Introduction
Bile duct cancers are characterized by horizontal
advancement along the bile duct wall. When a lesion extends toward
the liver side and reaches the intrahepatic region, hepatectomy is
necessary for resection (1).
However, the resection of intrahepatic bile duct has limitations.
Further resection is not possible if cancer cells are still present
(1). Furthermore, residual cancer
may be observed in the bile duct stump on postoperative
histopathological examination, and postoperative adjuvant therapy
may be required (2).
Photodynamic therapy (PDT) is a method used to treat
tumors by utilizing photodynamic reactions between photosensitive
substances with tumor affinity and lasers (3–5). For
inoperable bile duct cancer, PDT has been demonstrated to
effectively resolve cancer-associated stenosis and improve patient
prognosis (3–5). However, the effects of PDT on bile duct
cancer that have advanced into the intrahepatic bile duct have not
been reported. This is due to two difficulties; the first is that
it remains unclear whether PDT may be safely applied in the liver,
as photosensitive substances accumulate there. When PDT impairs
normal bile duct epithelial cells, outflow obstruction of bile
occurs in a thin intrahepatic bile duct, causing segmental
cholangitis and biloma (6,7). Furthermore, impairment of endothelial
cells in the hepatic artery and portal vein, which are parallel to
the bile duct, may cause thrombus formation and vascular breakage,
inducing hepatic infarction and hemorrhage (6,7). To
overcome this, a novel second-generation photosensitive substance,
talaporfin sodium, was used in the present study. Talaporfin sodium
accumulates very rapidly in tumors compared with conventional
photosensitive substances, and is excreted by normal tissues
(8–10). The second difficulty is that the
charge-coupled device (CCD) camera-equipped cholangioscope
typically used for treatment has a large diameter and cannot be
advanced into intrahepatic bile ducts. To overcome this, a
parallel-type ultra-small composite optical fiberscope (COF) was
developed, through which transpapillary PDT was able to be applied
to peripheral intrahepatic bile duct cancer (11,12). The
COF used in the present study was equipped with a camera for
observation with ordinary light and a laser irradiation hole, with
a diameter <1 mm. By advancing the COF upstream of the stenoic
region using the conventional duodenoscope as a parent scope,
observation of and laser irradiation to intrahepatic bile ducts
becomes possible. The aim of the present study was to investigate
the efficacy of talaporfin sodium and COF.
Materials and methods
Animals
A total of 2 female Japanese white rabbits (mean
body weight, 2.57 kg; age, 13 weeks old) were obtained from Sankyo
Labo Service Co., Inc., (Tokyo Japan). Rabbits were fed with
standard feed (CR-1; CLEA Japan, Inc., Tokyo, Japan) and
adminsitered 150 g/day and rabbits had free access to water. A
total of 2 female miniature swines (mean body weight, 8.4 kg; age,
5 months old) were obtained from Orienttal Yeast Co., Ltd. (Tokyo,
Japan). Their feed (MP-A; Orienttal Yeast Co., Ltd.) was given
ad libitum following high-pressure steam sterilization and
swines had free access to water. Animals were individually housed
in cages in a temperature-controlled room (22–24°C) with a relative
humidity of 60–65% and subjected to a 12-h light/dark cycle at
Tokyo Medical University Hospital (Tokyo, Japan). All experiments
were approved by the Animal Care and Ethics Committee of Tokyo
Medical University.
Photosensitizer
Talaporfin sodium (Laserphyrin; Meji Seika, Ltd.,
Tokyo, Japan) is composed of aspartic acid conjugated to the D ring
of the chlorine structure with an absorption peak at 664 nm
(8,13). Laserphyrin was reconstituted as a 1.0
mg/ml solution to prevent degradation by light.
Laser
A high-power red laser diode system (Panasonic
Healthcare Co., Ltd., Tokyo, Japan), was used in the present study.
The laser wavelength was adjusted to 664 nm to match the absorption
bands of Laserphyrin, and the system has a power output of 10–500
mW/cm2 at the fiber tip in a continuous wave mode. The
delivered energy was adjustable from 50–1,000 J/cm2.
Laser probe one
Radial probes (ZH-L5041HJP; Panasonic Healthcare
Co., Ltd.) were used for laser radiation to the Japanese white
rabbits.
