Introduction
Avian influenza A (H5N1) virus is a highly
pathogenic contagious agent that causes severe impairment in
poultry and humans, particularly limited person-to-person
transmission (1). The cumulative
number of confirmed human cases of H5N1 from 14 countries between
November 2003 and July 2014 reached 667, 393 of which were fatal
according to a report issued by the World Health Organization
(2). In total, 47 individuals
infected with H5N1 were identified in China and 30 (63.8%)
succumbed to the H5N1 infection (2).
H5N1 causes primary viral pneumonia with rapid progression to lung
failure following invasion of epithelial cells in the upper and
lower respiratory tracts (3).
However, the exact mechanism for elucidating the severity of human
H5N1 infection remains unclear. Previously, apoptosis was not only
observed in the alveolar epithelial cells of 2 patients who
succumbed to H5N1 infection, but was also induced by H5N1 in
numerous cell types in vivo and in vitro (4–6). These
results indicated that apoptosis may be important in H5N1
pathogenesis in the human body.
At present, two main apoptotic pathways have been
documented; the tumor necrosis factor (TNF) receptor-mediated
extrinsic pathway and the intrinsic pathway mediated by
mitochondria/cytochrome c (7). The non-structural protein 1 (NS1),
which is encoded by the influenza virus NS segment, is able to
alter the host response and virulence of the virus in the case of
reassortment without prior adaptation (8,9), and is
associated with apoptosis regulation in mammalian cells. Previous
studies revealed that the H5N1 NS1 protein induced the TNF-mediated
extrinsic apoptosis pathway in human alveolar basal epithelial
cells (10–13). However, the susceptible cell lines of
the various pathogenic avian influenza viruses are different, which
causes them to vary in their responses to apoptosis (14,15).
As mentioned above, apoptosis induced by the H5N1
NS1 protein may vary in various cell lines. To further investigate
whether the other apoptotic pathways induced by H5N1 NS1 protein
exist, H5N1 NS1 protein was used to induce the human lung
epithelial cell line, NCI-H292.
Materials and methods
Construction of an NS1-expressing
plasmid
Highly pathogenic avian influenza A/Jiangsu/1/2007
(H5N1) viral RNA was extracted from supernatants of infected cell
cultures for use as a polymerase chain reaction (PCR) template for
amplifying the NS1 gene. Total RNA was extracted from cell lysate
using the QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany)
according to the manufacturer's instructions. The full-length NS1
gene was amplified using the SuperScript III One-Step Reverse
Transcription-PCR (RT-PCR) system with Platinum Taq High-Fidelity
Polymerase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) from H5N1 virus cDNA. The sense and antisense primers used
for NS1 (EU434690) were 5′-GTGCTCGAGATGGATTCCAACACTGTGTCA-3′ and
5′-CACGGTACCTCAAACTTCTGACTCAATTGT-3′, respectively. The PCR
conditions were 95°C for 15 min, followed by 34 cycles of 94°C for
30 sec, 58°C for 30 sec, and 72°C for 30 sec. The cloning insert
was ligated into the pMD18-T vector (D101A; Takara Biotechnology
Co., Ltd., Dalian, China) by quick ligase (M2200S; NEB Beijing
Ltd., Beijing, China) incubating for 30 min at room temperature.
pMD18-T-NS1 was subcloned into the expression plasmid
pXJ40-hemagglutinin (HA) (Invitrogen; Thermo Fisher Scientific,
Inc.) using XhoI (D1094A) and KpnI (D1068A) (both
from Takara Biotechnology Co., Ltd., Dalian, China) sites to
produce the recombinant HA-tagged construct, pXJ40-HA-NS1. The
construction of plasmid pXJ40-HA-NS1 followed standard cloning
procedures. Competent Escherichia coli TOP10 cells
(Invitrogen; Thermo Fisher Scientific, Inc.) were transformed using
pXJ40-HA-NS1 plasmids, and the plasmids were amplified and purified
using a high-purity plasmid purification kit (Invitrogen; Thermo
Fischer Scientific, Inc.). Clones were then screened by restriction
enzyme digestion and sequence analysis using the version 3.1 BigDye
Terminator ready reaction cycle sequencing kit (Applied Biosystems;
Thermo Fischer Scientific, Inc.) according to the manufacturer's
instructions.
