Introduction
It has been demonstrated that autogenous bone
grafting has limited availability and accompanying risks include
infection and pain as well as injury to nerves and vessels
(1–3). Thus, bone graft substitutes, such as
Bio-Oss® blocks were considered for bone transplantation
in the clinic. Bio-Oss, a natural and porous bone mineral matrix
with no antigenic properties, is produced by removing all organic
components from bovine bone. Bio-Oss is physically and chemically
comparable to the mineralized matrix of human bone due to its
natural structure (4). Moreover,
Bio-Oss provides a scaffold for osteogenic cell migration due to
its macroscopic and microscopic structure with an interconnected
pore system (4). It also has been
reported that Bio-Oss is a non-resorbable bone substitute (5,6). Several
studies have clearly suggested that Bio-Oss contributes to
osteogenesis by providing a scaffold for osteogenic cells (7,8).
However, Bio-Oss does not have osteoinduction capability and does
not significantly promote the proliferation and differentiation of
osteoblasts. These disadvantages have limited its clinical
application in restoration of large bone defects.
Calcitonin gene-related peptide (CGRP), a
neuropeptide abundant in sensory neurons, regulates processes
associated with migraine and colitogenic responses (9,10). CGRP
also has an important role in bone innervation and has two
isoforms, α-CGRP and β-CGRP (11).
β-CGRP is not considered osteogenic (12). Han et al (13) showed that α-CGRP promotes rat
osteoblast proliferation and indicated that it may be essential in
bone remodeling. α-CGRP has also been shown to be involved in the
physiological activation of bone formation (13). Moreover, α-CGRP may inhibit apoptosis
of human osteoblasts, thus favoring local bone regeneration
(14). Overexpression of α-CGRP in
mice increased the rate of bone formation due to osteoblast
activity, which further resulted in the increase of trabecular bone
volume (15). α-CGRP knock-out in
mice leads to decreased bone formation and osteopenia (16). α-CGRP has a crucial role in
inhibiting bone resorption of osteoclasts and stimulating the
division of osteoblasts (17,18). In
addition, α-CGRP promotes osteogenesis by increasing the number and
the size of bone colonies in cultured rat bone marrow leukocytes
(19). Thus, α-CGRP has a potential
application in promoting osteogenesis.
Vacuum freeze-drying is a technique of freezing
water-bearing material to form a solid, followed by dehydration
under low temperature and pressure. Moreover, vacuum freeze-drying
does not affect the physical, chemical and biological properties of
certain materials. It has been reported that it is feasible to load
biological activity factors onto the surface of bone substitute
materials by using a vacuum freeze-drying technique (20).
In general, modified bone tissue engineering has
great potential for repairing bone defects that result from trauma,
surgical resection and congenital deformity corrections (21–23). In
the present study, α-CGRP was loaded onto the surface of Bio-Oss
using a vacuum freeze drying technique to modify the biological
activity of Bio-Oss in order to promote the activity of
osteoblasts. By evaluating osteogenesis for bone formation by
α-CGRP-Bio-Oss, the present study provided a potential clinical
application of Bio-Oss in restoring large bone defects.
Materials and methods
Bio-Oss modified by α-CGRP
The CGRP used in the present study was α-CGRP
(Anaspec, Fremont, CA, USA), which differs from β-CGRP by one amino
acid. CGRP was dissolved in distilled water to a final
concentration of 10−5 M, and was stored at −20°C. CGRP
was diluted to the appropriate concentration in culture medium
prior to use.
A total of 100 mg Bio-Oss (Geistlich Biomaterials,
Sweden, Switzerland) was added to 2 ml phosphate-buffered saline
(PBS; BestBio, Inc., Shanghai, China) containing the optimal
concentration of α-CGRP (10−7 M), followed by reaction
at room temperature for 24 h. Then Bio-Oss was then washed with
distilled water and freeze-dried. α-CGRP consists of 37 amino acids
and amino acids 2 and 7 are involved in the formation of a
disulfide bridge (24), which is
essential for biological activity (25,26).
Numerous peptide and non-peptide CGRP antagonists have been
described. These were initially based on peptide fragments lacking
the N-terminal disulfide-bonded loop, of which the best
characterized is CGRP8-37 (27–29). In
the present study, a mimic-α-CGRP, in which the disulfide bond was
disturbed, provided by GL Biochem Ltd. (Shanghai, China), was used.
Amino acids 1–7 of the mimic-α-CGRP were
H-Thr-Thr-Thr-Thr-Ala-Thr.
Scanning electron microscopy
(SEM)
The samples were placed on a conductive object
stage, sprayed with gold sputter in a vacuum spray apparatus
(MSP-1S; Hitachi, Ltd., Tokyo, Japan) and then scanned by SEM
(Hitachi S-3400N; Hitachi, Ltd.) at different magnifications. The
surface morphology of Bio-Oss bone substitute in different groups
was observed with the electron acceleration voltage set to 10
kV.
Primary osteoblast isolation and cell
culture
Primary osteoblast isolation from the calvaria of
neonatal Sprague Dawley rats (Medical Animal Experimental Center of
Guangdong, Guangzhou, China) was performed as previously described
(27). According to the rules of
Committee on Animal Research and Ethics of Guangzhou Medical
University (Guangzhou, China), the present research project was
reviewed and approved to be appropriate and humane by the Animal
Care and Use Committee of Guangzhou Medical University. In brief, 4
neonatal rats (age, 3 days old) were sacrificed and sterilized with
75% ethanol. The skin and brain tissue were carefully removed from
the skull, the jaw was cut and excess connective tissue and
vascular tissue was scrapped from around the edge of the calvaria.
The calvaria was washed three times with PBS and cut into 1×1
cm2 blocks. The calvaria was then incubated in 1%
trypsin (4 ml; containing EDTA; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 10 min at 37°C, followed by three
washes with PBS and subsequent incubation with 0.1% collagenase II
(4 ml; Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) at 37°C for
30 min. The collagenase solution was then replaced with fresh
solution, followed by incubation for a further 20 min at 37°C.
Cells digested by collagenase were collected and cultured in
Dulbecco's modified Eagle's medium supplemented with 10% (v/v)
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml; Gibco; Thermo Fisher Scientific, Inc.),
streptomycin (100 mg/ml, Gibco; Thermo Fisher Scientific, Inc.),
ascorbic acid (10 mg/ml; Sigma-Aldrich; Merck KGaA) and
β-glycerophosphate (10 mM; Sigma-Aldrich; Merck KGaA) in a
humidified atmosphere at 37°C with 5% CO2.
Immunofluorescence staining
At the second passage (P2), the osteoblasts cultured
in the 6-well plates were washed three times with PBS and then
fixed with 4% paraformaldehyde (BestBio, Inc.) for 15 min, followed
by washing with PBS containing 0.1% Triton X-100 (BestBio, Inc.)
for 5 min. Rhodamine phalloidin (300 µl/well; Sigma-Aldrich; Merck
KGaA) was used for F-actin staining and Hoechst 33342 (30 µg/ml;
Sigma-Aldrich; Merck KGaA) for cell nuclear staining. The
fluorescence images were captured using a fluorescence microscope
(Olympus Corp., Tokyo, Japan).