Laser probe two
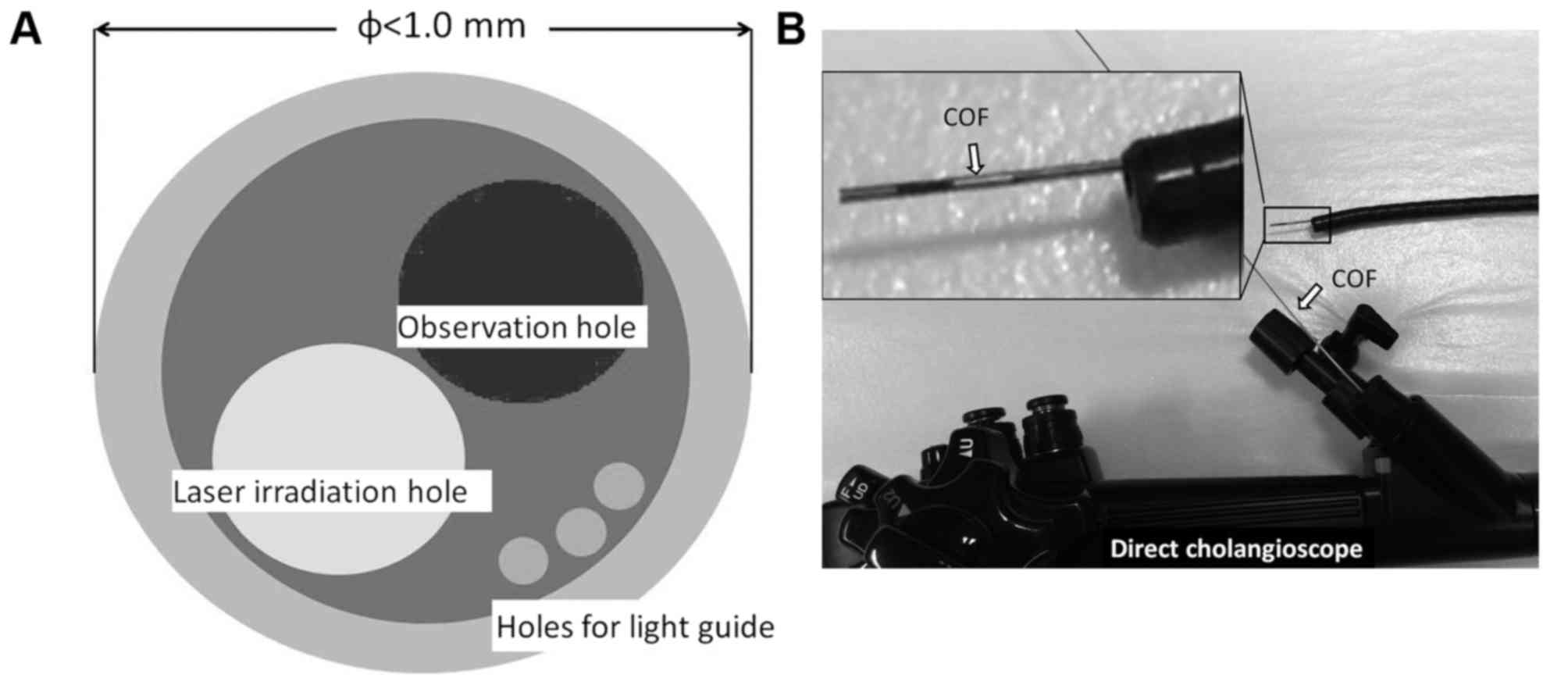
COF (OK Fiber Technology Co., Ltd., Kyoto, Japan)
was used for laser radiation to miniature swine. This COF was
independently developed as an endoscope by co-author Dr. Oka for
this study as previously described (11,12), but
with a smaller outer diameter. The outer diameter of the COF is
<1 mm, and the scope has a light guide, holes for observation
(10,000 pixels) and a photodynamic laser with a diameter of 0.4 mm
(Fig. 1A). The COF was used with the
transpapillary approach. Firstly, the target peripheral bile duct
was identified by endoscopic retrograde cholangiopancreatography
and a guide wire was inserted. A guide sheath (disposal guide
sheath SG-200C; Olympus Corp., Tokyo, Japan; outer diameter, 1.95
mm; translucent with a slightly bent tip and high bile duct
selectivity) was applied into which the COF was inserted. The guide
sheath was used to avoid excess bending of the COF and saline was
perfused into the sheath in order to secure a visual field in the
bile duct. The COF is able to be directly inserted through the
forceps hole of a duodenoscope, cholangioscope, SpyScope and
next-generation direct cholangioscope (Fig. 1B).