Cell line culture and transient
transfection
Non-small cell lung cancer cell lines, NCI-H1299 and
NCI-H292 (Cell Bank; Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences; Shanghai China), were separately grown
as a monolayer in Dulbecco's modified Eagle's medium (DMEM)
(Invitrogen; Thermo Fischer Scientific, Inc.) supplemented with 10%
fetal bovine serum (Invitrogen Thermo Fischer Scientific, Inc.) at
37°C and in a 5% CO2 incubator. The two cell lines were
used for different purposes, NCI-H1299 was used to observe the
localization of the NS1 protein in the cell whereas NCI-H292 was
used to confirm the extent of apoptosis induced by the NS1
protein.
NCI-H1299 and NCI-H292 were transfected with
pXJ40-HA-NS1 or control plasmids (pXJ40-HA-vector; Invitrogen;
Thermo Fischer Scientific, Inc.) using Lipofectamine 2000 reagent
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc). After 4 h, Lipofectamine 2000-DNA
complexes were removed, and the cell culture DMEM medium was
replaced with fresh DMEM with or without 0.025 nM staurosporine
(STS; Sigma-Aldrich; Merk KGaA, Darmstadt, Germany) at 37°C for 24
h. Cells were collected after 24 h, washed with phosphate-buffered
saline (PBS) and trypsinized with 0.125% trypsin/EDTA solution.
Immunofluorescence staining
NCI-H1299 cells were fixed in 4% (w/v)
paraformaldehyde at room temperature for 30 min and permeablized in
0.5% (w/v) Triton X-100, followed by incubation with primary and
secondary antibodies for 1 h at room temperature sequentially.
Anti-HA serum (AH158; Beyotime Institute of Biotechnology, Haimen,
China) with 1:200 was used for the control and Alexa 488-conjugated
secondary antibody (1:500, A-11017; Invitrogen; Thermo Fisher
Scientific, Inc.) were used to probe for the NS1 protein at room
temperature for 1 h. Following protein staining, anti-cytochrome c
monoclonal antibody (1:1,000, BD556432; BD Biosciences, Franklin
Lakes, NJ, USA) and Alexa 555-conjugated secondary antibody (1:500,
A-21427; Invitrogen; Thermo Fisher Scientific, Inc.) were utilized
to probe the NCI-H1299 cellular morphology. Finally,
4′,6-diamidino-2-phenylindole (DAPI, 1:1,000, D1306; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to dye the cell nucleus at
room temperature for 1 h. Triple-fluorescence stained cells were
observed with a confocal microscope at a high-power magnification
of ×100 (FV10-ASW, version 01.07.03.00; Olympus Corporation, Tokyo,
Japan).
Cell viability assay
MTT cell viability assays were performed according
to the manufacturer's instructions (Sigma-Aldrich; Merck KGaA).
Briefly, 20 µl 5 mg/ml MTT was added to the culture medium.
Following incubation at 37°C for 3 h, 100 µl acidic isopropanol
(0.1 nM HCl in acidic isopropanol) was added to the NCI-H292 cells
and then the absorbance of each sample was measured at 570 nm with
an automated plate reader (SpectraMax Paradigm; Molecular Devices,
LLC, Sunnyvale, CA, USA) compared to the same number of control
cells.
Flow cytometric analysis
In order to determine the apoptotic rate,
~1×106 NCI-H292 cells/ml were stained with fluorescein
isothiocyanate (FITC)-conjugated Annexin V and propidium iodide
(556547, Annexin V-FITC apoptosis detection kit, BD Pharmingen; BD
Biosciences) in a volume of 100 µl on ice for 30 min in the dark.
The cells were washed 3 times with PBS. Finally, 400 µl binding
buffer was added to the cells. Additionally, the mixture was
analyzed with a BD FACSCalibur flow cytometer and the percentage of
apoptosis of 10,000 cells was determined. The data were analyzed
using BD CellQuest™ Pro software (version 5.2.1; BD Biosciences)
and the percentage cells with apoptosis per group were
calculated.
Determination of mitochondrial
membrane potential (MMP)
At 24 h following transfection, the culture medium
of the NCI-H292 cells was replaced with DMEM that did not include
phenol red and was supplemented with 5 mg/l JC-1 dye (Beyotime
Institute of Biotechnology) in the dark for 20 min at 37°C.
Subsequently, the cells were washed twice with PBS and placed in
fresh medium without serum. Finally, MMP was analyzed by
calculating the ratio of fluorescence intensity at 555–488 nm in
triplicate.
