Introduction
Parkinson's disease (PD) is a common age-dependent
neurodegenerative disorder characterized by selective loss of the
substantia nigra dopaminergic neurons, which results in significant
reduction of the dopamine content in the striatum. The incidence of
this disease is increasing year by year and affects 1% of the
population over the age of 65 years (1). Although the exact etiology and
underlying mechanisms of PD remain unclear, the contributions of
oxidative stress, mitochondrial dysfunction, accumulation of iron
ions, activation of the apoptotic cascade, and the degeneration and
death of dopaminergic neurons in the development of PD have been
reported (2,3). The formation of reactive oxygen species
(ROS), loss of mitochondrial membrane potential, depletion of ATP,
and activation of caspase-3 and caspase-9 have been observed in the
substantia nigra and cerebrospinal fluid of PD patients (4–6).
Multiple stimuli-induced oxidative stress, free radical damage and
ubiquitin proteasome also serve an important role in the
pathogenesis of PD (7). In addition,
increasing evidence demonstrated that antioxidants, as scavengers
of ROS and free radicals, are vital in the prevention of PD
(8). The MPP+ ion, also
known as 1-methyl-4-phenylpyridinium, is the neurotoxic form of
methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPP+
can been taken up by dopaminergic neurons, leading to mitochondrial
dysfunction, oxidative stress and programmed cell death, which
simulates the parkinsonian syndrome in cell and animal models
(9).
Isoflavones are bioactive compounds with different
physiological and pharmacological properties, including
antioxidation, anti-free radical, anticancer, cardiovascular
protection and neuroprotective effects, and are widely applied in
the medicinal, food and cosmetic sectors (10–12).
Tectorigenin is a type of natural isoflavone, derived from the
flower of Pueraria thunbergiana (Leguminosae) which is used
in traditional Chinese medicine. The antioxidant, anticancer and
anti-inflammatory effects of tectorigenin have been reported in a
variety of disease model (13–15). A
research performed by Kang et al revealed that tectorigenin
protects hamster lung fibroblast V79-4 cells against hydrogen
peroxide (H2O2)-induced damage by activating
the extracellular signal regulated kinase pathway, while it
simultaneously enhances the activities of antioxidant enzymes
including superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GSH-Px) (16). In addition, Park et al
demonstrated that tectorigenin exerts antioxidant effects
characterized by inhibition of ROS production and upregulation of
antioxidant enzyme activity in H2O2-treated
C6 glioma cells and rat primary astrocytes (17). These findings suggest that
tectorigenin may have beneficial effects on MPP+-induced
neurotoxicity through regulation of oxidative stress.
In present study, it was initially demonstrated that
tectorigenin exerted a neuroprotective effect against
MPP+-elicited cytotoxicity and apoptosis in SH-SY5Y
cells. Furthermore, tectorigenin was observed to reverse the
MPP+-induced enhancement of the oxidative stress system
and inhibition of the antioxidant defense system. These results
suggest that the antioxidant effect of tectorigenin mediates its
protective role against MPP+-induced neurotoxicity.
Materials and methods
Materials
Tectorigenin, MPP+ iodide,
2′,7′-dichlorofluorescein diacetate (DCFH-DA), Dulbecco's modified
Eagle's medium (DMEM) and heat-inactivated fetal bovine serum (FBS)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Streptomycin/penicillin, BCA protein assay kit (P0009), Hoechst
33258 staining (C0003), BeyoECL Plus western blotting assay system
(P0018), RIPA lysis buffer (P0013B), Cell Counting kit-8 (CCK-8,
C0038) and lactate dehydrogenase (LDH) cytotoxicity detection kit
(C0017) were purchased from Beyotime Institute of Biotechnology
(Shanghai, China). Caspase-3 ELISA kit (SEA626Mu) was obtained from
USCN Business Co., Ltd. (Wuhan, China). The commercial SOD
(A001-1), CAT (A007-1) and GSH-Px (A005) bioluminescence ELISA kits
were provided by Jiancheng Bioengineering Institute (Nanjing,
China). An antibody against cytochrome c was purchased from
Abcam (Cambridge, UK, ab13575), and antibodies against NADPH
oxidase (NOX, no. 4301), Bax (no. 2774) and Bcl-2 (no. 15071) were
purchased from Cell Signaling Technology (Carlsbad, CA, USA).
Tubulin (60008-1-Ig) and secondary antibodies were purchased from
Proteintech (Danvers, MA, USA). All reagents were of high
purity.
Cell culture and treatment
Human neuroblastoma SH-SY5Y cells were obtained from
the Institute of Biochemistry and Cell Biology (Shanghai, China).
Cells were maintained in DMEM supplemented with 10% FBS and 1%
streptomycin/penicillin at 37°C in a humidified incubator with 5%
CO2. The cell culture medium was changed every 2–3 days.
The experimental groups were as follows: Untreated control, PD
model (treated with 0.5 mM MPP+), tectorigenin treatment
alone (10 µM), and tectorigenin treatment (0.1, 1 and 10 µM) in
MPP+ model.