Evaluation of Glisson's capsule
ensheathing the hepatic artery, portal vein, and bile ducts and
hepatocytes disorder by PDT (experiment one)
This experiment was performed to evaluate bile duct
and hepatic disorders associated with laser irradiation inside the
biliary ductal lumen. Japanese white rabbits were intraperitoneally
anesthetized with pentobarbital sodium (Kyoritsuseiyaku Corp.,
Tokyo, Japan) and underwent laparotomy 4 h following the
intravenous administration of Laserphyrin at 63 mg/kg, as
previously described (9,10). An incision was made in the duodenum
wall, and a 6 Fr sheath was inserted trans-transpapillary. Laser
probe one was inserted toward an intrahepatic bile duct using the
lumen of the sheath. Intraluminal laser irradiation (100
J/cm2) was performed with continuous application of
physiological saline into the lumen of the sheath. Following
irradiation, the fiber and sheath were removed and the duodenum
wall was closed with sutures. Rabbits were administered with Evans
blue stain solution via an ear vein and were stained blue at 48 h
after PDT. The liver and the biliary tract were harvested and fixed
for 2 days in 10% neutral buffered formaldehyde (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) at room temperature and embedded in
paraffin. Sections (2–3 µm thickness) were stained using
hematoxylin-eosin for histological diagnosis at a magnificaiton
×400 using an optical microscope (Olympus Corp.) following
sacrifice.
Evaluation of normal gallbladder
epithelial damage by PDT (experiment two)
A miniature swine was laparotomized under general
anesthesia, the gallbladder wall was incised and the guide sheath
was inserted. The COF was inserted and advanced to near the sheath,
and the gallbladder mucosa was observed while physiological saline
was applied. Laserphyrin was then intravenously administered at a
dosage of 10 mg/kg. A total of 4 h following Laserphyrin
administration, a laser was applied to the gallbladder mucosa at
the fundic and neck area of the gallbladder (100 J/cm2).
The irradiation region was marked with non-absorbable surgical
suture. The fundic area of the gallbladder alone was excised
immediately following irradiation. The lumen of the remnant
gallbladder was subsequently closed. Subsequently, the swine was
sacrificed and the remnant gallbladder was harvested at 4 weeks
post irradiation. The excised specimen was processed through the
standard course described above (n=2).
Results
Experiment one
Following PDT via the biliary ductal lumen, tissue
damage in the liver and biliary duct of Japanese white rabbits
(n=2) were assessed. All animals survived without apparent disorder
48 h following treatment. All organs were stained blue, and the
blood flow of the whole liver was confirmed macroscopically.
Histologically, the epithelium of the extrahepatic bile duct and
adjacent tissue and blood vessels were normal. No disorder or
damage was observed in the intrahepatic bile ducts, portal vein,
intrahepatic veins, hepatic artery or intrahepatic arteries around
the first branch of the right intrahepatic Glisson (Fig. 2A). Focal ischemic necrosis was
observed in hepatic cells around the extrahepatic Grison and those
at the most marginal site in the irradiation area (Fig. 2B).