Mitochondria isolation and calculation
of cytochrome c release
Cytosolic and mitochondrial isolation are performed
as described by Cheng et al (16). The percentage of cytochrome c
release was calculated using the following formula: The percentage
of cytochrome c release that is equal to the amount of
cytochrome c in the mitochondrial supernatant/the total
amount of cytochrome c in mitochondrial supernatant and
pellet.
Western blot analysis
Monolayers of cells transfected with DNA or
untransfected cells were lysed with ice-cold lysis buffer (150 mM
Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 1
tablet Complete Mini protein inhibitor mixture/10 ml buffer and 0.7
µg/ml pepstatin), and the total protein concentration was
determined using the bicinchoninic acid assay. A total of 7 µl
proteins with equivalent concentrations (1 µg/µl) were heated for 5
min at 100°C in lysis buffer containing β-mercaptoethanol, and then
resolved using 4–20% SDS-PAGE. The proteins were then transferred
onto a polyvinylidene difluoride membrane and blocked with 1%
powdered skimmed milk in Tris-buffered saline with 0.1% Tween-20
for 1 h at room temperature. Anti-cytochrome c MAb mouse
antibody (1:200, BD556432; BD Biosciences) was then used to probe
for cytochrome c overnight at 4°C. The membrane was also
probed for GADPH (1:1,000, A2066; Sigma-Aldrich; Merck KGaA) was
used as the loading control. Membranes were subsequently washed
with 150 mM PBS and incubated for 1 h at 4°C in 10% dried milk in
PBS. Membranes were washed 5 times with PBS and subsequently
incubated for 1 h at room temperature with anti-mouse
immunoglobulin G horseradish peroxidase-conjugated secondary
antibody (1:1,000, ZB2307; ZSGB BIO; OriGene Technologies, Inc.,
Rockville, MD, USA)., followed by visualization of positive bands
with the Pierce (Thermo Fisher Scientific, Inc.) enhanced
chemiluminescence procedure using Kodak BioMax film. Blots were
scanned and the protein ratios were calculated using the PDQuest
program (version 7.4.0; Bio-Rad Laboratories, Inc.). The results
shown are representative of 3independent experiments.
Statistical analysis
In the cell viability assays and MMP experiments,
all data were expressed as the mean ± standard deviation.
Statistical significance among groups was assessed using one-way
analysis of variance and the Tukey test. P<0.05 was considered
to indicate a statistically significant difference. Finally,
statistical analyses were performed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
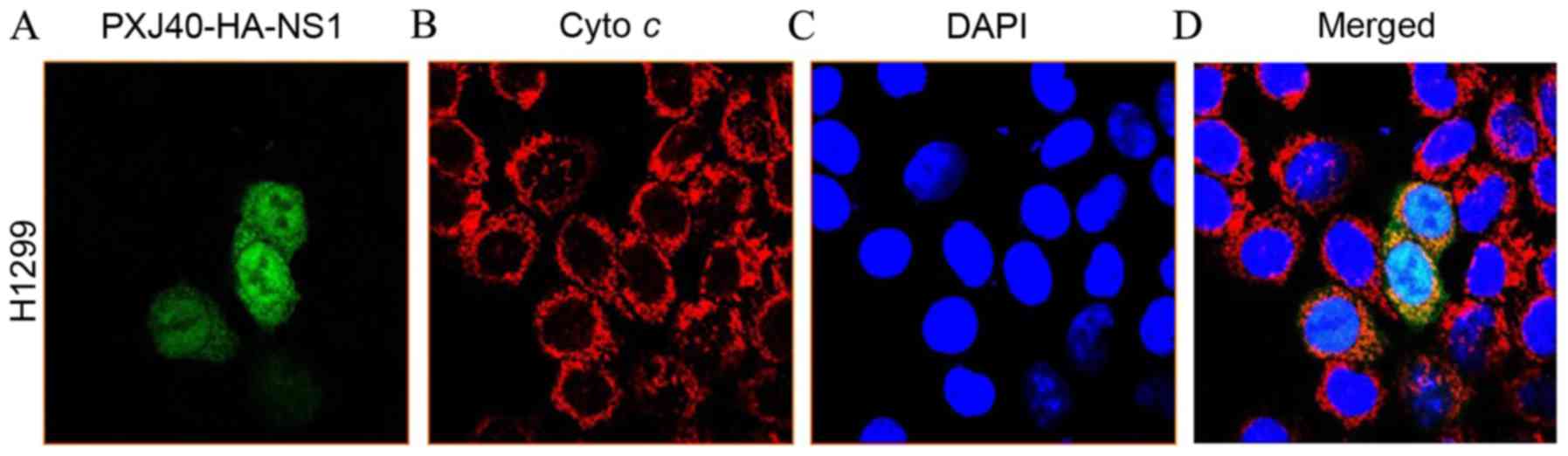
Location of H5N1 NS1 protein in
NCI-H1299 cells
The plasmid pXJ40-HA-NS1 was transfected into
NCI-H1299 cells, and the expression and location of the NS1 protein
was monitored. The plasmid pXJ40-NS1-HA includes the full-length NS
gene cloned from influenza H5N1. As demonstrated in Fig. 1, the NS1 protein began to express 24
h following transfection (Fig. 1A),
and predominantly remained in the nucleus (Fig. 1B-D).