Cell viability assay
SH-SY5Y cells that were grown in the logarithmic
growth phase were seeded in 96-well plates at a density of
3×103 cells/well. When 70–80% confluence was reached,
cells were treated as described earlier and the viability of cells
was examined by CCK-8 assay. In brief, after treatment for 24 h, 10
µl CCK-8 reagent was added into the culture medium of each well and
incubated for 2–3 h at 37°C. The absorbance at 570 nm was recorded
using a microplate reader (Varioskan; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and the viability of cells was recorded as
the percentage relative to the control.
LHD release assay
The effect of tectorigenin on
MPP+-induced cytotoxicity was evaluated based on the
degree of cytosolic LDH released into the culture medium (18), using an LDH Cytotoxicity Detection
kit according to the manufacturer's protocol. Briefly, cultured
SH-SY5Y cells were incubated with tectorigenin or MPP+
as previously described for 24 h; subsequently, 50 µl of medium
containing the released LDH was transferred to a new 96-well plate
and mixed with 50 µl reaction mixture. After 30 min of incubation
at room temperature, stop solution (50 µl) was added to terminate
the reactions, and the absorbance at 490 and 680 nm was measured
using a microplate reader to determine the LDH activity. LDH
release was represented as the percentage vs. the control
cells.
Hoechst 33258 nuclear staining
assay
SH-SY5Y cells (in logarithmic growth phase) were
seeded in 24-well plates at a density of 1×104
cells/well. When grown to 70–80% confluence, cells were treated
with MPP+ and/or tectorigenin as described earlier for
24 h. After washing three times with phosphate-buffered saline
(PBS) at 4°C, SH-SY5Y cells were fixed in 4% paraformaldehyde for
10 min at room temperature and then washed again. The cells were
subsequently incubated with 500 µl Hoechst 33258 at room
temperature for 10 min. Following further washing with PBS, the
fluorescence was detected using a fluorescence microscope (Olympus
FV1000; Olympus Corp., Tokyo, Japan).
ROS measurement
The level of cytosolic ROS was quantified using
DCFH-DA as a fluorescent probe. Following treatment with
aforementioned indicated reagents for 24 h, SH-SY5Y cells were
washed with PBS three times and incubated with 10 µmol/l DCFH-DA
for 30 min at 37°C. Subsequently, the cells were washed twice with
Hank's buffer salt solution (Invitrogen; Thermo Fisher Scientific,
Inc.), and the fluorescence of DCF was recorded at 485±10 nm
excitation and 530±12.5 nm emission wavelengths using an EnSpire
Multimode plate reader (PerkinElmer, Inc., Waltham, MA, USA).
Caspase-3 and antioxidant enzyme
activity detection
Logarithmic growth phase SH-SY5Y cells were seeded
in 6-well plates at a density of 1×105 cells/well. After
incubation with the aforementioned indicated treatments for 24 h,
the cells were harvested and lysed. The concentration of protein
was quantified by a BCA assay kit. The activities of caspase-3,
SOC, CAT and GSH-Px were measured using the corresponding
commercially available ELISA kits, according to manufacturer's
protocol. Caspase-3 activity was expressed as the fold of the
control group, while SOD, CAT and GSH-Px activities were expressed
as U/mg protein.
Western blot assay
After reaching ~70% conference, SH-SY5Y cells were
incubated with the indicated reagents for 24 h and then lysed using
RIPA lysis buffer. After centrifugation at 10,000 × g for 10 min at
4°C, the supernatant was collected and protein concentration was
quantified by the BCA protein assay kit following the
manufacturer's guide. An equal amount of protein (30–50 µg) was
separated by 10–12% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membrane. The membranes
were blocked with Tris-buffered saline/Tween 20 (TBST; containing
3.03 g Tris base, 18.8 g glycocine, 1 g SDS and 1 ml Tween-20 in
1,000 ml distilled water, pH 7.6) containing 5% non-fat milk for 2
h at room temperature. Next, samples were incubated with 1:1,000
dilution of cytochrome c, Bax, Bcl-2 and NOX primary
antibodies, respectively. After incubation overnight at 4°C, the
membranes were washed with TBST three times and incubated with a
horseradish peroxidase-conjugated secondary antibody (1:5,000
dilution in TBST) for 2 h at room temperature, followed by
detection with a BeyoECL Plus western blotting detection kit.
Protein blot images were quantized by ImageJ2× software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was performed in triplicates,
independently. The statistical significance of differences was
evaluated with one-way analysis of variance test, followed by
least-significant difference test, using GraphPad Prism 5 software
(GraphPad Inc., San Diego, CA, USA). All values are presented as
the mean ± standard error of the mean. P<0.05 was considered to
demonstrate differences that were statistically significant.
