Introduction
Heat shock factor 1 (HSF1) is a transcription factor
that is strongly conserved from yeast to humans (1). HSF1 has a key role in the cellular
response leading to the expression of heat shock proteins (Hsps),
which serve to protect cells from damage as a result of cellular
insults, including heat and oxidative stress (1,2).
However, the heat shock response often becomes deregulated in
tumors. HSF1 is expressed at a high level and has a role in
carcinogenesis (3). The expression
levels of Hsp90, Hsp70 and Hsp27, which are HSF1-target genes, are
increased in mammary tumors, leading to a decrease in cellular
apoptosis (4). Polo-like kinase 1
(PLK1) is a serine/threonine protein kinase that acts as an
important cell signaling regulator (5). The phosphorylation of HSF1 by PLK1 is a
vital step for HSF1 nuclear translocation in response to heat
stress (6). PLK1 expression is also
elevated in various types of human tumors (7–9). It has
been reported that depletion of PLK1 in oral squamous cell
carcinoma cells significantly inhibited the expression of HSF1 as
well as Hsp70 and Hsp90, and knockdown of PLK1 and HSF1 strongly
inhibited cell proliferation (10).
Furthermore, transduction of PLK1 small interfering (si)RNA or HSF1
short hairpin (sh)RNA into tumor cells reduced the migration and
invasive abilities of the cells (11,12).
Therefore, PLK1 and HSF1 may be potential targets in cancer
therapy.
Cationic liposomes have often been used for in
vivo siRNA delivery into tumors (13). However, following systemic injection
of siRNA/cationic liposome complexes (siRNA lipoplexes),
electrostatic interactions between positively charged lipoplexes
and negatively charged erythrocytes cause agglutination in the
blood, and the agglutinates contribute to high levels of entrapment
of lipoplexes in the highly extended lung capillaries (14). Recently, we demonstrated that
intravenous sequential injection of chondroitin sulfate plus siRNA
lipoplexes into mice with liver metastasis could deliver siRNA
efficiently to the metastasis without accumulation in the lungs,
and suppress the expression of a target gene in the tumor cells
(15,16). Therefore, the present study evaluated
the therapeutic efficacy of treatment with PLK1 or HSF1 siRNA using
a sequential injection method against liver metastasis.
Materials and methods
Reagents
1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP)
methyl sulfate salt was obtained from Avanti Polar Lipids Inc.,
(Alabaster, AL, USA). Cholesterol (Chol) and chondroitin sulfate C
sodium salt were purchased from Wako Pure Chemical Industries, Ltd.
(Osaka, Japan). All other chemicals were of the finest grade
available.
siRNA
Human PLK1 siRNA, human HSF1 siRNA, non-silencing
siRNA (Cont siRNA), and cyanine 5.5 (Cy5.5)-labeled luciferase
siRNA (Cy5.5-siRNA) were synthesized by Sigma Genosys (Tokyo,
Japan). The siRNA sequences for human PLK1 siRNA were as follows:
Sense strand 5′-CCUUGAUGAAGAAGAUCACTT-3′ and antisense strand
5′-GUGAUCUUCUUCAUCAAGGTT-3′ (17).
The siRNA sequences of the human HSF1 siRNA were: Sense strand
5′-GAACGACAGUGGCUCAGCAUU-3′ and antisense strand
5′-UGCUGAGCCACUGUCGUUCUU-3′ (17).
The siRNA sequences of the Cont siRNA as a negative control for
PLK1 siRNA or HSF1 siRNA were: Sense strand
5′-GUACCGCACGUCAUUCGUAUC-3′ and antisense strand
5′-UACGAAUGACGUGCGGUACGU-3′. The siRNA sequences of the luciferase
siRNA were: Sense strand 5′-GUGGAUUUCGAGUCGUCUUAA-3′ and antisense
strand 5′-AAGACGACUCGAAAUCCACAU-3. In Cy5.5-siRNA, Cy5.5 dye was
conjugated at the 5′-end of the sense strand.
Cell culture
Human breast cancer MDA-MB-231/Luc cells stably
expressing firefly luciferase were obtained from Cell
BioLabs, Inc., (San Diego, CA, USA). Tamoxifen-resistant human
breast cancer MCF-7-Luc (TamR-Luc#1) cells stably expressing
firefly pGL3 luciferase were donated by Dr Kazuhiro Ikeda
(Division of Gene Regulation and Signal Transduction, Research
Center for Genomic Medicine, Saitama Medical University, Saitama,
Japan). Human cervical carcinoma HeLa-Luc cells stably expressing
firefly pGL3 luciferase were obtained from Caliper Life
Sciences Co., (Hopkinton, MA, USA).
MCF-7-Luc cells were cultured in Dulbecco's modified
Eagle medium (DMEM; Wako Pure Chemical Industries, Ltd.)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 µg/ml kanamycin and 0.5
mg/ml G418 at 37°C in a 5% CO2 humidified atmosphere.
MDA-MB-231-Luc cells were cultured in DMEM supplemented with 10%
FBS and 100 µg/ml kanamycin at 37°C in a 5% CO2
humidified atmosphere. HeLa cells were cultured in Eagle's minimum
essential medium (Wako Pure Chemical Industries, Ltd.) supplemented
with 10% FBS and 100 µg/ml kanamycin at 37°C in a 5% CO2
humidified atmosphere.
Measurement of PLK1 and HSF1
expression level in vitro
For investigation of PLK1 and HSF1 mRNA expression
levels in tumor cells, total RNA was isolated from MDA-MB-231,
MCF-7 and HeLa cells using NucleoSpin RNA (Macherey-Nagel, GmbH,
Düren, Germany). First strand cDNA was synthesized from 2 µg of
total RNA using PrimeScript RTase (Takara Bio, Inc., Otsu, Japan).
For polymerase chain reaction (PCR), the 20-µl reaction volume
contained: 1 µl synthesized cDNA, 10 pmol of each specific primer
pair and 0.25 U Ex Taq DNA polymerase (Takara Bio, Inc.), with PCR
buffer containing 1.5 mM MgCl2 and 0.25 mM of each dNTP.