Alkaline phosphatase (ALP)
staining
The osteoblasts (P2) were cultured on slides (that
were not treated with lysin) for 1 week. For ALP staining, the
Gomori method was applied, following the instructions of the ALP
stain kit (D0001-2; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China).
Detection of ALP activity
Measurement of ALP activity was performed with an
ALP assay kit (A059-2; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China). In brief, osteoblasts were loaded onto a 24-well
culture plate containing Bio-Oss, α-CGRP-Bio-Oss, CGRP or
mimic-CGRP, respectively, at 2×104 cells per well. The
blank group consisted of osteoblasts that were loaded onto a
24-well culture plate at 2×104 cells per well. After
culturing for 7, 14 or 21 days, the media was removed. A total of
0.2 ml 0.1% Triton-100 was added and 30 µl cell lysate was used for
ALP activity detection. The optical density value of the culture
media was measured with a microplate reader (Thermo Fisher
Scientific, Inc.) at 490 nm. The protein concentration was
determined by bicinchoninic acid and bovine serum albumin (BestBio,
Inc.) was used as a standard. ALP activity was expressed as a
percentage over the negative control.
Alizarin Red-S staining
Osteoblasts at P3 were cultured in 6-well plates
with a seeding density of 1×105 cells/well if Bio-Oss (1
mg) was added or 2×104 cells/well if α-GGRP-Bio-Oss (1
mg) was used. When the cells reached 80% con-fluency, the cell
culture media was changed to mineralized induction solution, which
contained ascorbic acid (10 mg/ml), β-glycerophosphate (10 mM) and
dexamethasone (0.1 µmol/l; all from Sigma-Aldrich; Merck KGaA) for
culturing osteoblasts for up to 21 days. Subsequent to three washes
in PBS, cells were fixed with 4% paraformaldehyde for 10 min.
Alizarin Red-S solution (BestBio, Inc.) was added and cells were
cultured for 30 min at 37°C.
Analysis of cell proliferation
The effect of different concentrations of CGRP on
the proliferation of primary osteoblasts was determined by using a
Cell Counting Kit-8 (CCK-8; BestBio, Inc.). CCK-8 proliferation
analysis was performed according to the manufacturer's procedure.
In brief, osteoblasts were seeded into 24-well plates at a seeding
density of 1×104 cells/well and cultured with CGRP at
concentrations of 10−7, 10−8,
10−9, 10−10, 10−11 or 0 M, and the
medium was changed every 2 days. In another experiment, the wells
contained Bio-Oss, α-CGRP-Bio-Oss, CGRP or mimic-CGRP,
respectively, at 10−9 M, and the incubation time was 1,
3 or 7 days. The cells were then treated with CCK-8 reagent for 2 h
in the dark to assess the osteoblast proliferation rate. The
absorbance of the culture media was measured with a microplate
reader (Thermo Fisher Scientific, Inc.) at 450 nm.
RNA extraction and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
The seeding density of the osteoblasts was
105/well in 6-well plates. RNA was extracted from the
control, Bio-Oss, CGRP-Bio-Oss, CGRP and mimic-CGRP group using
TRIzol regent (Invitrogen; Thermo Fisher Scientific, Inc.). DNA was
further digested with gDNA Eraser (Takara, Otsu, Japan). The mRNA
expression levels of the osteogenic genes ALP, osteocalcin (OCN)
and Runt-related transcription factor-2 (RUNX2) were assessed by
RT-qPCR (Bio-Rad CFX96; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). For the reverse-transcription step, the reaction mixture
contained Prime Script RT Enzyme mix I (1.0 µl), RT Primer mix (1.0
µl), 5X Prime Script Buffer 2 (4.0 µl), RNase Free dH2O
(4.0 µl) and the reaction solution from step 1 (10.0 µl).
Reverse-transcription was performed according to the to the
manufacturer's instructions of the reverse transcription kit
(RR047; Takara Bio, Inc., Otsu, Japan). The reaction condition was
as follows: 15 min at 37°C, followed by 5 sec at 85°C and a holding
temperature of 4°C, using a T100™ Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Primers of these genes are
presented in Table I.
SYBR® Premix Ex TaqII (12.5 µl), forward primer (1.0
µl), reverse primer (1.0 µl), DNA (2.0 µl) and ddH2O
(8.5 µl) were used to provide a total reaction volume of 25 µl and
a SYBR-Green I fluorescence quantification PCR kit (RR820; Takara
Bio, Inc.) was used for qPCR. The reverse-transcribed nucleotides
were amplified using a CFX96™ Real-Time PCR Detection system
(Bio-Rad Laboratories, Inc.) and the following thermocycling
conditions: 30 sec at 95°C, followed by 40 cycles of 5 sec at 95°C
and 30 sec at 60°C. The relative expression level for each gene was
normalized to GAPDH and relative quantification of gene expression
was performed using the 2−∆∆Cq method (30).
 | Table I.Gene primers used for quantitative
polymerase chain reaction. |
Table I.
Gene primers used for quantitative
polymerase chain reaction.
| Gene | Direction | Sequence,
5′-3′ | Product size
(bp) | GenBank accession
no. |
|---|
| ALP | Forward |
CATCGCCTATCAGCTAATGCACA | 150 | NM_013059.1 |
|
| Reverse |
ATGAGGTCCAGGCCATCCAG |
|
|
| OCN | Forward |
CCCTCTCTCTGCTCACTCTGCT | 119 | NM_013414.1 |
|
| Reverse |
CTTACTGCCCTCCTGCTTGG |
|
|
| Runx2 | Forward |
CATGGCCGGGAATGATGAG | 148 | NM_001278483.1 |
|
| Reverse |
TGTGAAGACCGTTATGGTCAAAGTG |
|
|
| GAPDH | Forward |
GGCACAGTCAAGGCTGAGAATG | 143 | NM_017008.4 |
|
| Reverse |
ATGGTGGTGAAGACGCCAGTA |
|
|
Western blot analysis
To study the effects of CGRP on osteoblasts, western
blot analysis was used to determine the expression of ALP, OCN and
Runx2 on day. Briefly, osteoblasts were washed with ice-cold PBS
three times. A total of 100 µl radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology), containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40 and 0.1% SDS, was added
into each well. After incubation on ice for 15 min, the osteoblasts
were scraped and briefly centrifuged (12,000 × g at 4°C for 5 min).
The concentration of protein in the samples containing ALP, OCN and
Runx2 was measured by using G250 (Beyotime Institute of
Biotechnology). Radioimmunoprecipitation assay buffer was used to
adjust the concentration, for consistency. The nuclear extract was
collected, boiled with 5X SDS sample buffer (Beyotime Institute of
Biotechnology), and then subjected to SDS. A total of 10 µl protein
from each sample and 7 µl Marker (26616; Thermo Fisher Scientific,
Inc.) were loaded and separated using 10% SDS-PAGE. Samples were
subsequently blotted onto a polyvinylidene difluoride microporous
membrane (Beyotime Institute of Biotechnology) and the blot was
blocked for 1 h in 5% non-fat dry milk in Tris-buffered saline with
0.5% Tween-20 (PBST). Furthermore, the blot was incubated with
rabbit polycolonal anti-ALP (1:3,000; ab95462; Abcam, Cambridge,
UK), mouse monoclonal OCN (1:2,000; ab13418; Abcam) and rabbit
polyclonal Runx2 (1:2,000; ab23981; Abcam) antibodies at 4°C
overnight. GAPDH (1:8,000; ab181602; Abcam) was used as a control.