Results
Tectorigenin reverses
MPP+-induced cytotoxicity in SH-SY5Y cells
Firstly, in order to explore the protective effect
of tectorigenin on the MPP+-exhibited cell injury,
SH-SY5Y cells were pretreated with different concentrations of
tectorigenin (0.1, 1 and 10 µM) for 30 min and then cultured with
MPP+ (0.5 mM) for 24 h. The cell viability and the LDH
release were detected by CCK-8 assay and LDH release assay kit,
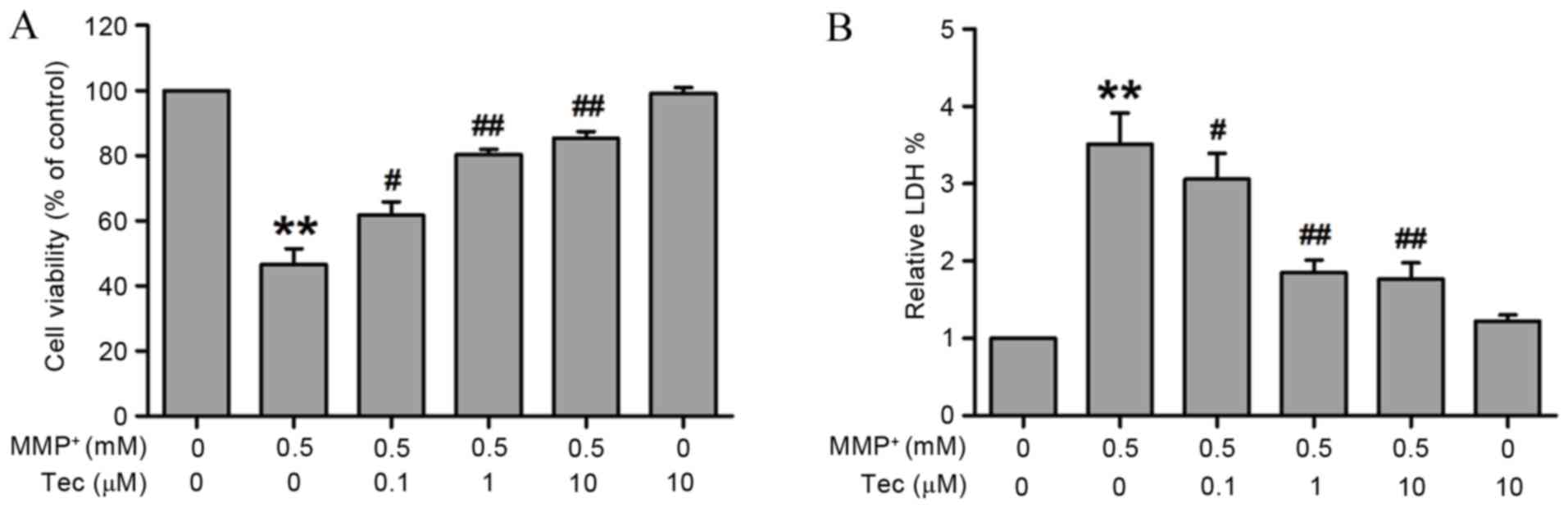
respectively. As shown in Fig. 1,
pretreatment of SH-SY5Y cells with tectorigenin significantly
upregulated the viability of cells in a concentration-dependent
manner as compared with the viability in the
MPP+-treated group (Fig.
1A). In addition, MPP+ alone for 24 h markedly
increased the LDH release, while pretreatment with tectorigenin
attenuated this LDH release in SH-SY5Y cells (Fig. 1B). Tectorigenin alone has no effects
on the viability of cells or the release of LDH in SH-SY5Y cells
(Fig. 1). These results suggest that
tectorigenin protects against MPP+-induced cell
damage.
Tectorigenin alleviates
MPP+-induced apoptosis in SH-SY5Y cells
To further investigate the effects of tectorigenin
on MPP+-induced neurotoxicity, the changes in apoptosis