The profile of PCR amplification for PLK1 and GAPDH cDNA consisted
of denaturation at 95°C for 0.5 min, primer annealing at 58°C for
0.5 min and elongation at 72°C for 1 min, for 30 cycles. For HSF1,
the thermocycling conditions consisted of denaturation at 95°C for
0.5 min, primer annealing at 63°C for 0.5 min and elongation at
72°C for 1 min, for 30 cycles. Human PLK1 cDNA (154 bp) was
amplified using the following primers: Human PLK1-FW
5′-CTCAACACGCCTCATCCTC-3′ and human PLK1-RW
5′-GTGCTCGCTCATGTAATTGC-3′ (18).
Human HSF1 cDNA (190 bp) was amplified using the primers: Human
HSF1-FW, (5′-CCGGCGGGAGCATAGACGAGAGG-3′) and human HSF1-RW
(5′-GACGGAGGCGGGGGCAGGTTCACT-3′) (19). Human GAPDH cDNA (820 bp) was
amplified using the primers: Human GAPDH-FW
(5′-ATGACCCCTTCATTGACCTC-3′) and human GAPDH-RW
(5′-AAGTGGTCGTTGAGGGCAAT-3′). Their PCR products were analyzed by
18% acrylamide gel electrophoresis in Tris-borate-EDTA buffer (Wako
Pure Chemical Industries, Ltd.), and were visualized by ethidium
bromide staining.
Transfection with siRNA
For knockdown of PLK1 or HSF1 mRNA by transfection
with PLK1 siRNA or HSF1 siRNA, MDA-MB-231 and HeLa cells were
plated into 6-well culture dishes at a density of 3×105
cells/well. The cells were transfected with 50 nM Cont, PLK1 or
HSF1 siRNA using Lipofectamine RNAiMax reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). A total of 24 h after transfection, total
RNA was isolated using NucleoSpin RNA and then first strand cDNA
was synthesized from 2 µg of total RNA using PrimeScript RTase.
Quantitative (q)PCR was performed using a Roche Light Cycler 96
system (Roche Diagnostics, Basel, Switzerland) and TaqMan Gene
expression assays (PLK1, Hs00983227_m1; HSF1, Hs00232134_m1; GAPDH,
Hs02786624_g1; all Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions consisted of an initial
denaturation at 95°C for 600 sec, and 45 cycles of denaturation at
95°C for 10 sec, and primer annealing and extension at 60°C for 30
sec (two step amplification). Samples were run in triplicate, and
the expression levels of PLK1 and HSF1 mRNA were normalized by the
amount of GAPDH mRNA in the same sample, and analyzed using the
2−ΔΔCq method (20).
Cell growth inhibition
MCF-7, MDA-MB-231 and HeLa cells were seeded in
96-well plates at a density of 2×104 cells per well 24 h
prior to transfection. Cells at confluences of 50% in the well were
transfected with 2.5, 5, 10, 20, 30, 40 and 50 nM Cont siRNA, PLK1
siRNA or HSF1 siRNA using Lipofectamine RNAiMax reagent and then
incubated for 48 h at 37°C. In combined treatment with doxorubicin
(DXR; LC Laboratories, Woburn, MA, USA), the cells were incubated
for 24 h at 37°C after transfection at 50 nM Cont siRNA, PLK1 siRNA
or HSF1 siRNA using Lipofectamine RNAiMax reagent, and then treated
with various concentrations (0.016–0.5 µM) of DXR as reported
previously (21). A total of 6 h
after incubation, the medium containing free DXR was changed for
fresh medium without DXR, and then incubated for another 18 h at
37°C. The cell number was determined using a Cell Counting kit-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Cell
viability was expressed relative to the absorbance of untreated
cells at 450 nm.
Preparation of liposomes and
lipoplexes
Cationic liposomes were prepared from DOTAP/Chol at
a molar ratio of 1:1 using a thin-film hydration method, as
reported previously (22). To
prepare siRNA/cationic liposome complexes (siRNA lipoplexes), the
cationic liposome suspension was mixed with siRNA by vortexing for
10 sec at a charge ratio (+:-) of 4:1, and left for 15 min at room
temperature. The theoretical charge ratio (+:-) of cationic
liposome to siRNA was calculated as the molar ratio of DOTAP
nitrogen to siRNA phosphate.
The particle size distributions of liposomes and
lipoplexes were measured using a light-scattering photometer
(ELS-Z2; Otsuka Electronics Co., Ltd., Osaka, Japan) at 25°C after
diluting the dispersion to an appropriate volume with water. The
ζ-potentials were measured using ELS-Z2 at 25°C after diluting the
dispersion with an appropriate volume of water. Cationic liposomes
were ~114 nm in size and had a ζ-potential of ~52 mV. The lipoplex
size was ~312 nm and the ζ-potential was ~40 mV.
Liver metastasis model
All animal experiments were performed with approval
from the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan). A total of 9 mice were housed in a
temperature-(24°C) and humidity-(55%) controlled room with a 12 h
light/dark cycle (lights on at 8:00 a.m.) with ad libitum
access to food and water. To generate mice with liver metastasis,
1.0×106 HeLa cells suspended in 50 µl PBS (pH 7.4)
containing 50% reconstituted basement membrane (Matrigel; BD
Biosciences, Franklin Lakes, NJ, USA) were inoculated into the
spleen of female SCID-Beige mice (18–20 g; 8 weeks old;
CB17.B6-PrkdcscidLystbg-J/Crl; Oriental Yeast
Co., Ltd., Tokyo, Japan).
Biodistribution of siRNA following
intravenous injection of siRNA lipoplexes into mice
For efficient delivery of siRNA into liver
metastasis, lipoplexes with 50 µg Cy5.5-siRNA were administered
intravenously into mice with HeLa-liver metastasis at 1 min after
the intravenous injection of 1 mg chondroitin sulfate (Wako Pure
Chemical Industries, Ltd.) at 10 days after inoculation of HeLa-Luc
cells, as reported previously (sequential injection method)
(15,16). A total of 1 h after injection, the
mice were sacrificed, and Cy5.5 fluorescent imaging of the tissues
was performed using a NightOWL LB981 NC100 system (Berthold
Technologies, Bad Wildbad, Germany). In Cy5.5 fluorescent imaging,
the excitation and emission filters were set at 630/20 and 680/30
nm, respectively. The exposure time for fluorescence was 5 sec. A
grayscale body-surface reference image was collected using a
NightOWL LB981 CCD camera (Berthold Technologies). The images were
analyzed using IndiGo2 software (version 2.0.1.0; Berthold
Technologies) provided with the in vivo imaging system.