H5N1 NS1 protein induced apoptosis in
NCI-H292 cells
Previous studies have revealed that the NS1 protein
of H5N1 is able to induce apoptosis in A549 cells and human airway
epithelial cells (17,18). The present study attempted to clarify
whether the expression of NS1 was sufficient to induce apoptosis
with or without STS, which was a confirmed apoptosis inducer, and
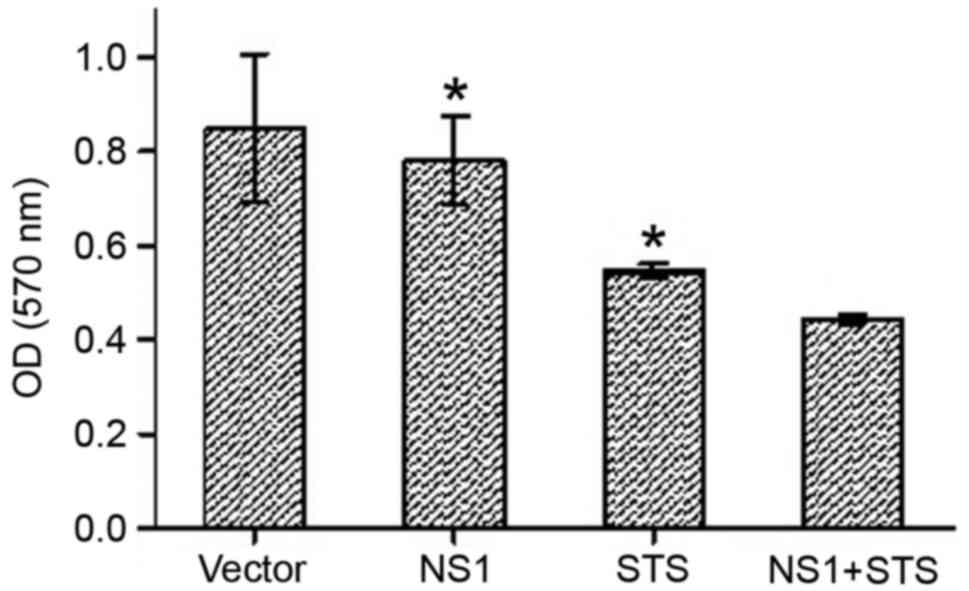
STS (0.025 nM) was used as a positive control. Initially, NS1
protein and STS were used to induce cytotoxicity, and the
cytotoxicity was then detected under various conditions using the
MTT assay. Compared with cells transfected with empty vector, the
viability of NS1-transfected cells was markedly lower (Fig. 2). However, the addition of STS
significantly decreased the viability of the cells compared with
NS1-transfected cells and STS-treated cells, which suggested an
increase in cytotoxicity (P<0.05).