under MPP+ treatment in the presence or absence of
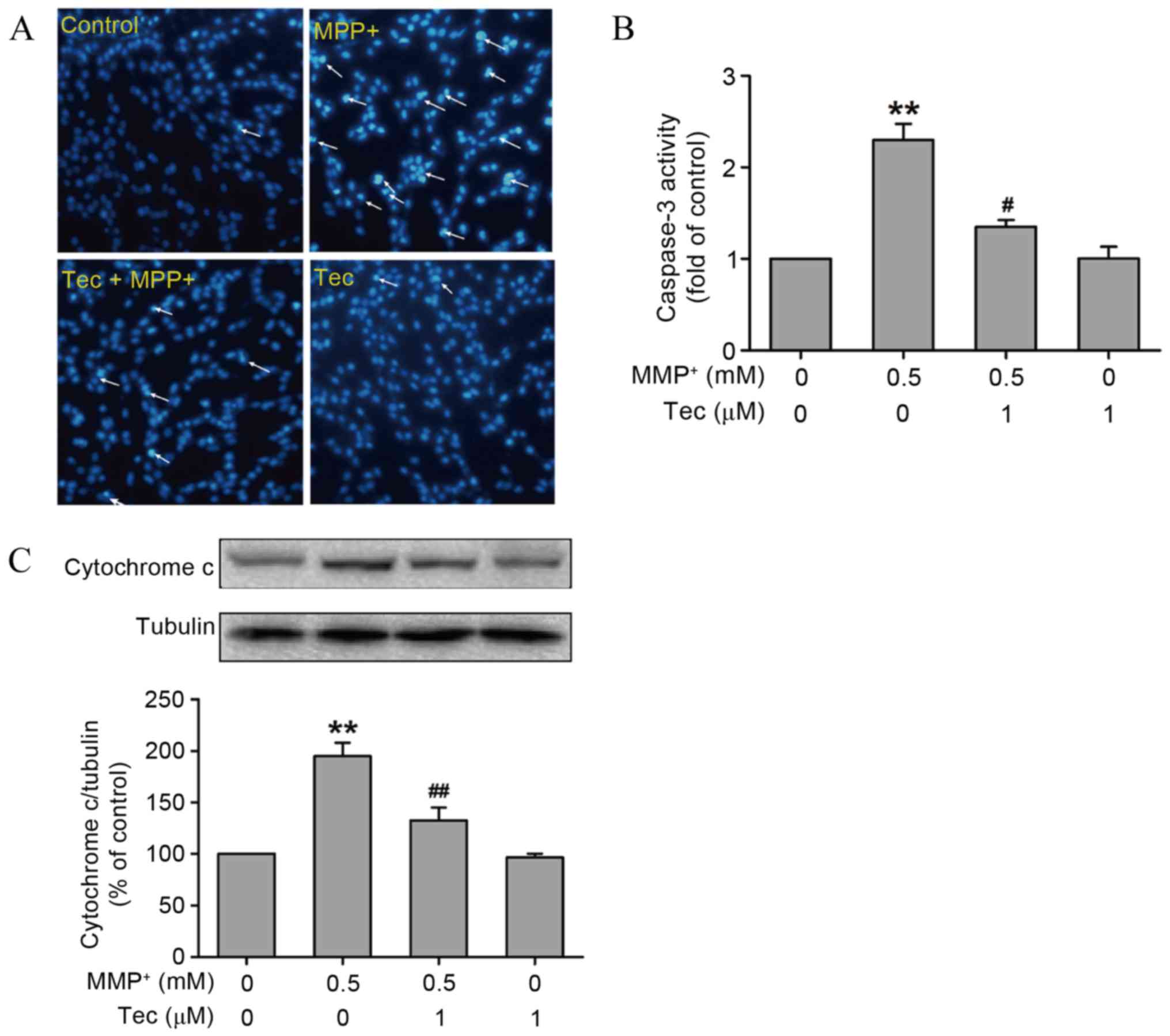
tectorigenin were investigated. The Hoechst 33258 staining results
revealed that the cells undergoing apoptosis, as indicated by dense
granular fluorescence, in the MPP+ (0.5 mM)-treated
alone group were increased in comparison with the control group.
However, this phenomenon was reversed by pretreatment with 1 µM
tectorigenin (Fig. 2A). Furthermore,
tectorigenin abolished the increase in the activity of caspase-3 (a
critical executioner of apoptosis) that was induced by
MPP+ incubation in SH-SY5Y cells (Fig. 2B). Western blot analysis results also
revealed that tectorigenin (1 µM) attenuated the upregulation of
cytochrome c levels induced by MPP+ (Fig. 2C). These findings indicate that
tectorigenin, which alone had no significant effect, produces
antiapoptotic effects in MPP+-treated SH-SY5Y cells.
Tectorigenin reverses
MPP+-induced changes of Bax and Bcl-2 expression levels
in SH-SY5Y cells
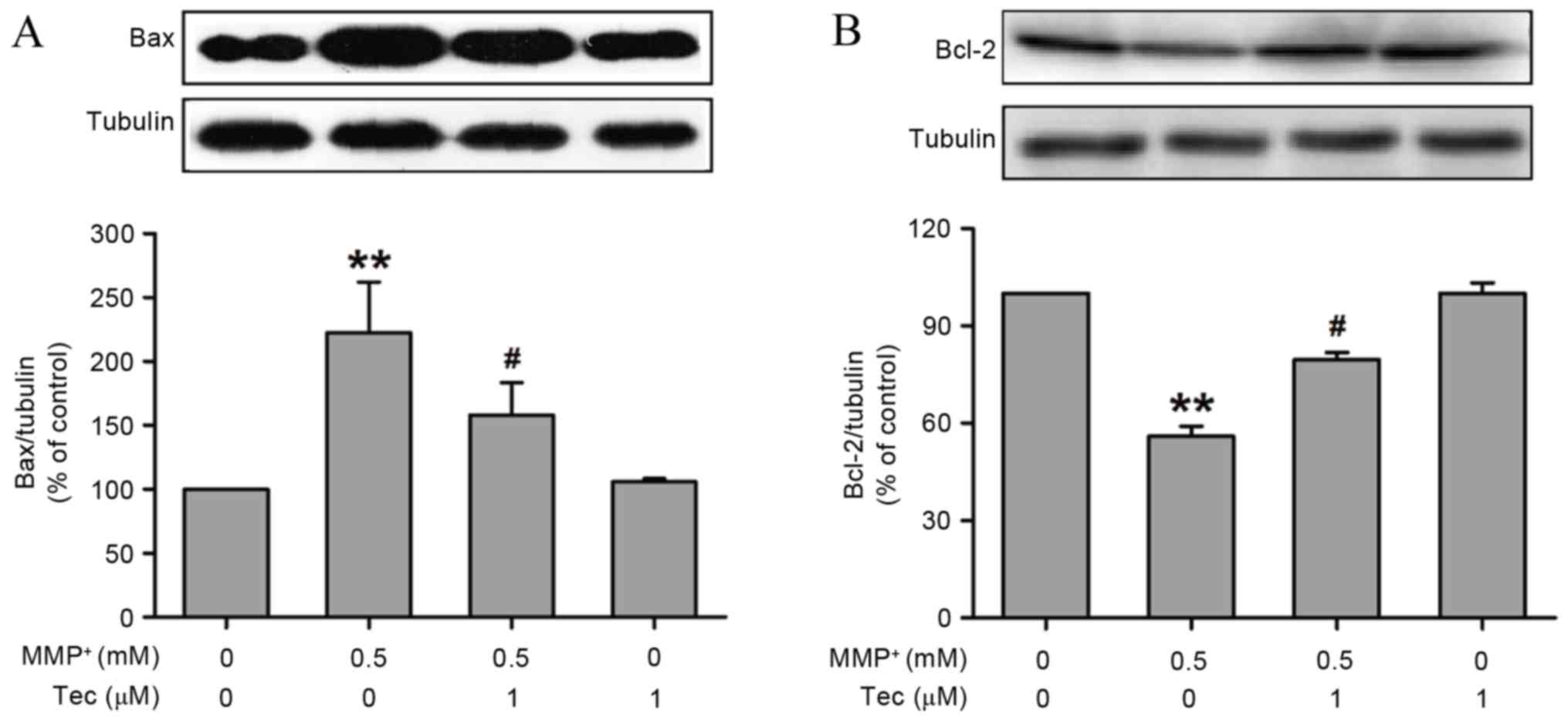
The present study also examined the expression
levels of apoptosis-associated proteins in SH-SY5Y cells that were
exposed to MPP+ (0.5 mM) in the presence or absence of