In vivo therapy for liver HeLa
metastasis
On days 8, 10, 12 and 14 after inoculation of HeLa
cells into the spleens of mice, 50 µg of Cont siRNA, PLK1 siRNA or
HSF1 siRNA were administered to mice using a sequential injection
method as described above (15,16). On
day 16 after inoculation, mice were sacrificed by cervical
dislocation, and then the excised livers and spleens were
weighed.
Statistical analysis
The statistical significance of differences between
mean values was determined using Student's t-test using GraphPad
Prism (version 4.0; GraphPad Software, Inc., La Jolla, CA, USA).
Multiple measurement comparisons were performed by one-way analysis
of variance on ranks with post hoc Tukey-Kramer's test. P<0.05
was considered to indicate a statistically significant
difference.
Results and Discussion
Expression level of HSF1 and PLK1
mRNA
For investigation of HSF1 and PLK1 mRNA expression
levels in cultured cells, three human tumor cell lines,
(MDA-MB-231, MCF-7 and HeLa cells) were used. As demonstrated in
Fig. 1A, PLK1 and HSF1 mRNA were
expressed strongly in MDA-MB-231 and HeLa cells, but weakly or not
at all in MCF-7 cells. Therefore, in subsequent experiments,
MDA-MB-231 and HeLa cells were used as the cell lines that
expressed HSF1 and PLK1 mRNA at high levels.
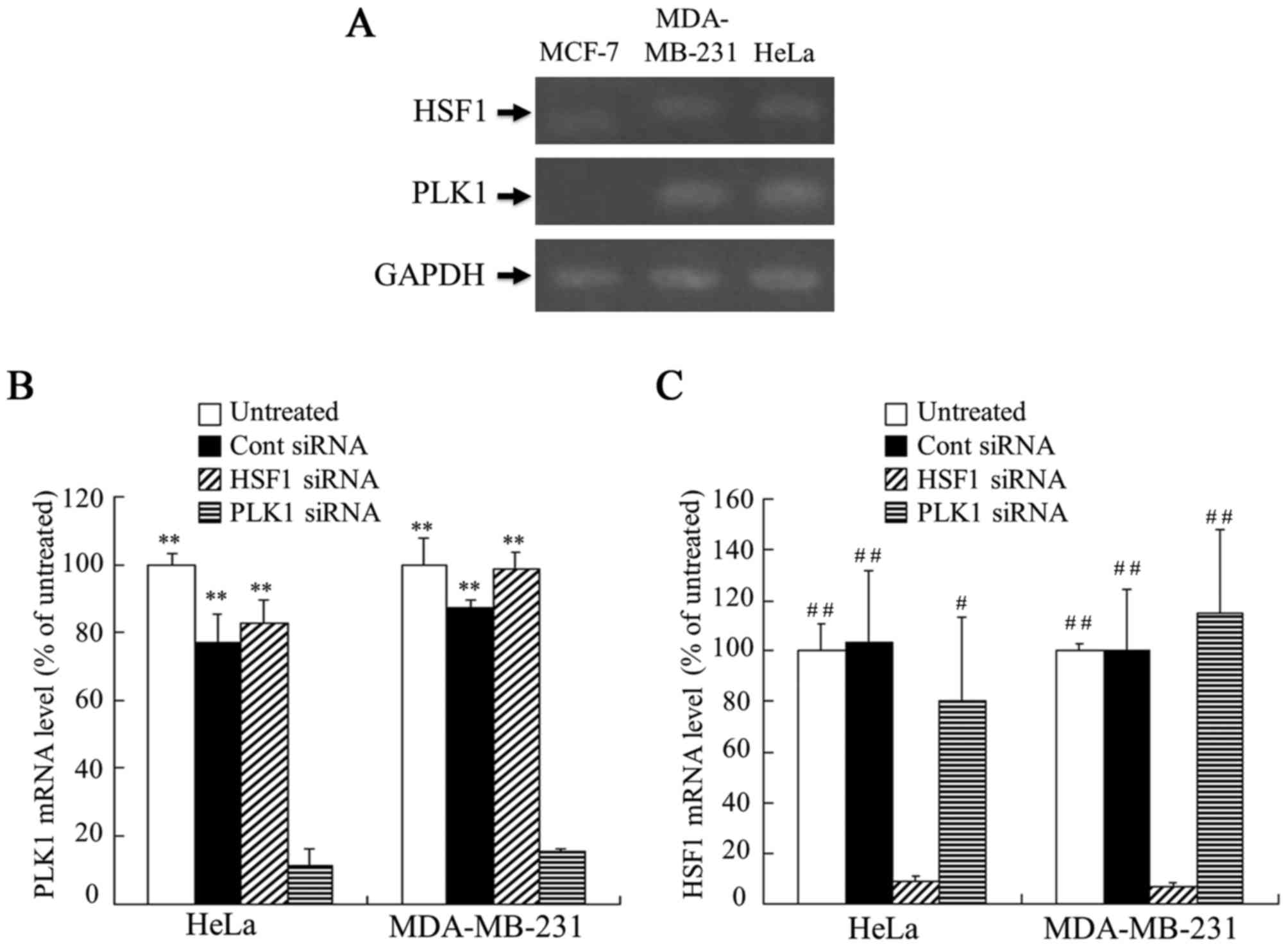 | Figure 1.Expression of PLK1 and HSF1 mRNA in
MCF-7, MDA-MB-231 and HeLa cells, and suppression of PLK1 or HSF1
mRNA expression by transfection with siRNA in MDA-MB-231 and HeLa
cells. (A) The expression levels of HSF1 and PLK1 mRNA in MCF-7,
MDA-MB-231 and HeLa cells were analyzed by RT-PCR. MDA-MB-231 and
HeLa cells were transfected with 50 nM Cont, HSF1 or PLK1 siRNA
using Lipofectamine RNAiMax reagent, and the expression levels of
(B) PLK1 and (C) HSF1 mRNA in the cells were analyzed by
RT-quantitative PCR. Data are presented as the mean + standard
deviation (n=3). **P<0.01 vs. PLK1 siRNA; #P<0.05
and ##P<0.01 vs. HSF1 siRNA. PLK1, polo-like kinase
1; HSF1, heat shock transcription factor 1; RT-PCR, reverse
transcription polymerase chain reaction; siRNA, small interfering
RNA; Cont, control. |
Subsequently, whether the expression level of PLK1
and HSF1 mRNA was decreased by transfecting PLK1 siRNA and HSF1
siRNA into the cells, respectively, was investigated. Lipofectamine
RNAiMax was used as an in vitro transfection reagent for
siRNA. When transfected into MDA-MB-231 and HeLa cells, PLK1 siRNA
and HSF1 siRNA significantly inhibited the expression of PLK1 and
HSF1 mRNA (P<0.01), respectively, compared with the levels in
the untreated or Cont siRNA groups (Fig.
