3σ gene repressors.
14-3-3 proteins are a family of highly conserved
proteins comprised of at least seven isoforms in mammalian cells
(4). Overexpression of the 14-3-3
isoforms β, γ, σ and θ has been identified in lung cancer (12). The present study focused on the
regulatory effect of HOTAIR on 14-3-3σ expression in NSCLC. Future
studies by our group will focus on the potential effects of HOTAIR
on the expression of 14-3-3 β, γ and θ, as inhibition of HOTAIR may
be an effective way of inhibiting the expression of 14-3-3σ and
other 14-3-3 proteins. In addition, as overexpression of HOTAIR and
14-3-3 protein has been detected in pancreatic cancer, lung
adenocarcinoma and breast tumor (8,9,10) and correlates with more aggressive
tumors and poor prognosis (11,24,25),
further studies investigating the association between HOTAIR and
14-3-3 proteins in other types of cancer are warranted.
In conclusion, the present results suggest that
HOTAIR promotes the expression of 14-3-3σ in NSCLC in vivo
and in vitro. To the best of our knowledge, the current
study is the first to identify a link between HOTAIR and 14-3-3σ
expression and further studies are now warranted to identify the
underlying mechanisms regarding the effects of HOTAIR on 14-3-3σ
expression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272584 and
81602030), China Postdoctoral Science Foundation (grant no.
2014RS4006) and The Hunan Province Natural Sciences Foundation of
China (grant no. 13JJ3039).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raungrut P, Wongkotsila A,
Lirdprapamongkol K, Svasti J, Geater SL, Phukaoloun M, Suwiwat S
and Thongsuksai P: Prognostic significance of 14-3-3γ
overexpression in advanced non-small cell lung cancer. Asian Pac J
Cancer Prev. 15:3513–3518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porter GW, Khuri FR and Fu H: Dynamic
14-3-3/client protein interactions integrate survival and apoptotic
pathways. Sem Cancer Biol. 16:193–202. 2006. View Article : Google Scholar
|
|
6
|
Morrison D: The 14-3-3 proteins:
Integrators of diverse signaling cues that impact cell fate and
cancer development. Trends Cell Biol. 19:16–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada T, Masuda N, Fukai Y, Shimura T,
Nishida Y, Hosouchi Y, Kashiwabara K, Nakajima T and Kuwano H:
Immunohistochemical expression of 14-3-3 sigma protein in
intraductal papillary-mucinous tumor and invasive ductal carcinoma
of the pancreas. Anticancer Res. 26:3105–3110. 2006.PubMed/NCBI
|
|
9
|
Shiba-Ishii A, Kano J, Morishita Y, Sato
Y, Minami Y and Noguchi M: High expression of stratifin is a
universal abnormality during the course of malignant progression of
early-stage lung adenocarcinoma. Int J Cancer. 129:2445–2453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boudreau A, Tanner K, Wang D, Geyer FC,
Reis-Filho JS and Bissell MJ: 14-3-3σ stabilizes a complex of
soluble actin and intermediate filament to enable breast tumor
invasion. Proc Natl Acad Sci USA. 110:E3937–E3944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neal CL and Yu D: 14-3-3ζ as a prognostic
marker and therapeutic target for cancer. Expert Opin Ther Targets.
14:1343–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi W, Liu X, Qiao D and Martinez JD:
Isoform-specific expression of 14-3-3 proteins in human lung cancer
tissues. Int J Cancer. 113:359–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radhakrishnan VM, Jensen TJ, Cui H,
Futscher BW and Martinez JD: Hypomethylation of the 14-3-3σ
promoter leads to increased expression in non-small cell lung
cancer. Genes Chromosomes Cancer. 50:830–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cetintas VB, Tetik A, Cok G, Kucukaslan
AS, Kosova B, Gunduz C, Veral A and Eroglu Z: Role of 14-3-3σ in
resistance to cisplatin in non-small cell lung cancer cells. Cell
Biol Int. 37:78–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, An Y, Liang Y and Xie XW: Role of
HOTAIR long noncoding RNA in metastatic progression of lung cancer.
Eur Rev Med Pharmacol Sci. 18:1930–1936. 2014.PubMed/NCBI
|
|
16
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greene FL and Sobin LH: The staging of
cancer: A retrospective and prospective appraisal. CA Cancer J
Clin. 58:180–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gospodarowicz MK, Miller D, Groome PA,
Greene FL, Logan PA and Sobin LH: The process for continuous
improvement of the TNM classification. Cancer. 100:1–5. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Shi Y, Chen L, Jiang Y, Mao C, Yan
B, Liu S, Shan B, Tao Y and Wang X: The ratio of FoxA1 to FoxA2 in
lung adenocarcinoma is regulated by LncRNA HOTAIR andchromatin
remodeling factor LSH. Sci Rep. 5:178262015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhuang Y, Wang X, Nguyen HT, Zhuo Y, Cui
X, Fewell C, Flemington EK and Shan B: Induction of long intergenic
non-coding RNA HOTAIR in lung cancer cells by type I collagen. J
Hematol Oncol. 6:352013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|