tectorigenin (1 µM). The Bax (pro-apoptotic protein) and Bcl-2
(anti-apoptotic protein) levels were determined by western blot
assay. As shown in Fig. 3,
MPP+ clearly promoted the expression of Bax (Fig. 3A) and inhibited the expression of
Bcl-2 (Fig. 3B), compared with that
of the control group. However, co-incubation of tectorigenin (1 µM)
and MPP+ (0.5 mM) evidently reversed the alterations of
Bax and Bcl-2 expression induced by treatment with MPP+
alone. These results suggest that tectorigenin decreases the ratio
of Bax/Bcl-2 and prevents MPP+-induced apoptosis.
Tectorigenin blocks
MPP+-induced ROS formation and NOX expression in SH-SY5Y
cells
Oxidative stress is known to serve an important role
in the pathological process of PD (19). To further investigate the underlying
protective mechanism of tectorigenin in MPP+-induced
neurotoxicity, the changes in oxidative stress under
MPP+ treatment in the presence or absence of
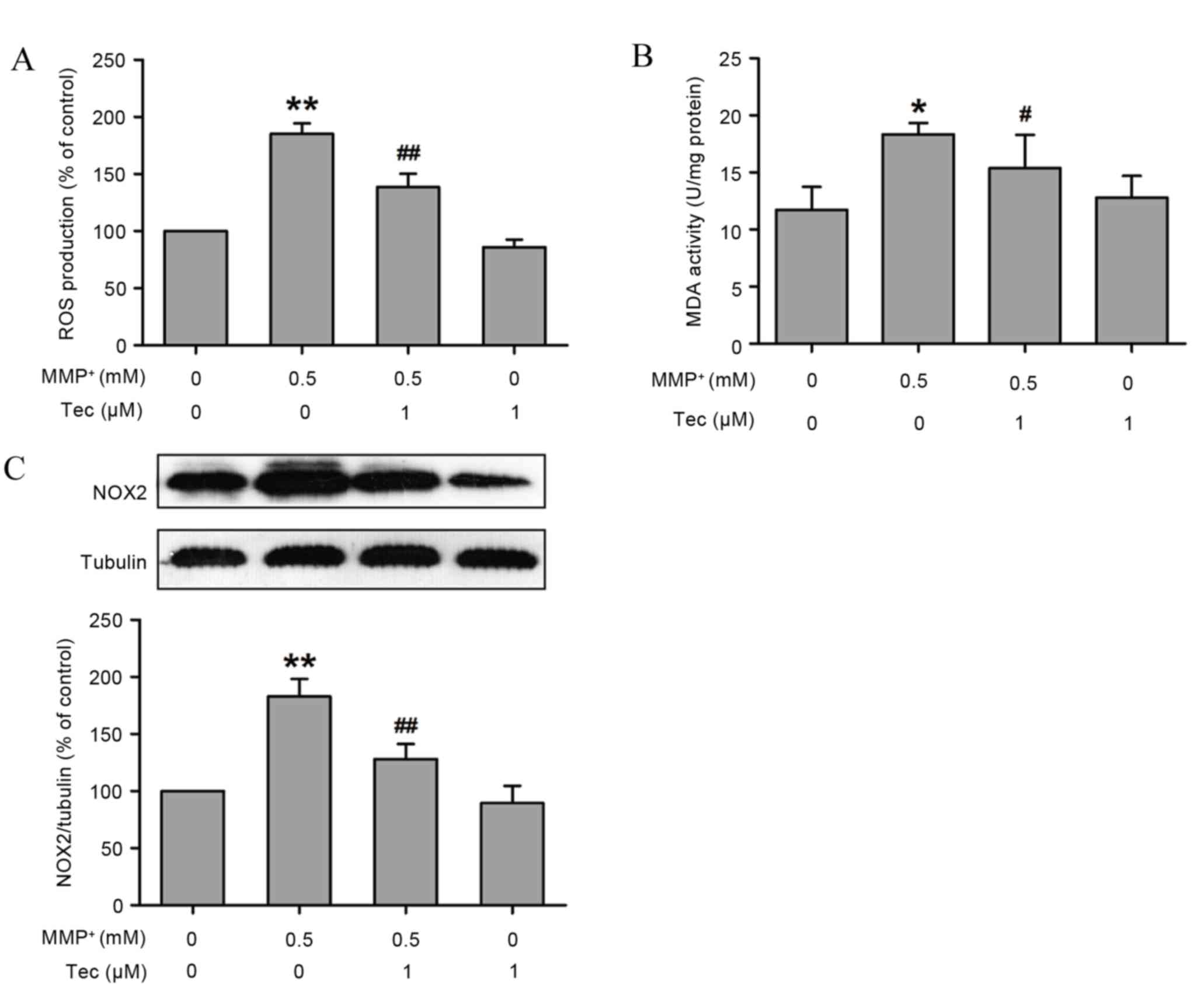
tectorigenin were examined. As shown in Fig. 4, compared with the control group,
MPP+ (0.5 mM) treatment alone led to an increase in ROS
formation in SH-SY5Y cells; however, this effect was evidently
reversed by pretreatment with tectorigenin (1 µM; Fig. 4A).