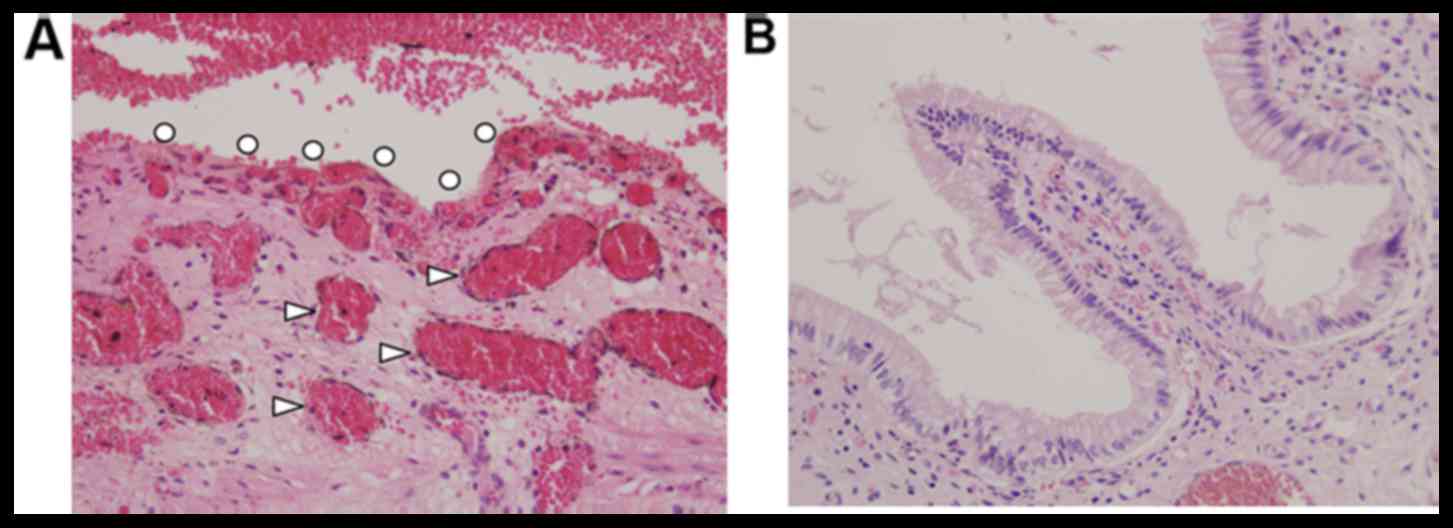
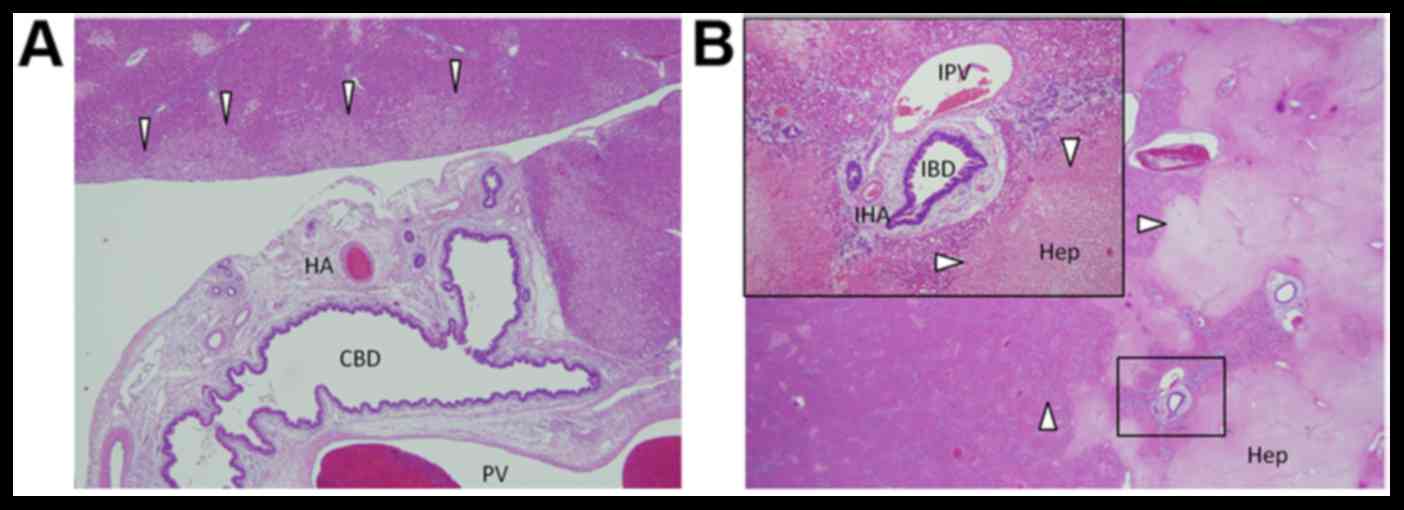 | Figure 2.Histological features of the extra-
and intrahepatic bile duct and adjacent tissue. (A) No damage was
observed to the bile duct epithelium, portal vein or hepatic
artery. In contrast, mild degeneration was observed in liver cells
adjacent to the irradiation field (white arrow heads). (B) Damage
to intrahepatic Glissons capsule was low compared with the
surrounding hepatocytes (white arrow heads). HA, hepatic artery;
CBD, common bile duct; PV, portal vein; IBD, intrahepatic bile
duct; IPV, intrahepatic portal vein; IHA, intrahepatic hepatic
artery; Hep, hepatocytes. (magnification, ×40). |
Experiment two
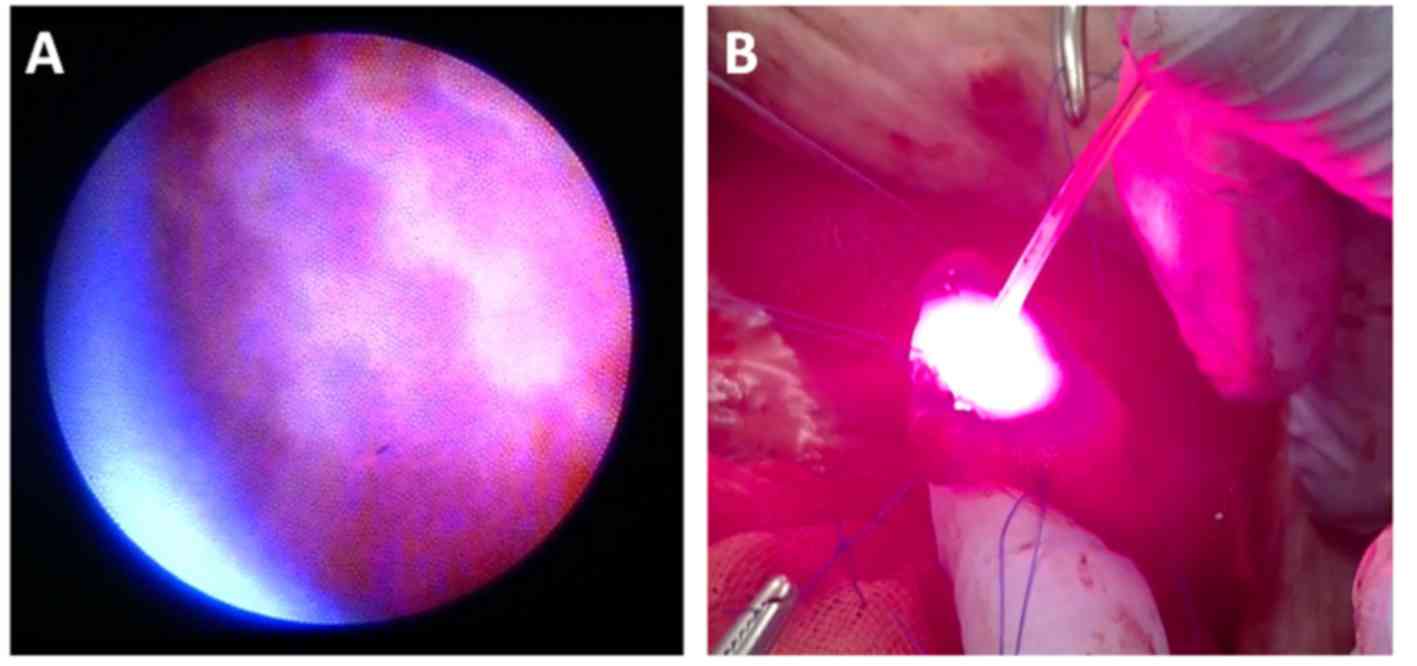
The mucosal folds of the gallbladder were observed
using the COF and saline circulation (Fig. 3). Congestion occurred immediately
following irradiation, and intra- and submucosal vasodilatation
occurred (Fig. 4A). At 4 weeks
post-irradiation, the mucosa appeared mostly normal (Fig. 4B).
Discussion
Transhepatic PDT was applied to 7 patients with
non-resected bile duct cancer (locally advanced cancer in 5
patients, and lesions were localized in the wall but not resected
due to another reason in 2 patients) at Tokyo Medical University in
1999, resulting in sufficient tumor control in the bile duct lumen
and conversion to cancer negativity on biopsy (6). In 2006, median survival time was
compared between patients treated with multidisciplinary treatment
including PDT (n=34) and untreated (n=23) (7). In 2006, a study by Witzigmann et
al (5) reported the 10-year
outcomes of PDT-treated patients (n=60) with unresectable bile duct
cancer. The 1-, 3- and 5-year survival rates were 69, 30 and 22%,
respectively, whereas the 5-year survival rates of patients
surgically treated with R0, R1 and R2 resections were 27, 10 and
0%, respectively. These results demonstrated that the 5-year
survival rate of patients treated with PDT exceeded those of
patients treated with R1 and R2 resections (5).