1B and C). In contrast, transfection of Cont siRNA or HSF1
siRNA did not significantly affect the expression of PLK1 mRNA in
MDA-MB-231 and HeLa cells (Fig. 1B),
and transfection of Cont siRNA or PLK1 siRNA did not significantly
affect HSF1 mRNA compared with the levels in the untreated cells
(Fig. 1C). These results indicated
that PLK1 siRNA and HSF1 siRNA could specifically suppress the
expression of PLK1 and HSF1 mRNA in the cells, respectively, and
suppression of PLK1 mRNA did not affect the expression level of
HSF1 mRNA in the cells.
Effect of HSF1 siRNA and PLK1 siRNA on
cell viability
To examine whether transfection of HSF1 siRNA or
PLK1 siRNA into tumor cells could inhibit tumor growth, cell
viability was measured 48 h after transfection of HSF1 siRNA or
PLK1 siRNA into MDA-MB-231, HeLa and MCF-7 cells. In MCF-7 cells,
transfection with HSF1 siRNA did not significantly affect cell
viability compared with transfection with Cont siRNA. However,
transfection with PLK1 siRNA significantly suppressed cell
viability at concentrations of 5, 10, 20, 30, 40 and 50 nM siRNA
compared with the viability of cells transfected with Cont siRNA
(Fig. 2). These results suggested
that HSF1 siRNA may not be effective for growth inhibition of MCF-7
cells. In contrast, MDA-MB-231 and HeLa cells transfected with HSF1
siRNA demonstrated a significant decrease in viability at some