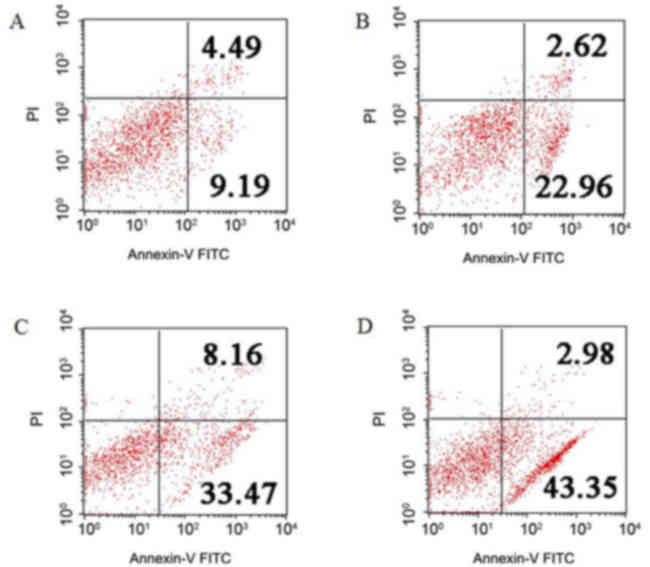
To further confirm cell apoptosis, which is
associated with NS1 protein expression, the NCI-H292 cells
expressing NS1 protein were stained with Annexin V-FITC and PI, and
detected using flow cytometry (Fig.
3). Compared with the empty vector-transfected cells (9.19%
Annexin V+/PI−, indicating early apoptosis,
and 4.49% Annexin V+/PI+, indicating late
apoptosis), the NS1-transfected cells were 22.96% Annexin
V+/PI− and 2.62% Annexin
V+/PI+. Following treatment with STS,
NS1-transfected cells were 43.35% Annexin
V+/PI− and 2.98% Annexin
V+/PI+, whereas empty vector-transfected
cells were 33.47% Annexin V+/PI− and 8.16%
Annexin V+/PI+. Compared with the empty
vector-transfected group, the number of apoptotic cells in the
NS1-plasmid transfected group was significantly increased
(P<0.05). Following combinational treatment of NS1 and STS, the
number of apoptotic cells was significantly increased compared with
the NS1 group (P<0.05). These results indicate that NS1 is
associated with the apoptosis of NCI-H292 cells, and that the
effect of apoptosis induced by NS1 protein may be enhanced by
STS.
Involvement of cytochrome c in
NS1-induced apoptosis
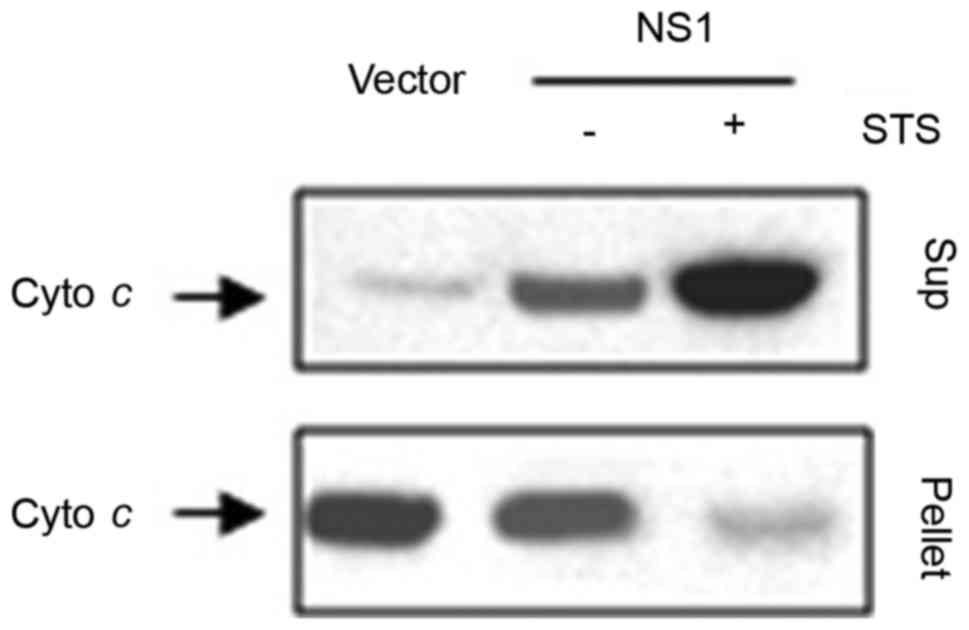
As demonstrated in Fig.