After washing in PBST, the blots were incubated in goat anti-rabbit
IgG horseradish peroxidase-conjugated secondary antibody (diluted
in 1:5,000; ab6721; Abcam) for 1 h at room temperature. All protein
concentrations were determined according to the recommendation of
Abcam. The membrane was then washed three times (10 min each time)
with Tris-buffered saline with 0.5% Tween-20 and exposed to film
following chemiluminescence reagent treatment with the enhanced
chemiluminescence and western blotting reagents (Beyotime Institute
of Biotechnology). Bands were quantified using densitometry of
digitalized images and the results were analyzed by Quantity One
software 4.52 (Bio-Rad Laboratories, Inc.). The results of the
present assay were confirmed by repeating the experiment three
times.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean from three independent experiments. Statistical analysis
was performed using SPSS statistical software v20.0 (International
Business Machines, Corp., Armonk, NY, USA). Data were evaluated
using analysis of variance followed by a least-significant
differences test or Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Bio-Oss modification
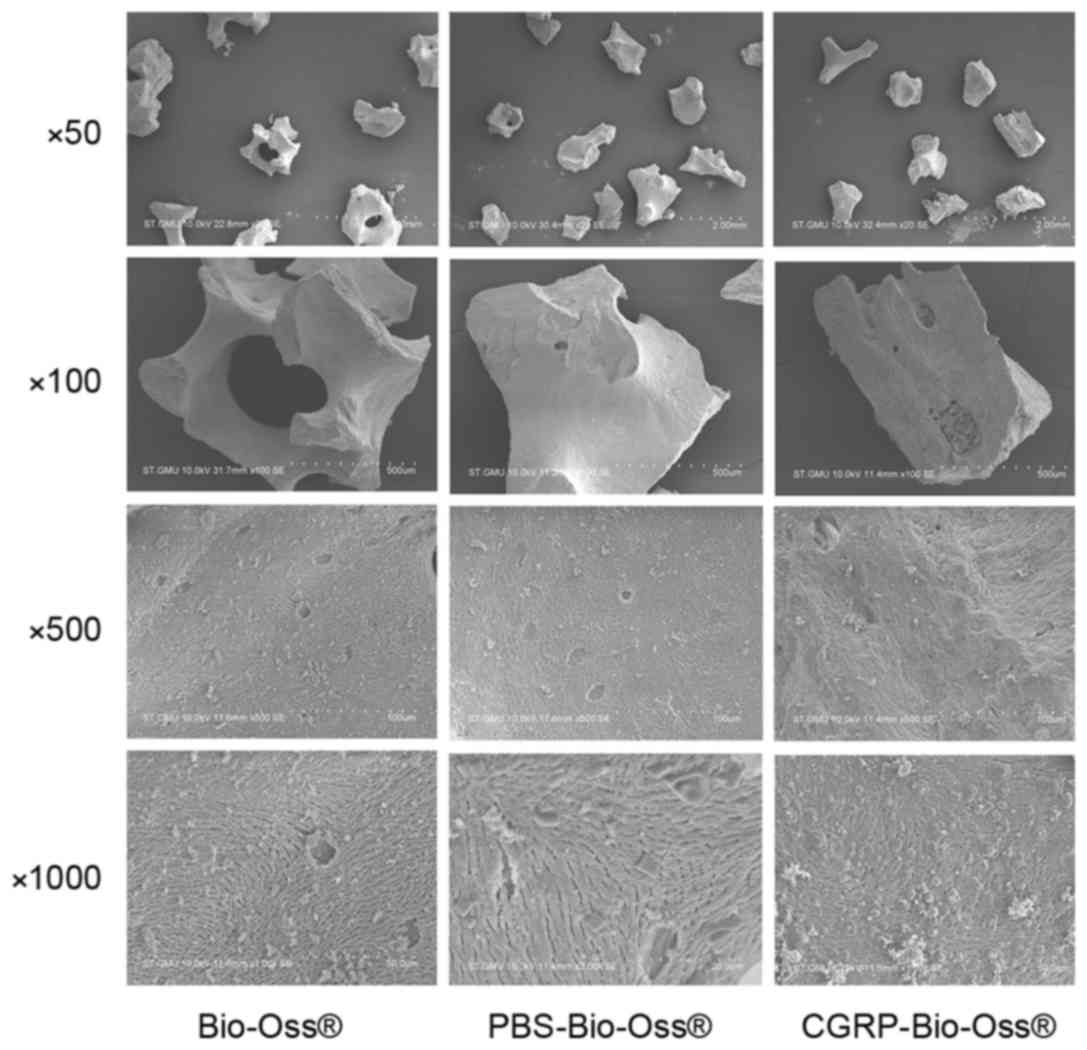
The surface morphology of the Bio-Oss, PBS-Bio-Oss
and CGRP-Bio-Oss was examined by SEM. No significant difference in
surface morphology was observed at different magnifications
(Fig. 1). These results demonstrated
that the vacuum freeze-drying technique did not affect the
physical, chemical and biological properties of the material.