NOX is the most important enzyme in cells resulting
in generation of ROS in the central nervous system (20). Following exposure of SH-SY5Y cells to
MPP+ for 24 h, the expression of NOX was significantly
increased (Fig. 4B). However,
pretreatment with tectorigenin (1 µM) blocked the
MPP+-caused upregulation of NOX levels in SH-SY5Y cells
(Fig. 4B). Notably, tectorigenin
alone had no effect on oxidative stress (Fig. 4). These results reveal that
tectorigenin prevents MPP+-induced oxidative stress,
which may contribute to the protective functions of
tectorigenin.
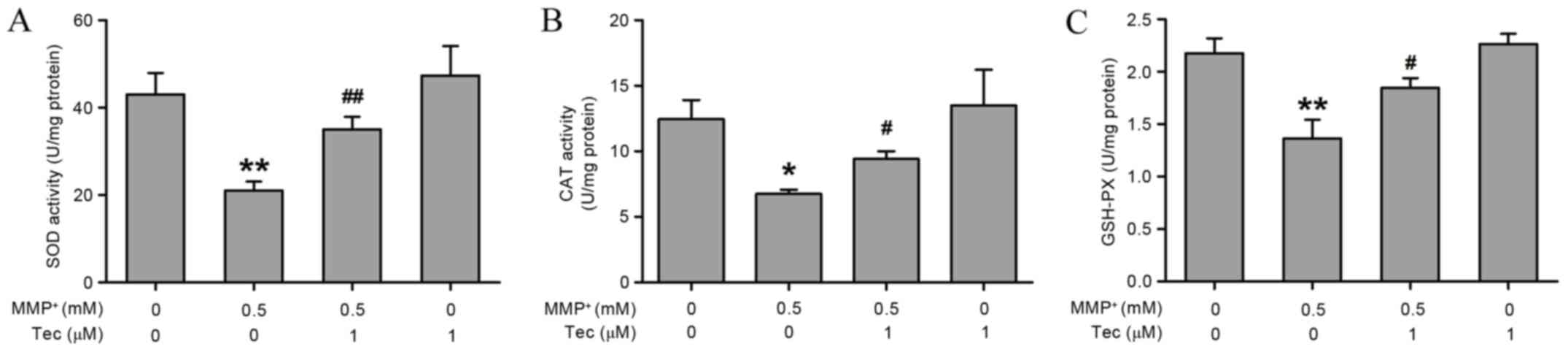
Tectorigenin restores
MPP+-induced decreases in antioxidant enzyme activities
in SH-SY5Y cells
Subsequently, the current study evaluated the
effects of tectorigenin on the activity of endogenous antioxidant
enzymes, which regulate the levels of ROS and free radicals in the
cells. As shown in Fig. 5, treatment
of SH-SY5Y cells with MPP+ (0.5 mM) significantly
decreased the activities of SOD (Fig.
5A) and CAT (Fig. 5B), as well
as the level of GSH-Px (Fig. 5C).
However, pretreatment with tectorigenin (1 µM) for 30 min, which
alone had no significant effect, prevented the
MPP+-induced decreases in antioxidant enzyme activities
(Fig. 5). These results indicate
that enhancement of antioxidant defense by tectorigenin may be
involved in the protective effects against MPP+-induced
neurotoxicity.
Discussion
The incidence of progressive neurodegeneration in PD
with increasing age involves the occurrence of oxidative stress.
However, there are currently no effective therapeutic drugs to
eliminate the excessive production of ROS and free radicals in the
nigrostriatal pathway of this disease. In the present study, the
in vitro PD model demonstrated that tectorigenin, an
antioxidant compound, abolished the neurotoxicity caused by
MPP+ treatment in SH-SY5Y cells. Tectorigenin reversed
the MPP+-induced reduction of cell viability and
increase of cell apoptosis. Notably, the underlying protective
mechanism of tectorigenin may be mediated through the attenuation
of ROS production and NOX expression, and the enhancement of
antioxidant defense, as evidenced by the downregulation of SOD, CAT
and GSH-Px levels.