4, NS1 together with STS induced the release of cytochrome
c from the mitochondria to the cytosol. It is known that
cytochrome c release from the mitochondria to the cytosol is
a signal for apoptosis (19). This
implies the possibility of NS1 protein being associated with
apoptosis by activating the intrinsic pathway through cytochrome
c. In order to confirm this, MMP was initially detected by
JC-1 staining. Normal cells stained with JC-1 emitted mitochondrial
orange-red fluorescence with a slight green fluorescence, whereas
the ratio of orange-red and green fluorescence was inverted when
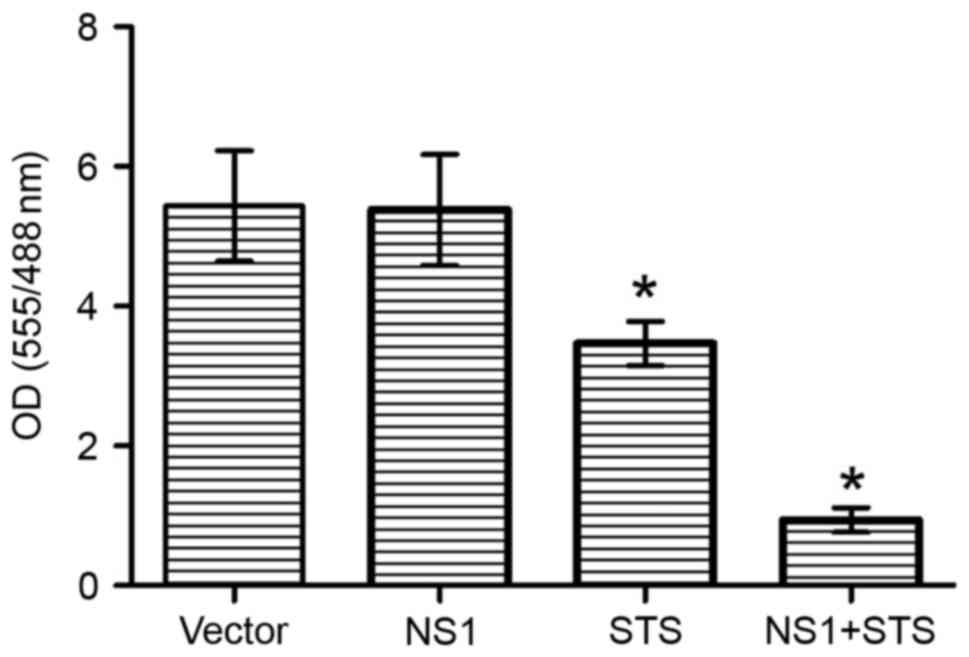
cell apoptosis was induced by NS1 protein. As shown in Fig. 5, in comparison with the empty vector
transfected cells group, NS1 transfected cells significantly
initiated apoptosis with STS (the ratio of fluorescence intensity
at 555/488 nm was ~1.00; P<0.05). However, without STS
treatment, the NS1-expressing NCI-H292 cells have no significant
difference with the empty vector.
To further corroborate the effect of the
mitochondria/cytochrome c on the intrinsic apoptosis pathway
inducted by the NS1 protein, western blotting was performed to
detect the expression of cytochrome c in mitochondrial
pellets and supernatants. The majority of cytochrome c
expressed in mitochondrial pellets was isolated from the cells
transfected with empty vector. It is noteworthy that the amount of
cytochrome c was markedly decreased in the mitochondrial
pellets following NS1-transfection for 24 h, whereas the amount of
cytochrome c was markedly increased in the mitochondrial
supernatant (Fig. 4). These results
indicate that NS1 may trigger the release of cytochrome c
from mitochondria and that this effect is enhanced by STS.
Discussion
The results of the present study indicate that the
NS1 protein of the H5N1 highly pathogenic avian influenza A virus
strain is associated with apoptotic activation of NCI-H1299 through
the intrinsic mitochondrial pathway. The MTT assay and flow
cytometric analysis revealed that the NS1 protein-induced apoptosis
of NCI-H1299 cells and its activity may be enhanced by STS.
Additionally, the NS1 protein together with STS was able to cause
the release of cytochrome c from the mitochondria to the
cytosol, and the change of MMP. During the intrinsic mitochondrial
apoptosis process, the permeabilization of the mitochondrial outer
membrane is the critical step, which results in the release of
several apoptogenic factors from the intermembrane space of
mitochondria (20–22). Cytochrome c is one of these
factors, which binds to the adaptor apoptotic peptidase-activating
factor 1 that subsequently recruits cytosolic pro-caspase-9 into a
heptameric apoptosome (23). The
intrinsic mitochondrial apoptosis pathway is one of the major
pathways during viral pathogenesis that include human
immunodeficiency virus and severe acute respiratory syndrome
coronavirus (24,25).