The objective of the present study was to
investigate local treatment with PDT as a surgical
resection-associated neo-adjuvant or postoperative adjuvant
therapy. Although there is no specification of PDT as adjuvant
therapy, PDT was applied to stump-positive cases predicted to have
residual cancer on the cut surface of the bile duct as adjuvant. In
2000, a study by Berr et al (14) applied PDT to a tumor of the common
bile duct at 250 J/cm2, and the bilateral bile ducts at
250 J/cm2, and performed a hepatectomy 23 days later
(14). It was observed that the
tumor at a 4-mm depth was completely necrotized, hepatic
duct-jejunum anastomosis formation completed, and no stenosis of
the anastomosed region occurred even at 18 months post-treatment.
This was the initial report on neo-adjuvant PDT for bile duct
cancer (14). In 2003, a study by
Wiedmann et al (15)
performed surgery following PDT at 242 J/cm2 and
achieved complete necrosis of the tumor at a 4-mm depth, with only
mild inflammation of the tumor-free bile duct and mild
complications of hepatocholangiojejunostomy in 4 patients (15). Based on these reports, surgery
performed following PDT does not seriously impair hepatic
duct-jejunum anastomosis. In the present study, PDT applied to the
gallbladder epithelium in miniature swine caused congestion and
edema immediately following irradiation; however, the epithelium
recovered to almost normal after 4 weeks. A study by Nanashima
et al (16) applied
Photofrin-PDT to the bile duct in 8 patients following non-curative
surgical resection. The recurrence-free period was 17.6 months,
significantly longer compared with the patients who were not
treated with PDT (8 months). A total of 4 stump-positive patients
were subsequently treated with Laserphyrin-PDT. Liver metastasis
occurred in 1 patient (without local recurrence), however
6–13-month recurrence-free survival was confirmed in the remaining
3 patients (16).
Photofrin, a first-generation photosensitive
substance, requires an ~4-week shading period in a dimly-lit room
(100–300 lux) and sunlight and intensive light must be avoided
following administration to the human body (7,17). Early
discovery of intrahepatic bile duct cancer is difficult, and so
lesions are typically already advanced cancer at the point of
discovery (17). Photofrin-PDT
cannot be additionally applied for a prolonged period following
PDT, making it unsuitable for the treatment of intrahepatic bile
duct cancer (17). In contrast, this
shading period is reduced to 3–7 days with Laserphyrin. The 664-nm
long wavelength laser used with Laserphyrin penetrates tissue well,
for which a strong PDT effect is expected. Nanashima et al
(16) initiated Photofrin-PDT in
2001 and subsequently adopted Laserphyrin-PDT. They also compared
the cytocidal effects between the combinations of Laserphyrin-PDT
with cisplatin, oxaliplatin, gemcitabine and fluorouracil, and PDT
alone. All agents potentiated the effect. The excimer dye laser
used in Photofrin-PDT is a large device, whereas the PD laser is
much smaller (18).
The most important thing to consider when applying
PDT through the inside of the bile duct is its influence on
important organs, i.e., the portal vein, hepatic artery and bile
duct. Destruction or damage to these blood vessels may cause
hemorrhage following PDT, and damage to the thick bile duct may
cause obstructions and cholangitis (7). In experiment one of the present study,
PDT was applied via the intrahepatic bile duct lumen employing the
transpapillary approach, and the intra- or extrahepatic Glisson's
capsule (hepatic artery, portal vein and bile duct) was not
affected, suggesting that Laserphyrin in the bile does not damage
the bile duct epithelium. It was assumed that, although Laserphyrin
is present in blood, excited Laserphyrin rapidly moves through the
blood vessels and does not damage the vascular endothelium in the
irradiated region. In contrast, hepatocytes that incorporated a
large amount of Laserphyrin and bile components were impaired by
Laserphyrin. However, this does not cause complications due to the
regenerative ability of hepatocytes unless hemorrhage, abscess or
bile congestion occurs (10). Based
on the above basic experimental findings (10), liver disorders caused by Laserphyrin
accumulating in hepatocytes were expected; however, based on
clinical experience of radiofrequency irradiation, it was concluded
that this may be avoided by adjusting the laser irradiation
dose.