concentrations compared with those transfected with Cont siRNA
(P<0.05; Figs. 3 and 4). Transfection with PLK1 siRNA
significantly inhibited cell growth at all concentrations in
MDA-MB-231 and HeLa cells compared with those transfected with Cont
siRNA (P<0.01; Figs. 3 and
4), indicating that suppression of
PLK1 expression strongly affected in vitro growth in tumor
cells that expressed PLK1 mRNA at high levels.
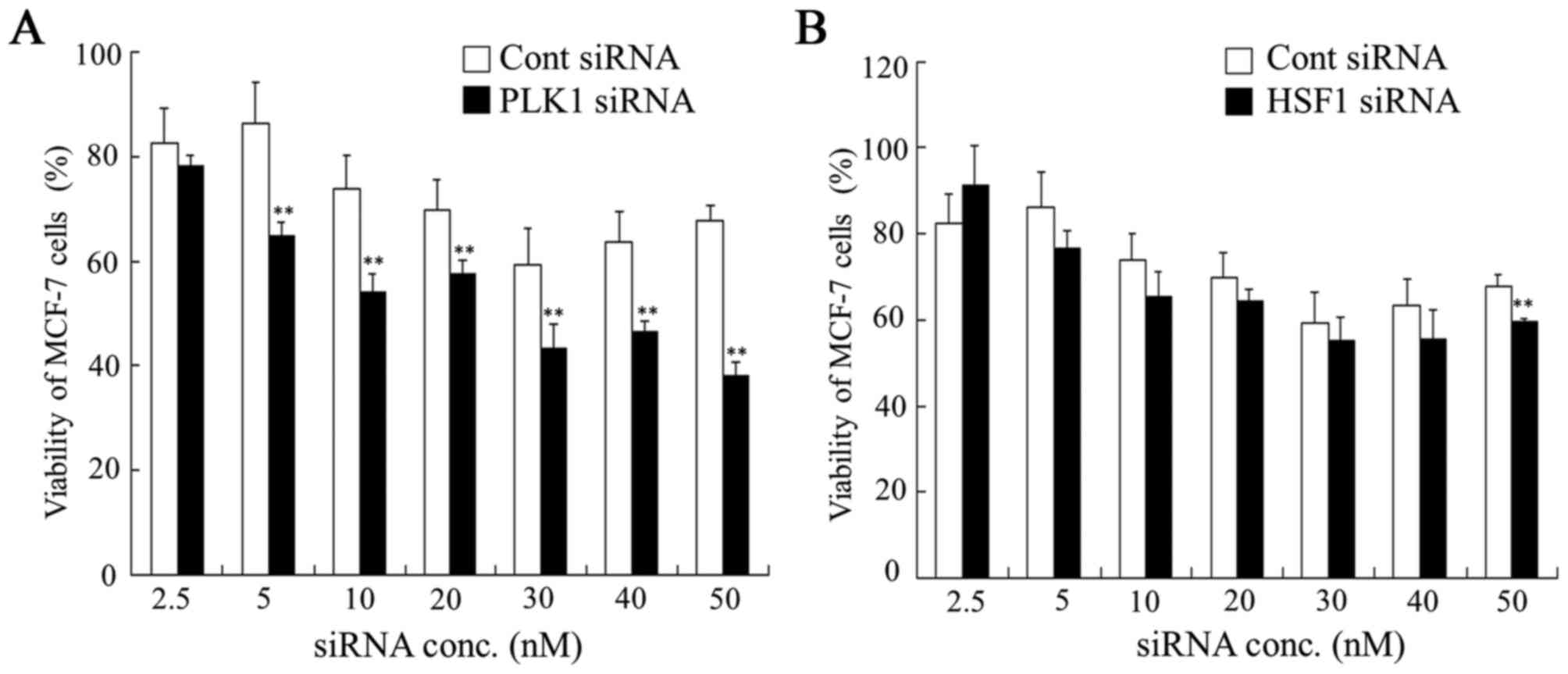 | Figure 2.Dose dependence of anti-proliferative
activities 48 h after transfection with PLK1 or HSF1 siRNA into
MCF-7 cells. At 48 h after transfection with (A) PLK1 or (B) HSF1
siRNA at 2.5, 5, 10, 20, 30, 40 and 50 nM, cell viability was
measured. Data are presented as the mean + standard deviation
(n=4). **P<0.01 vs. Cont siRNA. PLK1, polo-like kinase 1; HSF1,
heat shock transcription factor 1; siRNA, small interfering RNA;
Cont, control; conc, concentration. |
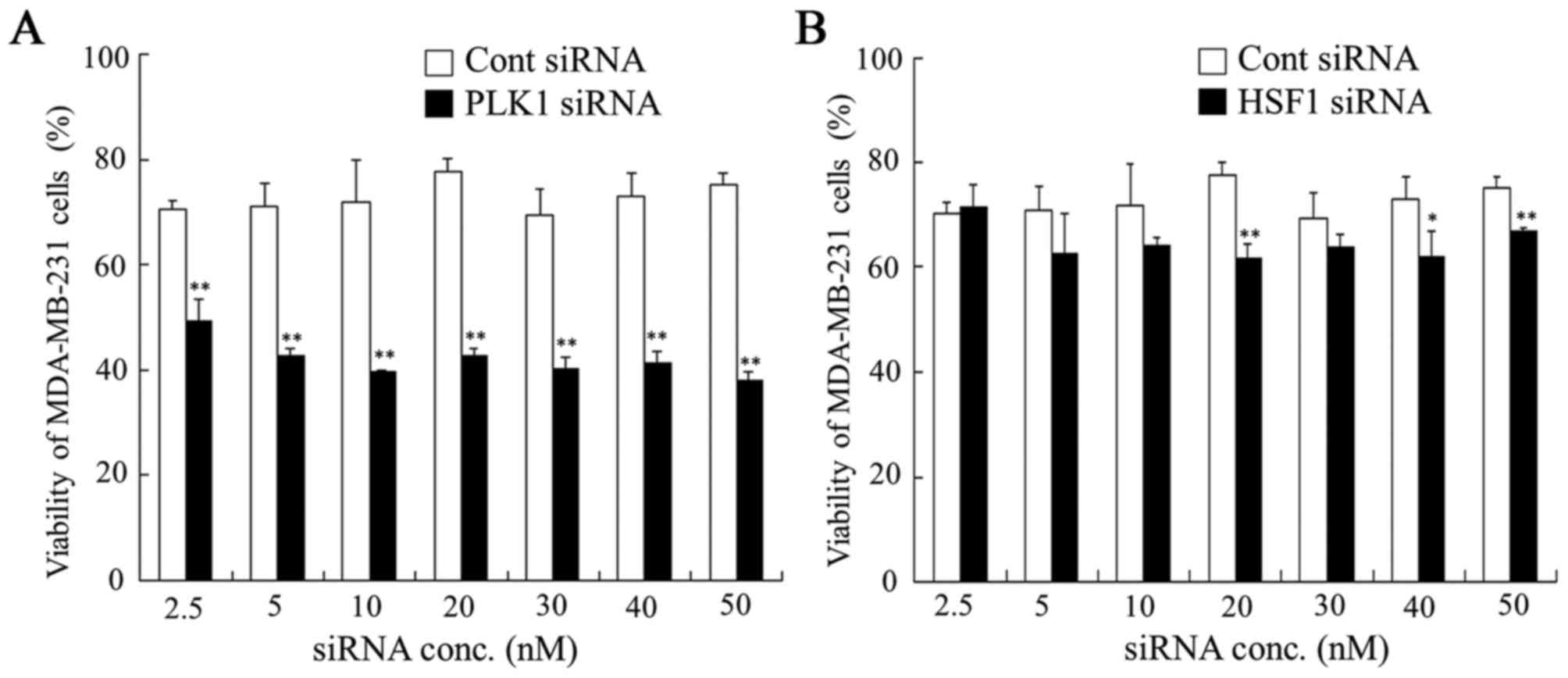 | Figure 3.Dose dependence of anti-proliferative
activities 48 h after transfection with PLK1 or HSF1 siRNA into
MDA-MB-231 cells. At 48 h after transfection with (A) PLK1 or (B)
HSF1 siRNA at 2.5, 5, 10, 20, 30, 40 and 50 nM, cell viability was
measured. Data are presented as the mean + standard deviation
(n=4). *P<0.05 and **P<0.01 vs. Cont siRNA. PLK1, polo-like
kinase 1; HSF1, heat shock transcription factor 1; siRNA, small