In the 1980s, MPTP was reported to cause
Parkinsonian symptoms (21), and it
was later observed that it is biotransformed into toxic
MPP+ by glial cells, which can cross the blood-brain
barrier and enter dopaminergic neurons, resulting in mitochondrial
damage, the generation of oxidative stress and the activation of
pro-apoptotic pathways (22). In
agreement with these previous studies, the present study observed
that treatment of SH-SY5Y cells with MPP+ induced loss
of cell viability, occurrence of apoptotic features, enhancement of
caspase-3 activity and cytochrome c, which was associated
with the increases in the ratio of Bax/Bcl-2 and the elevation of
ROS levels in SH-SY5Y cells. Increasing evidence revealed that
oxidative stress serves an important role in the pathogenesis of PD
and causes death of dopamine neurons in the substantia nigra in
various ways (23). In recent years,
the beneficial effects of naturally occurring phytochemicals with
potent antioxidant properties on neurodegenerative disorders have
received increasing attention (24,25). It
has been reported that traditional Chinese medicines, particularly
plant-derived constituents, may have a potential clinical value in
alleviating the pathologic processes of PD (26). In the present study, tectorigenin,
which is a natural isoflavone derived from the flower of
Pueraria thunbergiana (Leguminosae), was found to attenuate
MPP+-induced cytotoxicity, apoptosis and ROS production
in SH-SY5Y cells. These results are in agreement with the
observations of a previous study, reporting that pretreatment with
Puerariae flos decreased the viability of cells, the
occurrence of apoptotic features and the mRNA expression of
caspase-3 in ethanol-treated human neuroblastoma SK-N-MC cell line
(27).
Excessive accumulation of ROS and free radicals, as
well as an imbalance of the oxidative stress and antioxidative
stress system, induce lipid peroxidation and damage DNA,
subsequently resulting in cell functional disorder and even
apoptosis (28,29). In addition, oxidative stress-induced
apoptosis participates in the development of nervous system
diseases, whereas intervention with antioxidants reduces the
apoptosis via inhibiting the generation of ROS and thereby
resulting in neuroprotective effects (19). Multiple studies have reported that
ROS contributes to the apoptosis-associated mechanism of
MPP+-mediated neurotoxicity (30). Therefore, in the present study, it is
hypothesized that the protective effects of tectorigenin against
MPP+-induced apoptosis is associated with modulation of
the oxidative stress and antioxidative stress system. Furthermore,
emerging evidence has demonstrated that NOX enzymes transport
electrons across the plasma membrane to promote the generation of
ROS, contributing to neuronal death in the pathological processes
of PD (20). In the present study,
it was also observed that pretreatment with tectorigenin
significantly mitigated the MPP+-caused upregulation of
NOX protein in SH-SY5Y cells, further suggesting the inhibitory
effect of tectorigenin on the oxidative stress system, which may
contribute to the neuroprotective effects of tectorigenin. However,
in order to verify this hypothesis, further investigations
involving the use of NOX enzyme inhibitors need to be
conducted.
Kang et al revealed that tectorigenin
enhances the activities of cellular antioxidant enzymes, including
SOD, CAT and GSH-Px, while it also increases the expression of
their protein levels, thus protecting V79-4 cells against
H2O2-mediated damage (16). Similarly, the present study indicated
that pretreatment with tectorigenin markedly abolished the
MPP+-induced inhibition of SOD, CAT and GSH-Px
activities in SH-SY5Y cells, which indicates that the protective
effect of tectorigenin against MPP+-induced
neurotoxicity may be mediated by upregulation of the antioxidant
defense system. However, a limitation of the present study is that
the inhibition of antioxidant enzymes was not used to further
verify this hypothesis.
In conclusion, using MPP+-treated SH-SY5Y
cells to simulate PD symptoms, the present study determined that
tectorigenin exerted a neuroprotective effect against PD. The
mechanism underlying this protective effect was further
investigated and found to be associated with the antioxidant
activity of tectorigenin, as indicated by the tectorigenin-induced
downregulation of oxidative stress and enhancement of antioxidant
defense.
References
|
1
|
Lo Bianco C, Schneider BL, Bauer M, Sajadi
A, Brice A, Iwatsubo T and Aebischer P: Lentiviral vector delivery
of parkin prevents dopaminergic degeneration in an alpha-synuclein
rat model of Parkinson's disease. Proc Natl Acad Sci USA.
101:17510–17515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jellinger KA: The pathology of Parkinson's
disease. Adv Neurol. 86:55–72. 2001.PubMed/NCBI
|
|
3
|
Bales A, Peterson MJ, Ojha S, Upadhaya K,
Adhikari B and Barrett B: Associations between betel nut (Areca
catechu) and symptoms of schizophrenia among patients in Nepal:
A longitudinal study. Psychiatry Res. 169:203–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beal MF: Mitochondria, oxidative damage,
and inflammation in Parkinson's disease. Ann N Y Acad Sci.
991:120–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon HE and Paek SH: Mitochondrial
dysfunction in Parkinson's disease. Exp Neurobiol. 24:103–116.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blesa J, Trigo-Damas I, Quiroga-Varela A
and Jackson-Lewis VR: Oxidative stress and Parkinson's disease.
Front Neuroanat. 9:912015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim GH, Kim JE, Rhie SJ and Yoon S: The
role of oxidative stress in neurodegenerative diseases. Exp
neurobiol. 24:325–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turski L, Bressler K, Rettig KJ, Loschmann
PA and Wachtel H: Protection of substantia nigra from
MPP+ neurotoxicity by N-methyl-D-aspartate antagonists.