Transpapillary PDT is typically performed using a
circumferential probe with a non-X-ray permeable marker under
fluoroscopy (7). However,
application under direct vision may be advantageous for the effect
and safety if it is possible. In 2011, a study by Choi et al
(19) inserted a nasal endoscope
into the common bile duct using an auxiliary balloon-equipped
over-tube (5 Fr) or 5.5-mm tube for insertion, and PDT was applied
under direct vision with a success rate of 77.8% (7/9). With this
endoscope, the limit of the application site is the lower bile
duct, and a fiber with an extra-fine diameter is necessary for
insertion into the liver side. For the fiber with a transpapillary
insertable extra-fine diameter, SpyGlass system, which may be
inserted through the conventional duodenoscope, has been clinically
used since 2011 (20). This system
is comprised of a child scope, SpyGlass (outer diameter, 0.8 mm;
light source and fiber for observation, 6,000 pixels) and SpyScope
(outer diameter, 3.3 mm; two holes for water supply, one hole for
device water absorption and one hole for SpyGlass) serving as an
outer frame. SpyScope is capable of transporting SpyGlass with an
ultrafine diameter into a deep bile duct without damaging it, as
well as sufficiently washing and removing bile in the bile duct
through the independent water supply and absorption holes. In the
present study, COF was applied instead of the SpyGlass. A study by
Shigetomi et al (21)
reported insertion and laser irradiation of COF in the uterus
following hysterectomy. A guiding catheter was used to insert COF
into the uterine cavity via an orifice in the uterus, similar to
the present study. The uterine cavity was filled with saline and
observed. It was reported that the image quality of COF was
compatible compared with a conventional uteroscope. However, the
obtained image was inferior compared with CCD (21). In the present study, the well-known
normal fold-like protuberance of the reticular pattern was visually
recognized on the gallbladder mucosa via the COF. It was decided
that this level of accuracy was sufficient for viewing the mucosal
lesions. In conclusion, Laserphyrin did not induce necrotic changes
in the normal bile duct in the present study. In addition, the COF
was capable of acquiring high quality images. PDT applied to the
superficial layer of bile duct cancer under direct vision may be a
suitable technique for the treatment of intractable tumors,
including hilar and intrahepatic cholangiocarcinomas.
Acknowledgements
The present study was supported by the Grant-in-Aid
for Scientific Research (C) of the Japan Society for the Promotion
of Science (grant no. 25462126).
Glossary
Abbreviations
Abbreviations:
|
PDT
|
photodynamic therapy
|
|
COF
|
parallel-type ultra-small composite
optical fiberscope
|
References
|
1
|
Ebata T, Watanebe H, Ajioka Y, Oda K and
Nimura Y: Pathological appraisal of lines of resection for bile
duct carcinoma. Br J Surg. 89:1260–1267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakanishi Y, Kondo S, Zen Y, Yonemori A,
Kubota K, Kawakami H, Tanaka E, Hirano S, Itoh T and Nakanuma Y:
Impact of residual in situ carcinoma on postoperative survival in
125 patients with extrahepatic bile duct carcinoma. J Hepatobiliary
Pancreat Sci. 17:166–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ortner ME, Caca K, Berr F, Liebetruth J,
Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J and
Lochs H: Successful photodynamic therapy for nonresectable
cholangiocarcinoma: A randomized prospective study.
Gastroenterology. 125:1355–1363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiedmann M, Berr F, Schiefke I, Witzigmann
H, Kohlhaw K, Mössner J and Caca K: Photodynamic therapy in
patients with non-resectable hilar cholangiocarcinoma: 5-year
follow-up of a prospective phase II study. Gastrointest Endosc.
60:68–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witzigmann H, Berr F, Ringel U, Caca K,
Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J,
Hauss J and Wiedmann M: Surgical and palliative management and
outcome in 184 patients with hilar cholangiocarcinoma: Palliative
photodynamic therapy plus stenting is comparable to r1/r2
resection. Ann Surg. 244:230–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeda K, Shinohara Y, Takei K, Itoi T,
Nakamura K, Saito T, Okunaka T and Kato H: Photodynamic therapy for
bile duct cancer. Endoscopia Digestiva. 14:1171–1176. 1999.(In
Japanese).
|
|
7
|
Itoi T, Sofuni A, Itokawa F, Tsuchiya T,
Kurihara T, Fukuzawa K, Shinohara Y, Takeda K, Takahashi T,
Hashimoto T and Moriyasu F: Multidisciplinary therapy in patients
with unresectable hilar bile duct carcinomas. Endoscopia Digestiva.