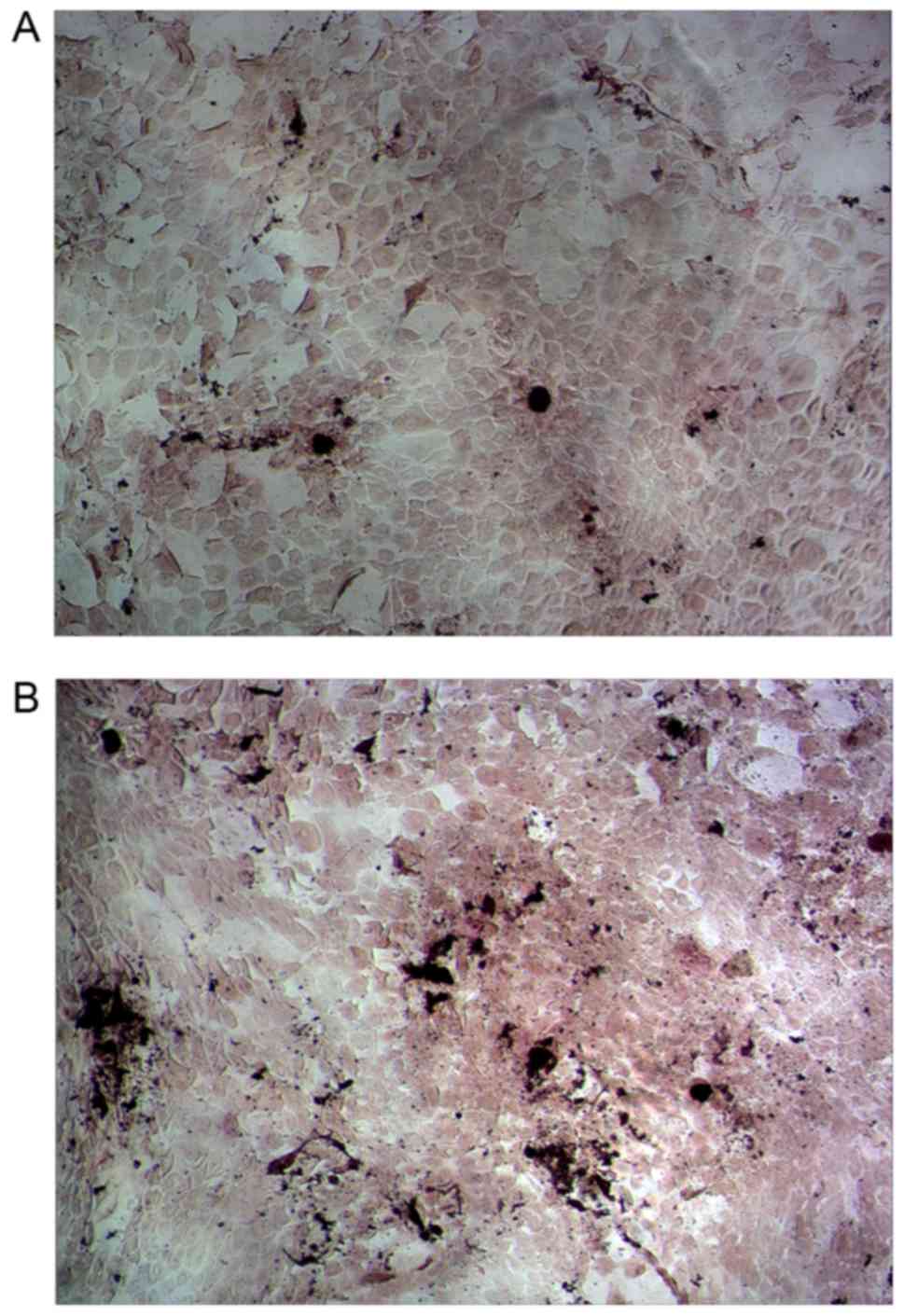
Osteoblastic characteristics of
primary osteoblasts
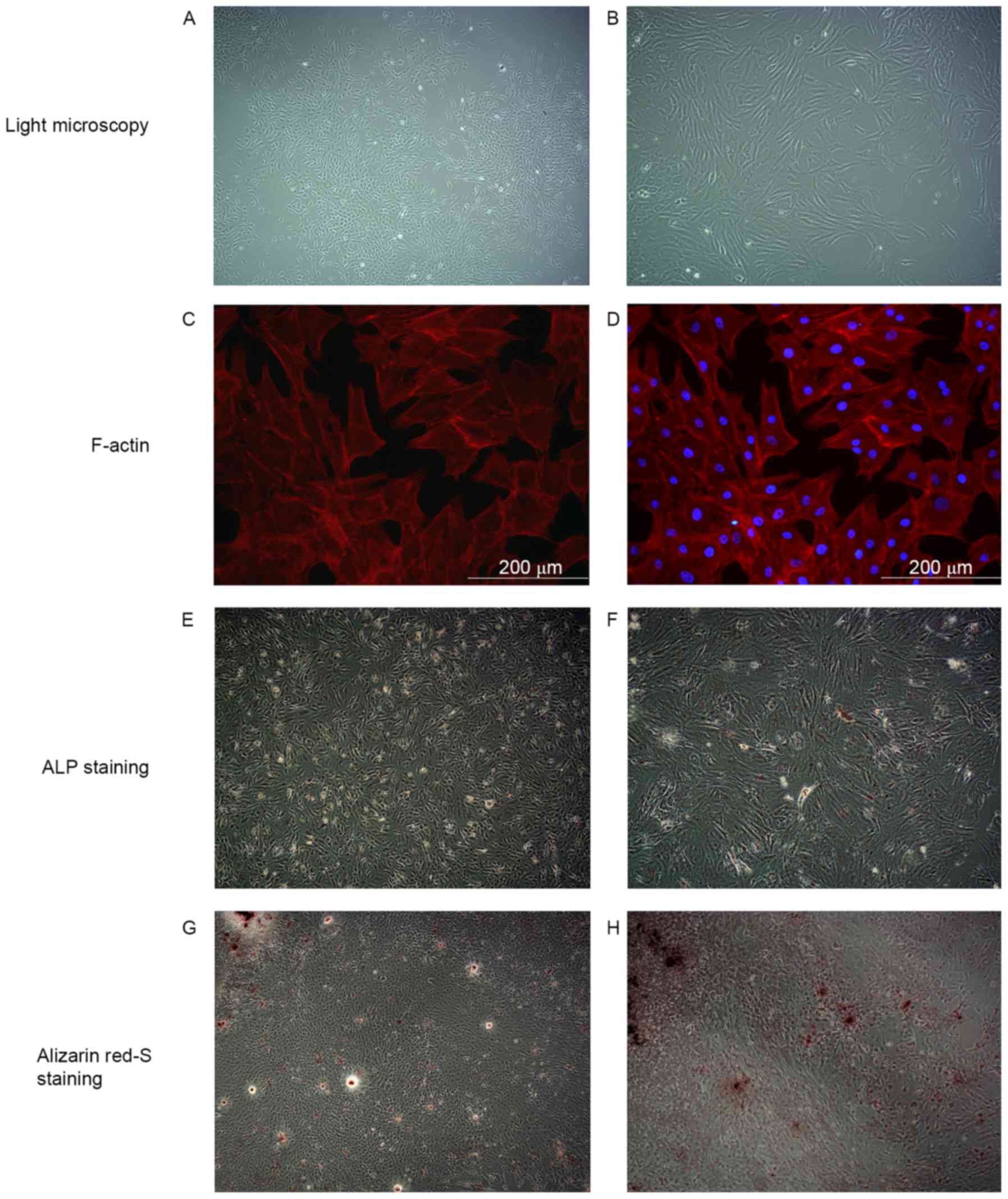
The osteoblastic characteristics of primary
osteoblasts were demonstrated by light microscopy, F-actin
staining, ALP and alizarin red staining. The morphology of cells
was mostly irregular, polygonal and had protrusions (Fig. 2A and B). Fluorescence staining
further confirmed the above results. The cell skeleton marker
F-actin was stained red and the nuclei were stained blue color
(Fig. 2C and D). ALP is the specific
marker enzyme of osteoblasts (31).
ALP-positive osteoblasts were displayed as black or gray black
particles in the cytoplasm after Gomori staining. As displayed in
Fig. 2E and F, a vast majority of
cells were positive. The mineralized nodules were dyed red after
Alizarin Red-S staining. As presented in Fig. 2G and H, red positive nodules were
variable in size with deep staining in the center, which gradually
became shallow towards the edges. Primary osteoblasts were passed
to the third passage for the subsequent experiments.