interfering RNA; Cont, control; conc, concentration. |
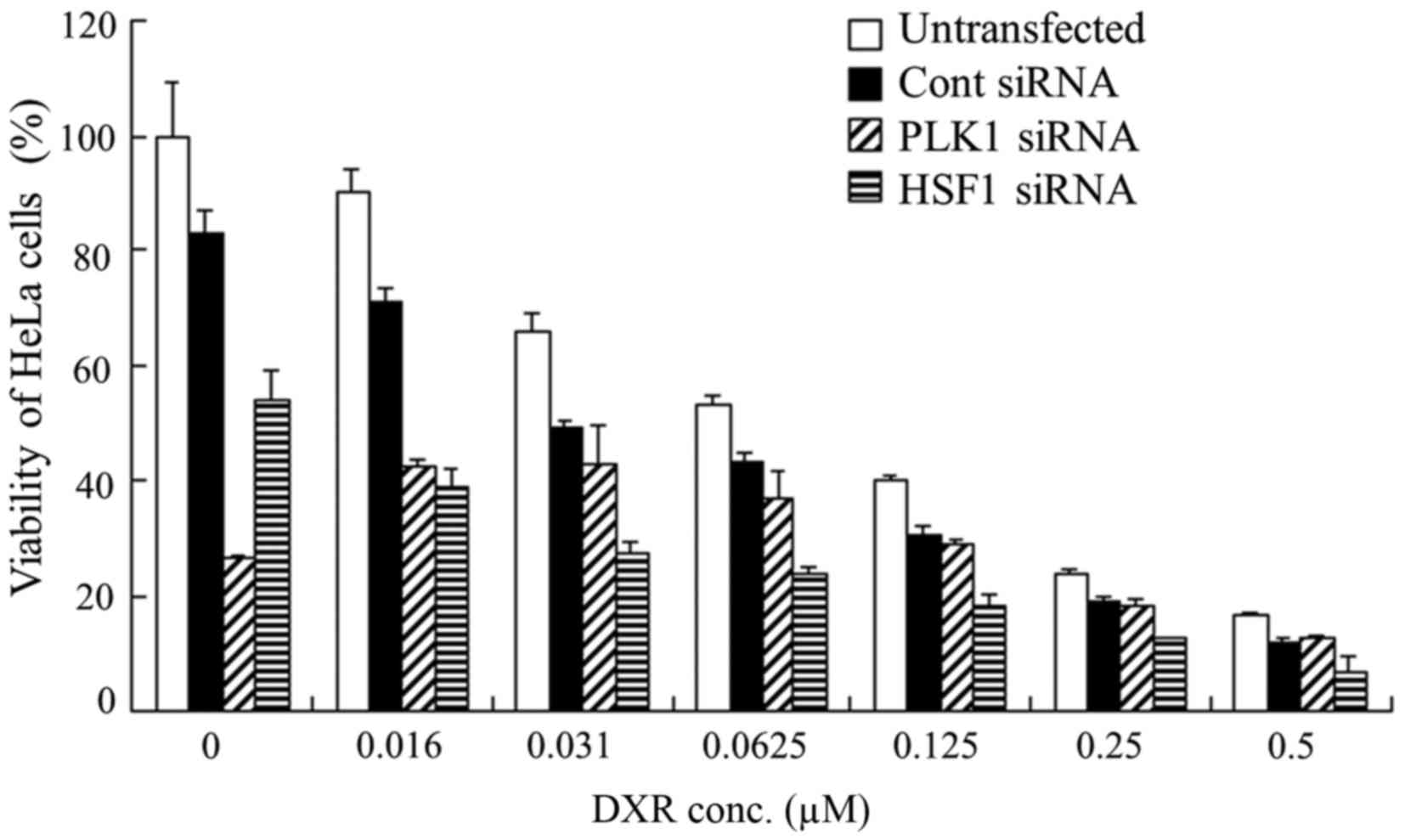
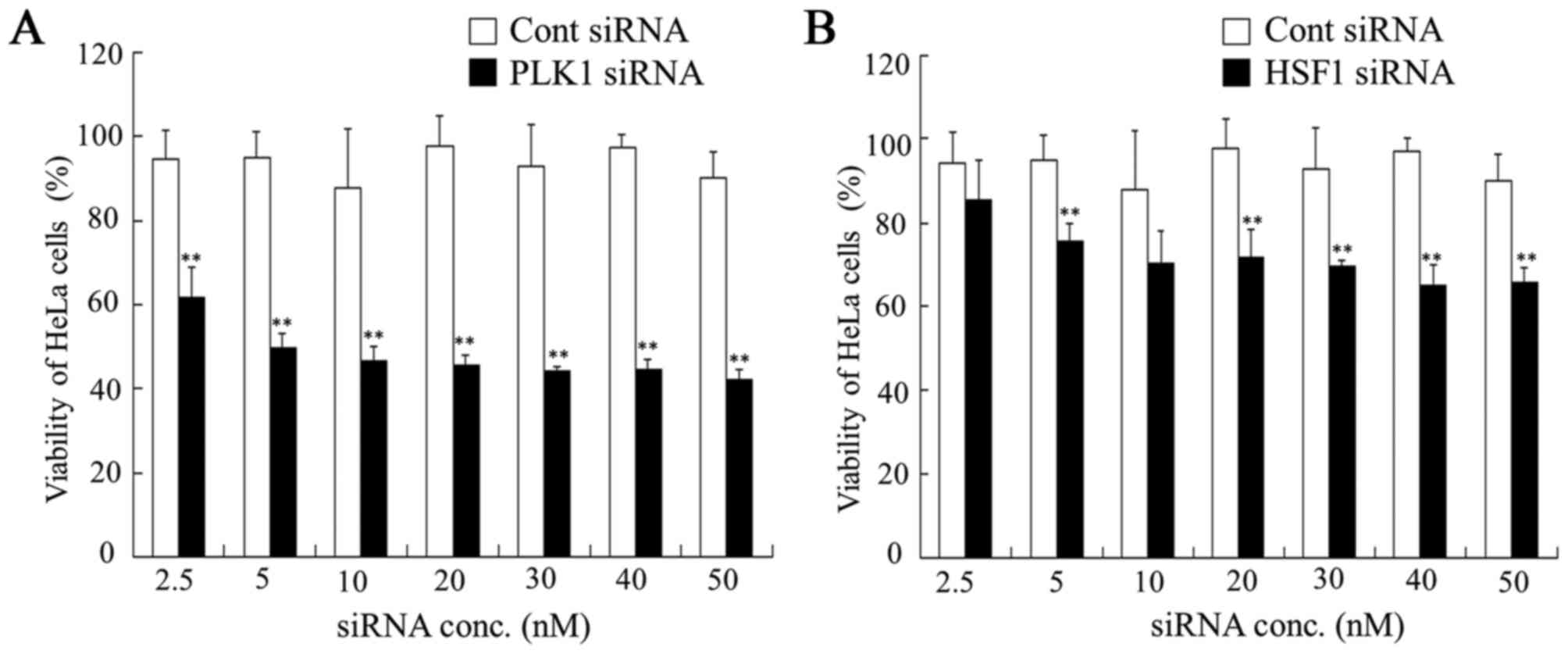 | Figure 4.Dose dependence of anti-proliferative
activities 48 h after transfection with PLK1 or HSF1 siRNA into
HeLa cells. At 48 h after transfection with (A) PLK1 or (B) HSF1
siRNA at 2.5, 5, 10, 20, 30, 40 and 50 nM, cell viability was
measured. Data are presented as the mean + standard deviation
(n=4). **P<0.01 vs. Cont siRNA. PLK1, polo-like kinase 1; HSF1,
heat shock transcription factor 1; siRNA, small interfering RNA;
Cont, control; conc, concentration. |
Following this, it was evaluated whether
transfection with PLK1 siRNA or HSF1 siRNA could increase the
growth inhibitory effect of DXR treatment in HeLa cells. The
combined treatment of HSF1 siRNA with DXR exhibited additive
cytotoxicity in HeLa cells compared with HSF1 siRNA treatment alone
(Fig. 5). However, the combined
treatment of PLK1 siRNA with DXR did not exhibit a significant
synergistic or additive cytotoxicity in HeLa cells compared with
PLK1 siRNA treatment alone (Fig. 5).
These data indicated that a reduction in PLK1 or HSF1 expression
did not increase the sensitivity to DXR in HeLa cells. Therefore,
for the in vivo experiment for evaluation of therapeutic
efficacy for tumor metastasis, we decided to inject PKL1 siRNA or
HSF1 siRNA without DXR.