Nature. 349:414–418. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singer TP and Ramsay RR: Mechanism of the
neurotoxicity of MPTP. An update. FEBS Lett. 274:1–8. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han YO, Han MJ, Park SH and Kim DH:
Protective effects of kakkalide from Flos puerariae on
ethanol-induced lethality and hepatic injury are dependent on its
biotransformation by human intestinal microflora. J pharmacol Sci.
93:331–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutierrez-Zepeda A, Santell R, Wu Z, Brown
M, Wu Y, Khan I, Link CD, Zhao B and Luo Y: Soy isoflavone
glycitein protects against beta amyloid-induced toxicity and
oxidative stress in transgenic Caenorhabditis elegans. BMC
Neurosci. 6:542005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan D, Xie YY, Bai X, Wu X, Yang JY and
Wu CF: Inhibitory activity of isoflavones of Pueraria flowers on
nitric oxide production from lipopolysaccharide-activated primary
rat microglia. J Asian Nat Prod Res. 11:471–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han T, Cheng G, Liu Y, Yang H, Hu YT and
Huang W: in vitro evaluation of tectoridin, tectorigenin and
tectorigenin sodium sulfonate on antioxidant properties. Food Chem
Toxicol. 50:409–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park EK, Shin YW, Lee HU, Lee CS and Kim
DH: Passive cutaneous anaphylaxis-inhibitory action of
tectorigenin, a metabolite of tectoridin by intestinal microflora.
Biol Pharm Bull. 27:1099–1102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KT, Sohn IC, Kim DH, Choi JW, Kwon SH
and Park HJ: Hypoglycemic and hypolipidemic effects of tectorigenin
and kaikasaponin III in the streptozotocin-lnduced diabetic rat and
their antioxidant activity in vitro. Arch Pharm Res. 23:461–466.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang KA, Lee KH, Chae S, Zhang R, Jung MS,
Kim SY, Kim HS, Kim DH and Hyun JW: Cytoprotective effect of
tectorigenin, a metabolite formed by transformation of tectoridin
by intestinal microflora, on oxidative stress induced by hydrogen
peroxide. Eur J pharmacol. 519:16–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JS, Jung JS, Jeong YH, Hyun JW, Le
TK, Kim DH, Choi EC and Kim HS: Antioxidant mechanism of isoflavone
metabolites in hydrogen peroxide-stimulated rat primary astrocytes:
Critical role of hemeoxygenase-1 and NQO1 expression. J neurochem.
119:909–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Racher AJ, Looby D and Griffiths JB: Use
of lactate dehydrogenase release to assess changes in culture
viability. Cytotechnology. 3:301–307. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katunina EA, Titova NV, Malykhina EA,
Gasanov MG, Makarova AA, Voronina TA, Nerobkova LN, Valdman EA and
Avakyan GN: Oxidative stress and Parkinson's disease: Mechanisms
and perspectives of treatment. Zh Nevrol Psikhiatr Im S S
Korsakova. 115:141–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorce S and Krause KH: NOX enzymes in the
central nervous system: From signaling to disease. Antioxid redox
signal. 11:2481–2504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Markey SP, Johannessen JN, Chiueh CC,
Burns RS and Herkenham MA: Intraneuronal generation of a pyridinium
metabolite may cause drug-induced parkinsonism. Nature.
311:464–467. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khwanraj K, Phruksaniyom C, Madlah S and
Dharmasaroja P: Differential expression of tyrosine hydroxylase
protein and apoptosis-related genes in differentiated and
undifferentiated SH-SY5Y neuroblastoma cells treated with
MPP+. Neurol Res Int. 2015:7347032015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramaniam SR and Chesselet MF:
Mitochondrial dysfunction and oxidative stress in Parkinson's
disease. Prog Neurobiol. 106–107:17–32. 2013. View Article : Google Scholar
|
|
24
|
Houghton PJ and Howes MJ: Natural products
and derivatives affecting neurotransmission relevant to Alzheimer's
and Parkinson's disease. Neurosignals. 14:6–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mythri RB, Harish G and Bharath MM:
Therapeutic potential of natural products in Parkinson's disease.
Recent Pat Endocr Metab Immune Drug Discov. 6:181–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu W, Zhuang W, Zhou S and Wang X:
Plant-derived neuroprotective agents in Parkinson's disease. Am J
Transl Res. 7:1189–1202. 2015.PubMed/NCBI
|
|
27
|
Jang MH, Shin MC, Kim YJ, Chung JH, Yim
SV, Kim EH, Kim Y and Kim CJ: Protective effects of Puerariae
flos against ethanol-induced apoptosis on human neuroblastoma
cell line SK-N-MC. Jap J Pharmacol. 87:338–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stadtman ER: Role of oxidant species in
aging. Curr Med Chem. 11:1105–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohr JB and Browning JA: Free radical
involvement in neuropsychiatric illnesses. Psychopharmacol Bull.
31:159–165. 1995.PubMed/NCBI
|
|
30
|
Di Monte D, Sandy MS, Ekström G and Smith
MT: Comparative studies on the mechanisms of paraquat and
1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem
Biophys Res Commun. 137:303–309. 1986. View Article : Google Scholar : PubMed/NCBI
|