Effect of CGRP on osteoblast
proliferation
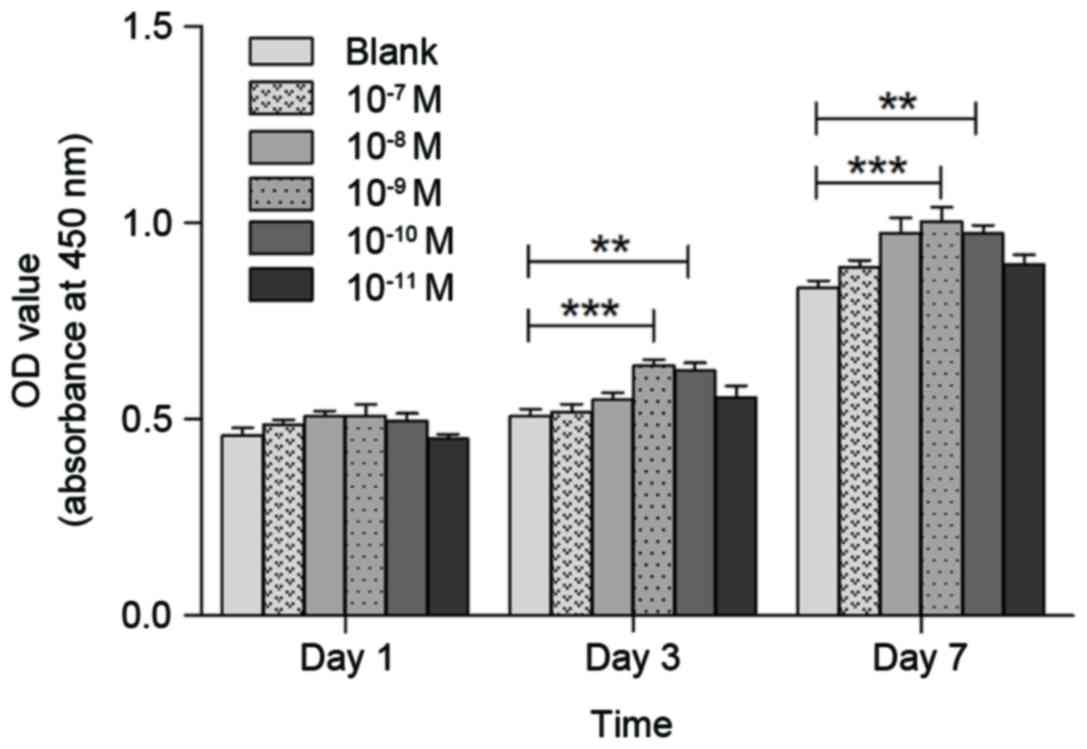
The effects of CGRP on osteoblast proliferation were
assessed using the CCK-8 assay. CGRP at different concentrations
(10−7, 10−8, 10−9,
10−10, 10−11 or 0 M) was tested on
osteoblasts after incubation for 1, 3 or 7 days. As shown in
Fig. 3, 10−9 M α-CGRP
resulted in the highest increase in the proliferation rate compared
to that in the control group (P<0.01).
Effects of CGRP on ALP activity in
osteoblasts
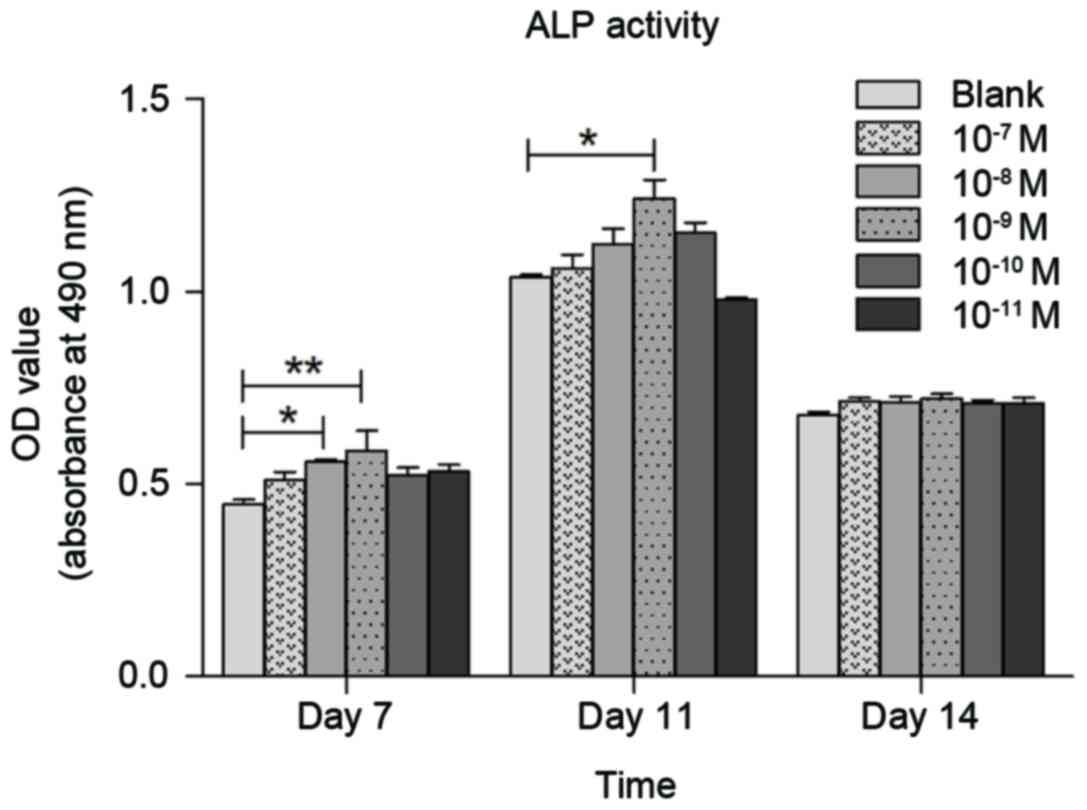
Effects of CGRP on ALP activity in osteoblasts were
assessed using the ALP Kit assay. Various concentrations of CGRP
(10−7, 10−8, 10−9,
10−10, 10−11 or 0 M) were tested on
osteoblasts at days 7, 11 and 14. As shown in Fig. 4, 10−9 M α-CGRP resulted in
the greatest increase in the ALP activity rate at day 7 (P<0.01)
and 11 (P<0.05), compared to the control groups. Thus,
10−9 M α-CGRP was selected to modify Bio-Oss.
Furthermore, the concentration at which mimic-α-CGRP was used was
10−9 M.