Biodistribution of siRNA following
injections of siRNA lipoplexes
Recently, we demonstrated that intravenous
sequential injection of chondroitin sulfate plus siRNA lipoplexes
into mice with liver metastasis could deliver siRNA efficiently to
the metastasis without accumulation in the lungs, and suppress the
expression of a target gene in the tumor cells (15,16).
Therefore, the present study evaluated the therapeutic efficacy of
treatment with PLK1 siRNA or HSF1 siRNA using the sequential
injection method against HeLa-liver metastasis. First, the
biodistribution of siRNA following sequential injections of
chondroitin sulfate plus siRNA lipoplexes into mice with HeLa-liver
metastasis was investigated. To generate mice with liver
metastasis, HeLa cells were inoculated into the spleens of mice. As
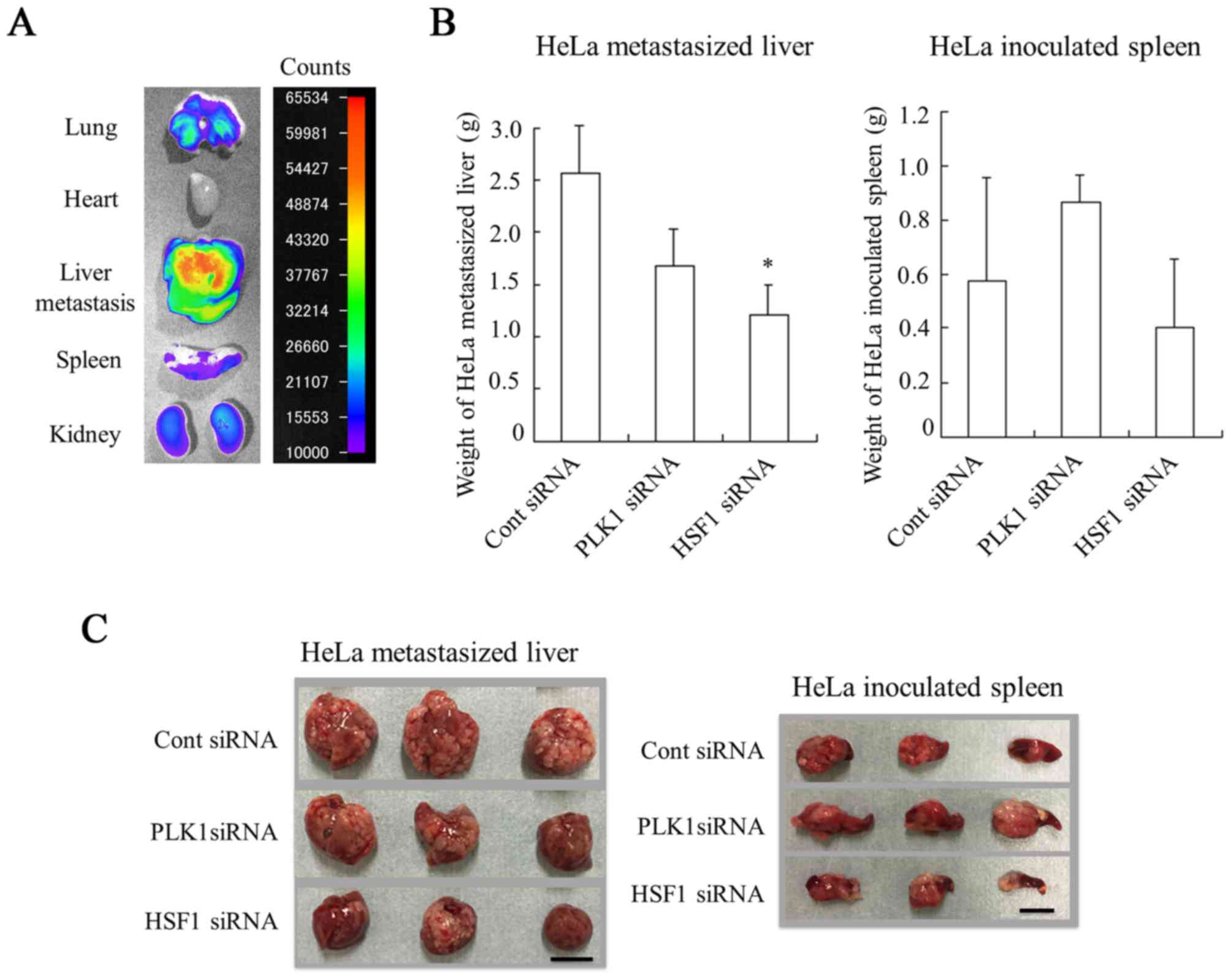
demonstrated in Fig. 6A, siRNA was
largely accumulated in HeLa-metastasized liver. These results
indicated that sequential injections could deliver siRNA
effectively into HeLa-metastasized livers of mice.
Therapeutic efficacy against liver
HeLa-metastasized tumors
Finally, the present study evaluated the in
vivo efficacy of PLK1 siRNA or HSF1 siRNA in inhibiting the
growth of liver HeLa-metastasized tumors. Injection of PLK1 siRNA
or HSF1 siRNA was performed a total of four times, with 2 days
between each injection. The antitumor effect on metastasis was
evaluated by measurement of liver weight (mg) (Fig. 6B and C). Injections of PLK1 siRNA
suppressed the increase in weight of HeLa-metastasized liver
(1,677±342 mg) compared with those injected with Cont siRNA
(2,567±453 mg); however, this difference was not significant. In
contrast, injections of HSF1 siRNA significantly inhibited the
increase in weight of metastasized livers (1,217±280 mg) compared
with those injected with Cont siRNA (P<0.05). Regarding the
weight of HeLa-inoculated spleens, significant differences in the
weight of spleens were not observed among the different groups
(Fig. 6B and C). These results
suggested that injection of HSF1 siRNA could inhibit tumor
metastasis to the liver and/or tumor growth in liver metastasis.
However, it was not clear why HSF1 siRNA did not exhibit strong
growth inhibition in vitro compared with PLK1 siRNA
(Fig. 4). HSF1 siRNA may be able to
inhibit invasion and metastasis of tumor cells rather than tumor
growth.