Although Zhang et al (10) demonstrated that the H5N1 NS1 protein
induced caspase-dependent apoptosis in human alveolar basal
epithelial cells, Neuman et al (20) revealed that the activation of caspase
is the common end event during apoptosis. Therefore, the study by
Zhang et al (10) did not
have enough evidence to support that the NS1 of H5N1 induced
apoptosis through an extrinsic pathway. In the present study, the
efficiency of NS1 in inducing apoptosis is lower than that of STS,
however, the increasing synergistic effect on inducing apoptosis
between STS and NS1 were observed. In addition, in the present
study, the plasmid with the NS1 gene was transfected with
Lipofectamine 2000 and the transfecting efficiency was lower than
the penetrating ability of STS. At this point, although the
capacity of inducing apoptosis of NS1 is weaker than STS in the
present study, the importance of each one needs to be investigated
further. Previous studies have revealed that the NS1 protein of
influenza A viruses is a multifunctional viral protein that
modulates the virus replication cycle and viral protein synthesis,
and HA and neuropilin-1 (NP1) of H5N1 also induce apoptosis of
airway epithelial cells (26,27).
In vivo, there is a need to determine whether the synergetic
effect on inducing apoptosis by NP1, nucleoprotein and HA exists.
In particular, these proteins induce apoptosis by either sharing
the same pathway or not.
In conclusion, the results of the present study
reveal that the intrinsic mitochondrial apoptosis pathway is
associated with the apoptosis induced by the NS1 protein of H5N1.
Therefore, this may be a novel mechanism in the ability of highly
pathogenic avian influenza A virus H5N1 causing severe impairment
in humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. NSFC 81302466),
Jiangsu Provincial ‘Twelfth five-year plan’ Key Provincial Talents
Program (grant no. H201118) and Project 333 Talents in Jiangsu
(grant no. BRA2015490).
References
|
1
|
Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R,
Huai Y, Dong J, Bao C, Wen L, et al: Probable limited
person-to-person transmission of highly pathogenic avian influenza
A (H5N1) virus in China. Lancet. 371:1427–1434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
http://www.who.int/influenza/human_animal_interface/2017_02_14_tableH5N1.pdf?ua=1Accessed.
October 2–2014.
|
|
3
|
Nicholls JM, Chan MC, Chan WY, Wong HK,
Cheung CY, Kwong DL, Wong MP, Chui WH, Poon LL, Tsao SW, et al:
Tropism of avian influenza A (H5N1) in the upper and lower
respiratory tract. Nat Med. 13:147–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tripathi S, Batra J, Cao W, Sharma K,
Patel JR, Ranjan P, Kumar A, Katz JM, Cox NJ, Lal RB, et al:
Influenza A virus nucleoprotein induces apoptosis in human airway
epithelial cells: Implications of a novel interaction between
nucleoprotein and host protein clusterin. Cell Death Dis.
28:e5622013. View Article : Google Scholar
|
|
5
|
Daidoji T, Koma T, Du A, Yang CS, Ueda M,
Ikuta K and Nakaya T: H5N1 avian influenza virus induces apoptotic
cell death in mammalian airway epithelial cells. J Viro.
82:11294–11307. 2008. View Article : Google Scholar
|
|
6
|
Sarmento L, Wasilenko J and
Pantin-Jackwood M: The effects of NS gene exchange on the
pathogenicity of H5N1 HPAI viruses in ducks. Avian Dis. 54 Suppl
1:S532–S537. 2010. View Article : Google Scholar
|
|
7
|
Herold S, Ludwig S, Pleschka S and Wolff
T: Apoptosis signaling in influenza virus propagation, innate host
defense, and lung injury. J Leukoc Biol. 92:75–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam WY, Tang JW, Yeung AC, Chiu LC, Sung
JJ and Chan PK: Avian influenza virus a/Hk/483/97(H5N1) NS1 protein
induces apoptosis in human airway epithelial cells. J Virol.
82:2741–2751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lam WY, Yeung AC and Chan PK: Apoptosis,
cytokine and chemokine induction by non-structural 1 (NS1) proteins
encoded by different influenza subtypes. Virol J. 8:5542011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Yang Y, Zhou X, Yang Z, Liu X,
Cao Z, Song H, He Y and Huang P: The NS1 protein of influenza a
virus interacts with heat shock protein hsp90 in human alveolar
basal epithelial cells: Implication for virus-induced apoptosis.