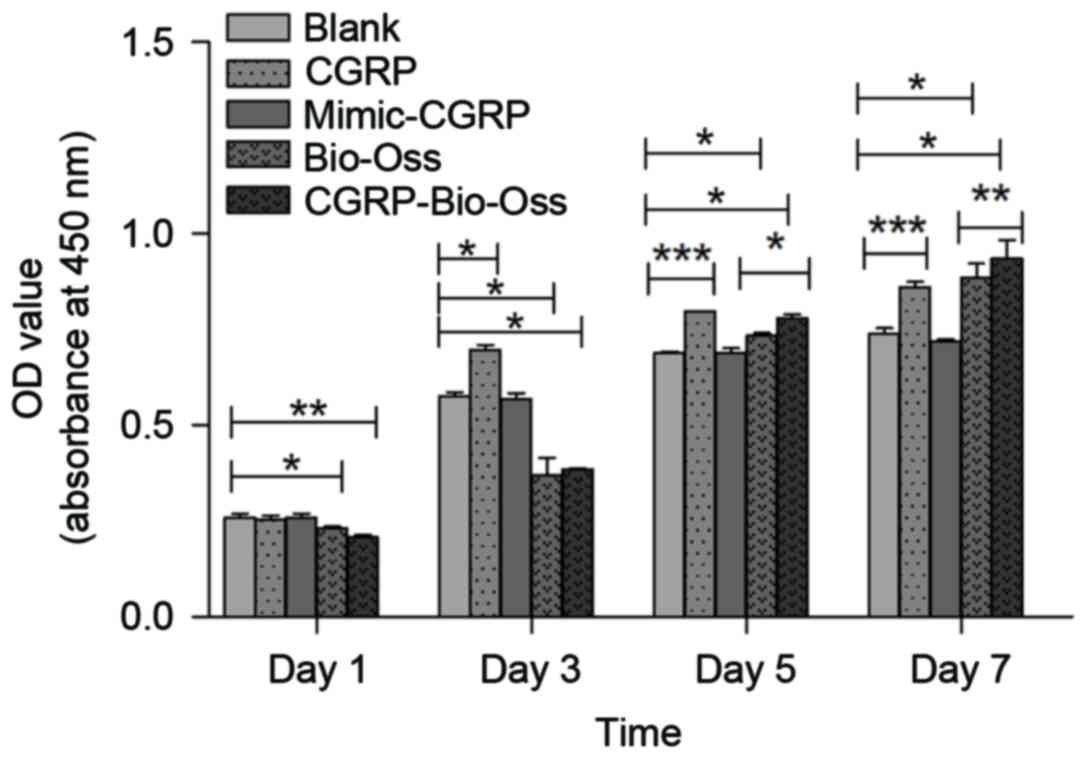
Assessment of osteoblast proliferation
rate
Compared with the control group, the cell
proliferation rate in the α-CGRP group was significantly increased
at days 3 (P<0.01), 5 (P<0.001) and 7 (P<0.001). However,
in Bio-Oss groups, the cell proliferation rate significantly
increased at day 5 and 7 (P<0.05; Fig. 5) compared with the control group. Of
note, the proliferation rate in the α-CGRP-Bio-Oss group was
significantly higher than that in the Bio-Oss group at days 5 and
7. By contrast, mimic-α-CGRP did not influence the osteoblast
proliferation (Fig. 5).
Effect of CGRP on osteoblast
mineralization capability
The effects of CGRP on osteoblast mineralization
capability were assessed using the Alizarin Red-S staining. As
shown in Fig. 6, the amount of
mineralization nodules in the CGRP-Bio-Oss group was obviously
higher than that in the Bio-Oss group.
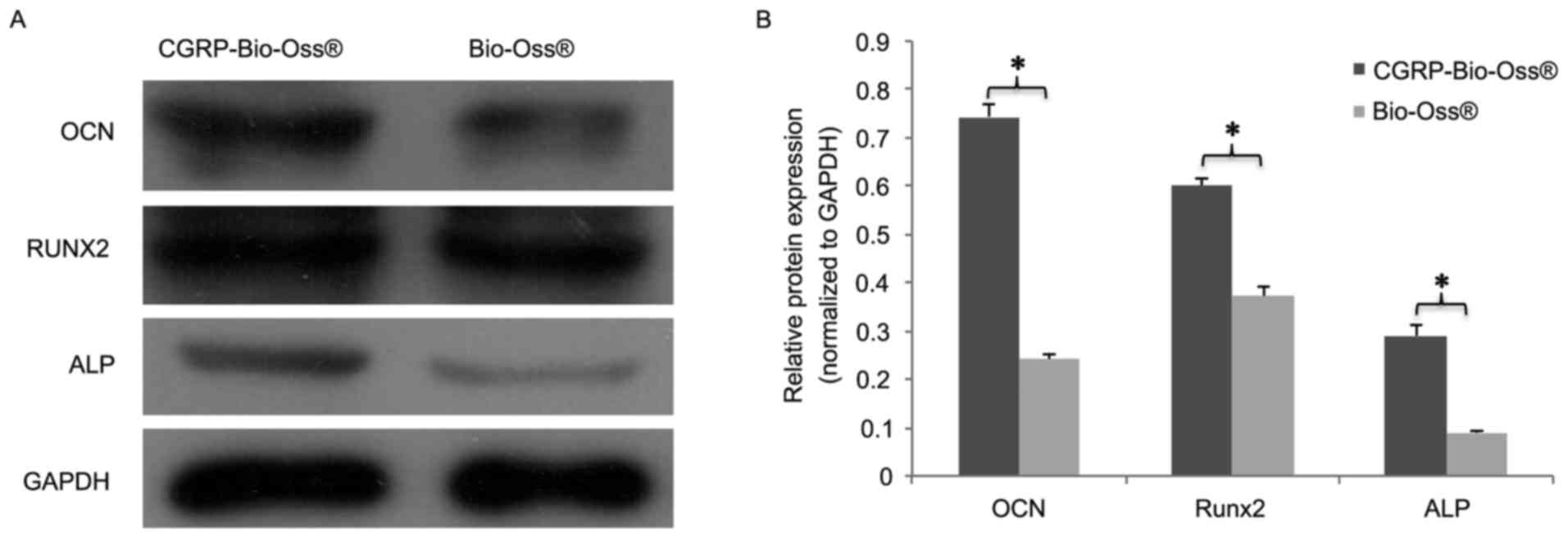
Assessment of osteogenesis-associated
genes and proteins
The expression of genes associated with
osteogenesis, including the osteogenic transcription factor Runx2,
the early osteogenic marker ALP and the late osteogenic marker OCN,
were evaluated by RT-qPCR on days 7, 14 and 21. The addition of
CGRP (10−9 M) to the culture media significantly
increased osteoblastic expression of ALP, as well as that of OCN at
day 14. Runx2 was also increased at days 7 and 14, but not at day
21 (Fig. 7). A significant
upregulation of ALP and OCN was also found in osteoblasts cultured
with α-CGRP-Bio-Oss compared with that in the Bio-Oss group. Gene
expression of Runx2 increased at day 7 but not on days 14 and 21 in
the α-CGRP-Bio-Oss compared to the Bio-Oss group. The detected
protein levels of Runx2, ALP and OCN corresponded to the mRNA
expression, which are presented in Fig.
8. Significant upregulation of Runx2, ALP and OCN was found in
osteoblasts cultured with α-CGRP-Bio-Oss compared to those in the
Bio-Oss group (P<0.05; Fig.
8).