It has been reported that inhibition of PLK1
expression suppressed invasion of tumor cells and decreased the
activity of cluster of differentiation (CD)44v6 (23), which is an important member of the
cell adhesion molecule CD44 family. Additionally, HSF1 serves
various important roles in cancer via regulating cell
proliferation, anti-apoptosis, epithelial-mesenchymal transition,
migration, invasion and metastasis (24). HSF1 may promote invasion and
metastasis of hepatocellular carcinoma by enhancing cell motility
through Hsp27 (25). Such findings
indicated that PLK1 and HSF1 may be involved in the regulation of
cancer metastasis.
Intravenous injection of PLK1 siRNA with a fusion
protein of human epidermal growth factor 2-single-chain fragmented
antibody and protamine peptide retarded breast tumor growth and
reduced metastasis (12).
Furthermore, it has been reported that melanoma cells infected with
adenovirus expressing HSF1 shRNA markedly reduced invasion and
metastasis in a subcutaneous xenograft model (11). In the present study, injections of
HSF1 siRNA or PLK1 siRNA with cationic liposomes inhibited tumor
progression in HeLa metastasis. These findings suggest that PLK1
and HSF1 are critical factors that influence metastasis and have an
important role in tumor progression.
In conclusion, injection of PKL1 siRNA or HSF1 siRNA
into mice with liver HeLa metastasis inhibited tumor metastasis.
PLK1 and HSF1 may, therefore, be considered as promising
therapeutic targets for tumor metastasis.
Acknowledgements
The present project was supported in part by a
Grant-in-Aid for Scientific Research (C) from the Japan Society for
the Promotion of Science (KAKENHI; grant nos. JP26460046 and
JP17K08251).
References
|
1
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morano KA and Thiele DJ: Heat shock factor
function and regulation in response to cellular stress, growth, and
differentiation signals. Gene Expr. 7:271–282. 1999.PubMed/NCBI
|
|
3
|
Wang Y, Theriault JR, He H, Gong J and
Calderwood SK: Expression of a dominant negative heat shock
factor-1 construct inhibits aneuploidy in prostate carcinoma cells.
J Biol Chem. 279:32651–32659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calderwood SK: Heat shock proteins in
breast cancer progression--a suitable case for treatment? Int J
Hyperthermia. 26:681–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmucker S and Sumara I: Molecular
dynamics of PLK1 during mitosis. Mol Cell Oncol. 1:e9545072014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SA, Yoon JH, Lee SH and Ahn SG:
Polo-like kinase 1 phosphorylates heat shock transcription factor 1
and mediates its nuclear translocation during heat stress. J Biol
Chem. 280:12653–12657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takai N, Hamanaka R, Yoshimatsu J and
Miyakawa I: Polo-like kinases (Plks) and cancer. Oncogene.
24:287–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knecht R, Elez R, Oechler M, Solbach C,
von Ilberg C and Strebhardt K: Prognostic significance of polo-like
kinase (PLK) expression in squamous cell carcinomas of the head and
neck. Cancer Res. 59:2794–2797. 1999.PubMed/NCBI
|
|
9
|
Takahashi T, Sano B, Nagata T, Kato H,
Sugiyama Y, Kunieda K, Kimura M, Okano Y and Saji S: Polo-like
kinase 1 (PLK1) is overexpressed in primary colorectal cancers.
Cancer Sci. 94:148–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SA, Kwon SM, Yoon JH and Ahn SG: The
antitumor effect of PLK1 and HSF1 double knockdown on human oral
carcinoma cells. Int J Oncol. 36:867–872. 2010.PubMed/NCBI
|
|
11
|
Nakamura Y, Fujimoto M, Fukushima S,
Nakamura A, Hayashida N, Takii R, Takaki E, Nakai A and Muto M:
Heat shock factor 1 is required for migration and invasion of human
melanoma in vitro and in vivo. Cancer Lett. 354:329–335. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao YD, Sun TM, Huang SY, Dou S, Lin L,
Chen JN, Ruan JB, Mao CQ, Yu FY, Zeng MS, et al: Targeted delivery
of PLK1-siRNA by ScFv suppresses Her2+ breast cancer
growth and metastasis. Sci Transl Med. 4:130ra482012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattori Y, Arai S, Kikuchi T, Ozaki K,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317. 2016.
View Article : Google Scholar
|
|
16
|
Hattori Y, Arai S, Okamoto R, Hamada M,
Kawano K and Yonemochi E: Sequential intravenous injection of
anionic polymer and cationic lipoplex of siRNA could effectively
deliver siRNA to the liver. Int J Pharm. 476:289–298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YJ, Kim EH, Lee JS, Jeoung D, Bae S,
Kwon SH and Lee YS: HSF1 as a mitotic regulator: Phosphorylation of
HSF1 by Plk1 is essential for mitotic progression. Cancer Res.
68:7550–7560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seth S, Matsui Y, Fosnaugh K, Liu Y, Vaish
N, Adami R, Harvie P, Johns R, Severson G, Brown T, et al:
RNAi-based therapeutics targeting survivin and PLK1 for treatment
of bladder cancer. Mol Ther. 19:928–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jacobs AT and Marnett LJ: HSF1-mediated
BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated
colon cancer cells via stabilization of anti-apoptotic Bcl-2
proteins. J Biol Chem. 284:9176–9183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakurai Y, Hatakeyama H, Akita H and
Harashima H: Improvement of doxorubicin efficacy using liposomal
anti-polo-like kinase 1 siRNA in human renal cell carcinomas. Mol
Pharm. 11:2713–2719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato M, Hattori Y, Kubo M and Maitani Y:
Collagenase-1 injection improved tumor distribution and gene
expression of cationic lipoplex. Int J Pharm. 423:428–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XG, Lu XF, Jiao XM, Chen B and Wu
JX: PLK1 gene suppresses cell invasion of undifferentiated thyroid
carcinoma through the inhibition of CD44v6, MMP-2 and MMP-9. Exp
Ther Med. 4:1005–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang S, Tu K, Fu Q, Schmitt DC, Zhou L,
Lu N and Zhao Y: Multifaceted roles of HSF1 in cancer. Tumour Biol.
36:4923–4931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang F, Chang R and Yang L: Heat shock
factor 1 promotes invasion and metastasis of hepatocellular
carcinoma in vitro and in vivo. Cancer. 118:1782–1794. 2012.
View Article : Google Scholar : PubMed/NCBI
|