Virol J. 8:1812011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehrhardt C, Wolff T, Pleschka S, Planz O,
Beermann W, Bode JG, Schmolke M and Ludwig S: Influenza A virus NS1
protein activates the Pi3k/Akt pathway to mediate antiapoptotic
signaling responses. J Virol. 81:3058–3067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukherjee S, Majumdar S, Vipat VC, Mishra
AC and Chakrabarti AK: Non structural protein of avian influenza a
(H1N1) virus is a weaker suppressor of immune responses but capable
of inducing apoptosis in host cells. Virol J. 9:1492012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang N, Hong X, Yang P, Ju X, Wang Y, Tang
J, Li C, Fan Q, Zhang F, Chen Z, et al: The 2009 pandemic
a/wenshan/01/2009 H1N1 induces apoptotic cell death in human airway
epithelial cells. J Mol Cell Biol. 3:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Meng D, Wei T, Zhang S, Hu Y and
Wang M: Apoptosis and pro-inflammatory cytokine response of mast
cells induced by influenza A viruses. PLoS One. 9:e1001092014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hui KP, Li HS, Cheung MC, Chan RW, Yuen
KM, Mok CK, Nicholls JM, Peiris JS and Chan MC: Highly pathogenic
avian influenza H5N1 virus delays apoptotic responses via
activation of STAT3. Sci Rep. 6:285932016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng EH, Wei MC, Weiler S, Flavell RA,
Mak TW, Lindsten T and Korsmeyer SJ: Bcl-2, Bcl-X(L) sequester BH3
domain-only molecules preventingbax- and BAK-mediated mitochondrial
apoptosis. Mol Cell. 8:705–711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Yang Y, Zhou X, Liu X, Song H, He
Y and Huang P: Highly pathogenic avian influenza A virus H5N1 NS1
protein induces caspase-dependent apoptosis in human alveolar basal
epithelial cells. Virol J. 7:512010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang CF, Jiang SW, Zhu HQ, Yang YT, Yang
ZX, Xu L, Zhao LX, Zhou XW and Huang PT: Cloning NS1 gene of H5N1
avian influenza virus and apoptosis induced by it in human
pulmonary carcinoma cell line A549. Bing Du Xue Bao. 23:360–365.
2007.(In Chinese). PubMed/NCBI
|
|
19
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potentials for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neumann S, El Maadidi S, Faletti L, Haun
F, Labib S, Schejtman A, Maurer U and Borner C: How do viruses
control mitochondria-mediated apoptosis? Virus Res. 209:45–55.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Li J, Huang H, Yang M, Zhuang D,
Cheng X, Zhang H and Fu X: Microcystin-LR induces
mitochondria-mediated apoptosis in human bronchial epithelial
cells. Exp Ther Med. 12:633–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Firsov AM, Kotova EA, Orlov VN, Antonenko
YN and Skulachev VP: A mitochondria-targeted antioxidant can
inhibit peroxidase activity of cytochrome c by detachment of the
protein from liposomes. FEBS Lett. 590:2836–2843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lauber K, Appel HA, Schlosser SF, Gregor
M, Schulze-Osthoff K and Wesselborg S: The adapter protein
apoptotic protease-activating factor-1 (Apaf-1) is proteolytically
processed during apoptosis. J Biol Chem. 276:29772–29781. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deniaud A, Brenner C and Kroemer G:
Mitochondrial membrance permeabilization by HIV-1 Vpr.
Mitochondrion. 4:223–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan YX, Tan TH, Lee MJ, Tham PY, Gunalan
V, Druce J, Birch C, Catton M, Fu NY, Yu VC and Tan YJ: Induction
of apoptosis by the severe acure respiratory syndrome coronavirus
7a protein is dependent on tis interaction with the Bcl-xL protein.
J Virol. 81:6346–6355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thulasi Raman SN and Zhou Y: Networks of
host factors that interact with NS1 protein of influenza A virus.
Front Microbiol. 7:6542016.PubMed/NCBI
|
|
27
|
Matsuoka Y, Matsumae H, Katoh M, Eisfeld
AJ, Neumann G, Hase T, Ghosh S, Shoemaker JE, Lopes TJ, Watanabe T,
et al: A comprehensive map of the influenza A virus replication
cycle. BMC Syst Biol. 7:972013. View Article : Google Scholar : PubMed/NCBI
|