18:87–94. 2006.(In Japanese).
|
|
8
|
Wong Kee, Song LM, Wang KK and Zinsmeister
AR: Mono-L-aspartyl chlorine e6 (NPe6) and hematoporphyrin
derivative (HpD) in photodynamic therapy administered to a human
cholangiocarcinoma model. Cancer. 82:421–427. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura K and Aizawa K: Photodynamic
therapy (PDT) of transplanted VX2 cells bile duct tumor in rabbits
using Mono-l-aspartyl chlorine e6 (ME2906). Tokyo Ika Daigaku
Zasshi (J Tokyo Med Univ). 58:502–510. 2000.(In Japanese).
|
|
10
|
Kasuya K, Shimazu M, Suzuki M, Kuroiwa Y,
Usuda J, Itoi T, Tsuchida A and Aoki T: Novel photodynamic therapy
against biliary tract carcinoma using mono-L:-aspartyl chlorine e6:
Basic evaluation for its feasibility and efficacy. J Hepatobiliary
Pancreat Sci. 17:313–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oka K, Seki T, Naganawa A, Kim K and Chiba
T: A novel ultrasmall composite optical fiberscope. Surg Endosc.
25:2368–2371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oka K, Seki T, Naganawa A, Yamashita H,
Kim K and Chiba T: The development of a composite-type optical
fiberscope system for fetoscopic laser photocoagulation of
chorionic plate anastomosing vessels (FLPC). Minim Invasive Ther
Allied Technol. 19:94–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato H, Furukawa K, Sato M, Okunaka T,
Kusunoki Y, Kawahara M, Fukuoka M, Miyazawa T, Yana T, Matsui K, et
al: Phase II clinical study of photodynamic therapy using
mono-L-aspartyl chlorin e6 and diode laser for early superficial
squamous cell carcinoma of the lung. Lung Cancer. 42:103–111. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berr F, Tannapfel A, Lamesch P, Pahernik
S, Wiedmann M, Halm U, Goetz AE, Mössner J and Hauss J: Neoadjuvant
photodynamic therapy before curative resection of proximal bile
duct carcinoma. J Hepatol. 32:352–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wiedmann M, Caca K, Berr F, Schiefke I,
Tannapfel A, Wittekind C, Mössner J, Hauss J and Witzigmann H:
Neoadjuvant photodynamic therapy as a new approach to treating
hilar cholangiocarcinoma: A phase II pilot study. Cancer.
97:2783–2790. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nanashima A, Abo T, Nonaka T, Nonaka Y,
Morisaki T, Uehara R, Ohnita K, Fukuda D, Murakami G, Tou K, et al:
Photodynamic therapy using talaporfin sodium
(Laserphyrin®) for bile duct carcinoma: A preliminary
clinical trial. Anticancer Res. 32:4931–4938. 2012.PubMed/NCBI
|
|
17
|
Belliner DA, Greco WR, Loewen GM, Nava H,
Oseroff AR and Dougherty TJ: Clinical pharmacokinetics of the PDT
photosensitizers porfimer sodium (Photofrin),
2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (Photochlor) and
5-ALA-induced protoporphyrin IX. Laser Surg Med. 38:439–444. 2006.
View Article : Google Scholar
|
|
18
|
Nanashima A, Yamaguchi H, Shibasaki S, Ide
N, Sawai T, Tsuji T, Hidaka S, Sumida Y, Nakagoe T and Nagayasu T:
Adjuvant photodynamic therapy for bile duct carcinoma after
surgery: A preliminary study. J Gastroenterol. 39:1095–1101. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi HJ, Moon JH, Ko BM, Min SK, Song AR,
Lee TH, Cheon YK, Cho YD and Park SH: Clinical feasibility of
direct peroral cholangioscopy-guided photodynamic therapy for
inoperable cholangiocarcinoma performed by using an ultra-slim
upper endoscope (with videos). Gastrointest Endosc. 73:808–813.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurihara T, Yasuda I, Isayama H,
Tsuyuguchi T, Yamaguchi T, Kawabe K, Okabe Y, Hanada K, Hayashi T,
Ohtsuka T, et al: Diagnostic and therapeutic single-operator
cholangiopancreatoscopy in biliopancreatic diseases: Prospective
multicenter study in Japan. World J Gastroenterol. 22:1891–1901.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shigetomi H, Oka K, Seki T and Kobayashi
H: Design and preclinical validation of the composite-type optical
fiberscope for minimally invasive procedures of intrauterine
disease. J Minim Invasive Gynecol. 22:985–991. 2015. View Article : Google Scholar : PubMed/NCBI
|