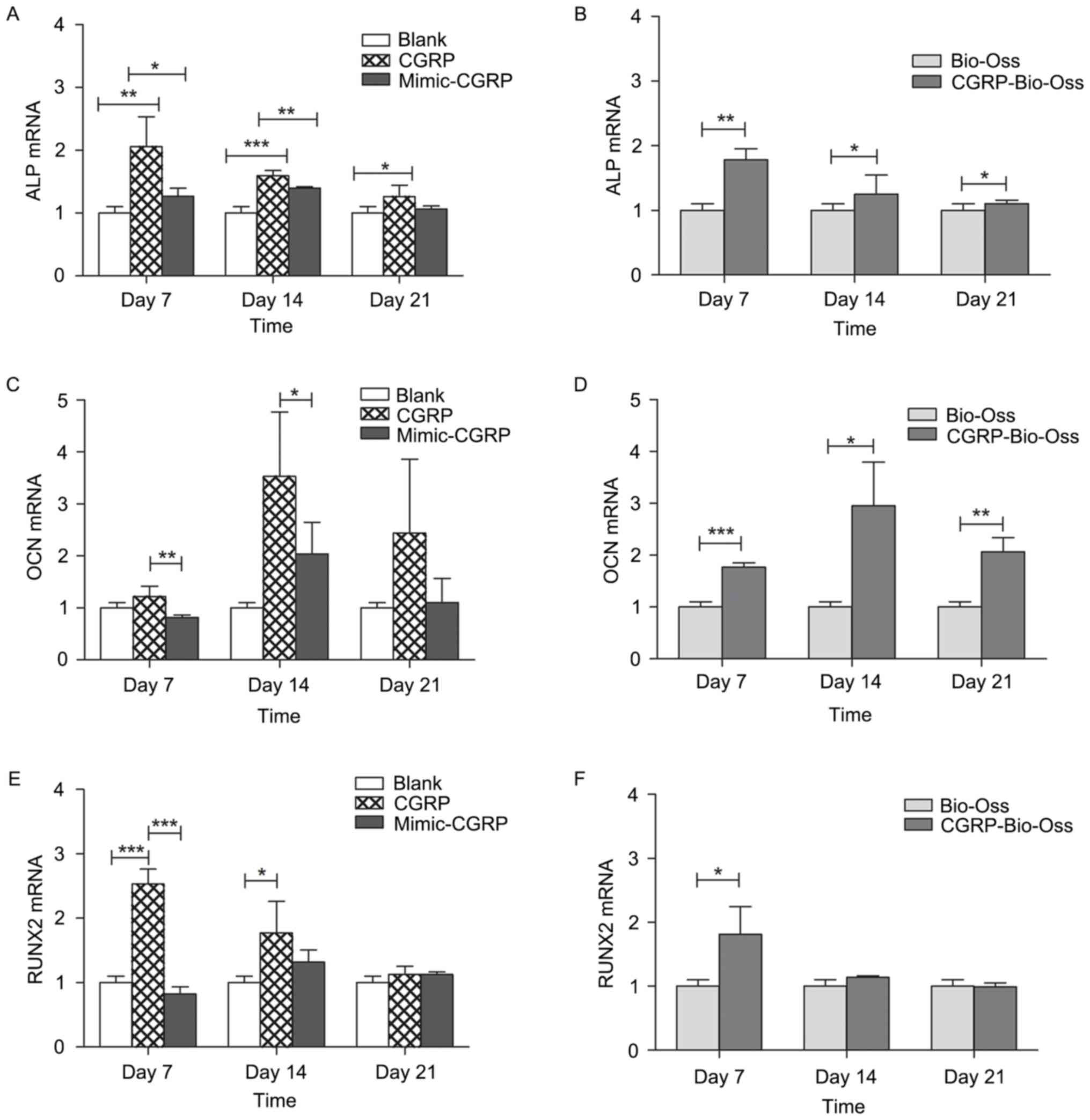 | Figure 7.Expression of the
osteogenesis-associated genes (A and B) ALP, (C and D) OCN and (E
and F) RUNX2 was evaluated by reverse-transcription quantitative
polymerase chain reaction analysis on days 7, 14 and 21. Addition
of CGRP (10−9 M) to the culture media significantly
increased osteoblastic expression of ALP, OCN at day 14 and Runx2
at days 7 and 14, but not on day 21. Significant upregulation of
ALP and OCN was found in the osteoblasts cultured with
α-CGRP-Bio-Oss® compared to that in the Bio-Oss group.
Gene expression of Runx2 increased at day 7 but not on day 14 and
21 in the α-CGRP-Bio-Oss compared to that in the Bio-Oss group.
*P<0.05, **P<0.01, ***P<0.001. ALP, alkaline phosphatase;
OCN, osteocalcin; Runx2, Runt-related transcription factor 2; CGRP,
calcitonin gene-related peptide. |
Discussion
The present study was the first, to the best of our
knowledge, to modify Bio-Oss with α-CGRP to analyze the specific
effect of α-CGRP-Bio-Oss on osteogenesis in vitro.
α-CGRP was loaded onto the surface of Bio-Oss using
a vacuum freeze-drying technique in order to provide Bio-Oss with
biological activity in order to promote the activity of
osteoblasts. The samples were scanned by SEM, revealing no
significant difference in surface morphology at different
magnifications. These results demonstrated that the vacuum
freeze-drying technique did not affect the physical, chemical and
biological properties of the material.
Due to its greatest effect on cell proliferation and
ALP activity, 10−9 M CGRP was chosen as the optimal
concentration to modify Bio-Oss. It was found that the
proliferation rate was significantly increased in the osteoblasts
cultured with α-CGRP-Bio-Oss compared to those in the Bio-Oss group
on days 5 and 7. In previous studies, three different
concentrations of CGRP (10−12, 10−10 and
10−8 M) were tested in bone marrow mesenchymal stem
cells at day 4 post-seeding, but only the 10−10 M
concentration significantly increased bromodeoxyuridine
incorporation by 36% (P<0.05), compared with vehicle-treated
control cultures (32). Cornish
et al (33) used different
concentrations of CGRP (10−8, 10−9 and
10−10 M) on primary rat osteoblasts, and the
proliferation activity was tested after a 24-h period. Their
findings indicated that 10−9 M CGRP was able to promote
cell proliferation. A similar observation was indicated in the
present study.
ALP activity has been widely used to demonstrate the
early and late differentiation of osteoblast-like cells (34,35). ALP
activity was enhanced in osteoblasts cultured with α-CGRP-Bio-Oss.
The expression levels of ALP, OCN and Runx2 were increased in the
α-CGRP-Bio-Oss group. However, in the mimic-α-CGRP group, disulfide
breakage in α-CGRP led to the reduction of proliferation, ALP
activity and expression alteration of osteogenic markers based on
the same culture conditions between α-CGRP and mimic-α-CGRP, which
suggested that this motif was essential for the function of α-CGRP
in osteogenesis. It was thus concluded that α-CGRP significantly
improved the osteogenesis function of Bio-Oss. However,
α-CGRP-Bio-Oss should be further assessed in vivo in future
studies.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that Bio-Oss modified by
α-CGRP significantly improved osteoblastic function. The strategy
of applying α-CGRP-Bio-Oss may be a promising method of
regenerating bone tissue in the clinic.
Acknowledgements
The present study was supported by grants of the
Science and Technology Bureau of Liwan District (grant no.
20140004), Science and Technology Planning Project of Guangdong
Province (grant no. 2013B021800278), Educational Commission of
Guangdong Province (grant no. B1531026), Medical Scientific
Research Foundation of Guangdong Province (grant no. B2015089) and
Youth Scientific Research Project of Guangzhou Medical University
(grant no. 2015A33).
References
|
1
|
Younger EM and Chapman MW: Morbidity at
bone graft donor sites. J Orthop Trauma. 3:192–195. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milinković ZB, Krneta O, Milicković S,
Dozić D and Curcić A: Are the additional grafts necessary? Acta
Chir Iugosl. 57:69–72. 2010. View Article : Google Scholar
|
|
3
|
Myeroff C and Archdeacon M: Autogenous
bone graft: Donor sites and techniques. J Bone Joint Surg Am.
93:2227–2236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tapety FI, Amizuka N, Uoshima K, Nomura S
and Maeda T: A histological evaluation of the involvement of
Bio-Oss in osteoblastic differentiation and matrix synthesis. Clin
Oral Implants Res. 15:315–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schlegel AK: Bio-Oss bone replacement
material. Long-term results with Bio-Oss bone replacement material.
Schweiz Monatsschr Zahnmed. 106:141–149. 1996.(In French, German).
PubMed/NCBI
|
|
6
|
Schlegel AK and Donath K: BIO-OSS-a
resorbable bone substitute? J Long Term Eff Med Implants.
8:201–209. 1998.PubMed/NCBI
|
|
7
|
Fulmer NL, Bussard GM, Gampper TJ and
Edlich RF: Anorganic bovine bone and analogs of bone mineral as
implants for craniofacial surgery: A literature review. J Long Term
Eff Med Implants. 8:69–78. 1998.PubMed/NCBI
|
|
8
|
Amerio P, Vianale G, Reale M, Muraro R,
Tulli A and Piattelli A: The effect of deproteinized bovine bone on
osteoblast growth factors and proinflammatory cytokine production.
Clin Oral Implants Res. 21:650–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diener HC: CGRP as a new target in
prevention and treatment of migraine. Lancet Neurol. 13:1065–1067.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Jong PR, Takahashi N, Peiris M, Bertin
S, Lee J, Gareau MG, Paniagua A, Harris AR, Herdman DS and Corr M:
TRPM8 on mucosal sensory nerves regulates colitogenic responses by
innate immune cells via CGRP. Mucosal Immunol. 8:491–504. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sample SJ, Hao Z, Wilson AP and Muir P:
Role of calcitonin gene-related peptide in bone repair after cyclic
fatigue loading. PLoS One. 6:e203862011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirt D and Bernard GW: CGRP-beta unlike
CGRP-alpha has no osteogenic stimulatory effect in vitro. Peptides.
18:1461–1463. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han N, Zhang DY, Wang TB, Zhang PX and
Jiang BG: Calcitonin gene-related peptide induces proliferation and
monocyte chemoattractant protein-1 expression via extracellular
signal-regulated kinase activation in rat osteoblasts. Chin Med J
(Engl). 123:1748–1753. 2010.PubMed/NCBI
|
|
14
|
Mrak E, Guidobono F, Moro G, Fraschini G,
Rubinacci A and Villa I: Calcitonin gene-related peptide (CGRP)
inhibits apoptosis in human osteoblasts by β-catenin stabilization.
J Cell Physiol. 225:701–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ballica R, Valentijn K, Khachatryan A,
Guerder S, Kapadia S, Gundberg C, Gilligan J, Flavell RA and
Vignery A: Targeted expression of calcitonin gene-related peptide
to osteoblasts increases bone density in mice. J Bone Miner Res.
14:1067–6574. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schinke T, Liese S, Priemel M, Haberland
M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel
RF, Emeson RB and Amling M: Decreased bone formation and osteopenia
in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner
Res. 19:2049–2056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valentijn K, Gutow AP, Troiano N, Gundberg
C, Gilligan JP and Vignery A: Effects of calcitonin gene-related
peptide on bone turnover in ovariectomized rats. Bone. 21:269–274.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han N, Zhang DY, Wang TB, Zhang PX and
Jiang BG: Calcitonin gene-related peptide induces proliferation and
monocyte chemoattractant protein-1 expression via extracellular
signal-regulated kinase activation in rat osteoblasts. Chin Med J
(Engl). 123:1748–1753. 2010.PubMed/NCBI
|
|
19
|
Shih C and Bernard GW: Calcitonin gene
related peptide enhances bone colony development in vitro. Clin
Orthop Relat Res. 335–344. 1997.PubMed/NCBI
|
|
20
|
Xiao H, Huang C, Feng Y and Li R: Research
situation and development of vacuum freeze-drying technology. Chin
Med Equip J. 13:30–32. 2010.
|
|
21
|
Schek RM, Wilke EN, Hollister SJ and
Krebsbach PH: Combined use of designed scaffolds and adenoviral
gene therapy for skeletal tissue engineering. Biomaterials.
27:1160–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang XQ, Chen JG, Gittens S, Chen CJ,
Zhang XL and Zhang ZY: The ectopic study of tissue-engineered bone
with hBMP-4 gene modified bone marrow stromal cells in rabbits.
Chin Med J (Engl). 118:281–288. 2005.PubMed/NCBI
|
|
23
|
Roldán JC, Detsch R, Schaefer S, Chang E,
Kelantan M, Waiss W, Reichert TE, Gurtner GC and Deisinger U: Bone
formation and degradation of a highly porous biphasic calcium
phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem
cells in an ectopic mouse model. J Craniomaxillofac Surg.
38:423–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durham PL: Inhibition of calcitonin
gene-related peptide function: A promising strategy for treating
migraine. Headache. 48:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watkins HA, Au M and Hay DL: The structure
of secretin family GPCR peptide ligands: Implications for receptor
pharmacology and drug development. Drug Discov Today. 17:1006–1014.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi T, Christopoulos G, Bailey RJ,
Christopoulos A, Sexton PM and Hay DL: Identification of N-terminal
receptor activity-modifying protein residues important for
calcitonin gene-related peptide, adrenomedullin, and amylin
receptor function. Mol Pharmacol. 74:1059–1071. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watkins HA, Rathbone DL, Barwell J, Hay DL
and Poyner DR: Structure-activity relationships for α-calcitonin
gene-related peptide. Br J Pharmacol. 170:1308–1322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiba T, Yamaguchi A, Yamatani T, Nakamura
A, Morishita T, Inui T, Fukase M, Noda T and Fujita T: Calcitonin
gene-related peptide receptor antagonist human CGRP-(8–37). Am J
Physiol. 256:E331–E335. 1989.PubMed/NCBI
|
|
29
|
Dennis T, Fournier A, Cadieux A, Pomerleau
F, Jolicoeur FB, St Pierre S and Quirion R: hCGRP8-37, a calcitonin
gene-related peptide antagonist revealing calcitonin gene-related
peptide receptor heterogeneity in brain and periphery. J Pharmacol
Exp Ther. 254:123–128. 1990.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mendonça G, Mendonça DB, Simões LG, Araújo
AL, Leite ER, Duarte WR, Aragão FJ and Cooper LF: The effects of
implant surface nanoscale features on osteoblast-specific gene
expression. Biomaterials. 30:4053–4062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cornish J, Callon KE, Bava U, Kamona SA,
Cooper GJ and Reid IR: Effects of calcitonin, amylin, and
calcitonin gene-related peptide on osteoclast development. Bone.
29:162–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turksen K, Bhargava U, Moe HK and Aubin
JE: Isolation of monoclonal antibodies recognizing rat
bone-associated molecules in vitro and in vivo. J Histochem
Cytochem. 40:1339–1352. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van den Beucken JJ, Walboomers XF, Boerman
OC, Vos MR, Sommerdijk NA, Hayakawa T, Fukushima T, Okahata Y,
Nolte RJ and Jansen JA: Functionalization of multilayered
DNA-coatings with bone morphogenetic protein 2. J Control Release.
113:63–72. 2006. View Article : Google Scholar : PubMed/NCBI
|