Introduction
Diffuse large B cell lymphoma (DLBCL), which
accounts for 30 to 40% of non-Hodgkin lymphoma (NHL) cases, is the
most common malignant lymphoma. Response rates to RCHOP treatment
(rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone) range from 80 to 90% in patients with low-risk disease
(1). However, the response rates for
refractory or relapsed patients range from 30 to 60% with frequent
relapses, and salvage chemotherapy is often inadequate for these
patients. Thus, there is an urgent need to develop new anti-tumor
drugs with better efficacy and lower toxicity that can enhance the
chemotherapeutic sensitivity of refractory and relapsed
patients.
Botanical drugs are pharmaceuticals of plant origin
that generally have multiple targets and fewer side effects than
those of traditional medicines. Artemisinin and its derivatives,
including artesunate, dihydroartemisnin and artemether (ART), are
well-known anti-malaria botanical drugs, and these sesquiterpene
lactone compounds contain specific endoperoxide bridges. Abundant
experimental and clinical studies have shown that artemisinin and
its derivatives are effective in treating malaria with little drug
resistance (2). In recent studies,
these artemisinin drugs have not only exhibited significant
cytotoxicity towards and inhibitory effects on different cancer
cells under experimental conditions (3–6), but
also increased the recurrence-free survival with well-toleration in
colorectal cancer patients and contributed to regression in
prostate carcinoma patients (7,8). These
findings suggest that artemisinin derivatives may also be promising
drugs for treating lymphoma patients. However, the effects of these
artemisinin drugs on lymphoma are still unclear.
Intracellular free iron is reported to be more
abundant in cancer cells than normal cells (9). Artemisinin and its derivatives can
react with intracellular free iron to form cytotoxic free radicals
and increase the activity of antioxidant enzymes, promoting
apoptosis in cancer cells (10).
Furthermore, the expression of genes involved in iron metabolism is
positively correlated with the sensitivity of cancer cells to
artemisinin treatment (11).
Additionally, tumor cells can secrete vascular endothelial growth
factor (VEGF) receptors to increase capillary permeability, promote
proliferation and migration of endothelial cells and contribute to
tumor angiogenesis. Capillary permeability and tumor angiogenesis
are reduced by inhibiting VEGF receptors (12). Artemisinin is also shown to inhibit
tumor angiogenesis by suppressing the expression of VEGF in
treatment of brain glioma (5).
Moreover, artemisinin inhibits the proliferation of tumor cells by
blocking the apoptosis pathway of P53-independent tumors (13). All these studies indicate that
artemisinin and its derivatives are potential anti-tumor drugs, but
the detailed mechanisms require further elucidation.
Here, we used two human DLBCL cell lines, SUDHL-4
and DB, to explore the anti-cancer effects of ART (a derivative of
artemisinin) on DLBCL cells. Our results showed that ART
significantly inhibited the proliferation of DLBCL cells by
suppressing the expression of cell cycle-related genes (CDK2, CDK4,
and Cyclin D1) and c-Myc, and induced DLBCL cells apoptosis by
activating the Caspase-3/PARP1 axis.
Materials and methods
Cell lines and cell culture
The DLBCL cell lines SUDHL-4 and DB were generously
provided by Shanghai Ruijin Hospital (Shanghai, China). Cells were
cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine
serum (Gibco, USA) at 37°C in a humidified incubator containing 5%
CO2, supplied with fresh medium every 3 days and
subcultured when confluence was reached.
Cell counting assays and Cell Counting
Kit-8 (CCK8) analysis
SUDHL-4 and DB cells were seeded into 12-well plates
at a density of 6×104 cells per well. ART, purchased
from Sigma-Aldrich Co. and dissolved in ethanol (Sinopharm Chemical
Reagent Co., Ltd.), was added to the medium to reach a final
concentration of 0.1 mM. Cells with an equal volume of ethanol were
used as negative controls. Cell counting assays were performed
after 24, 48, and 72 h of ART treatment. Cell proliferation was
assessed in 96-well plates at a density of 3×103 cells
per well using the CCK8 assay (DOJINDO, Japan).
Ki-67 detection
DLBCL cells were treated with ART (0.1 mM) and
collected after 48 h. Cells were fixed by 2% PFA for 30 min. After
permeabilization, the cells were further stained with Ki-67 (Abcam,
ab66155) at 37°C for 60 min and then stained with FITC-labeled Goat
Anti-Rabbit IgG (H+L) (Beyotime Biotechnology) at 37°C for 60 min
in the dark before flow cytometric analysis.
Cell cycle analysis
SUDHL-4 and DB cells were seeded into 12-well plates
at a density of 6×104 cells per well and treated with
ART (0.1 mM) for 48 h. Cells were washed twice with phosphate
buffered saline (PBS) and then resuspended with precooled 70%
ethanol overnight at 4°C. After centrifugation, the pellets were
washed twice with precooled PBS. Each sample was mixed with 500 µl
of staining buffer, 25 µl propidium iodide (PI) staining solution
and 10 µl RNase A (Beyotime Biotechnology) and incubated for 30 min
in the dark at 37°C. The cell cycle distribution was evaluated by
flow cytometry.
Cell apoptosis assay
SUDHL-4 and DB cells were seeded into 12-well plates
at a density of 6×104 cells per well and treated with
ART (0.3 mM) for 48 h. Cells with an equal volume of ethanol were
used as negative controls. All cells were collected after a 48 h
treatment and then washed twice with PBS. After centrifugation,
each sample was mixed with 195 µl staining buffer, 5 µl PI staining
solution and 5 µl Annexin V-FITC solution (KeyGEN Biotech.) and
incubated for 20 min in the dark at 37°C before flow cytometry
analysis.
Western blotting
RIPA buffer (KeyGEN Biotech.) was used to lyse the
cells, and protein concentration was quantified by the BCA method.
An equal amount of the extracted protein was separated by 10%
SDS-PAGE and transferred onto PVDF membranes. The membranes were
blocked with 3% BSA for 1 h at room temperature and then incubated
with the primary antibodies overnight at 4°C. After three washes
with TBST, the membranes were incubated with secondary antibodies
(1:3,000) for 1 h at room temperature. The membranes were washed
with TBST three times before visualization by a chemiluminescence
system. Antibodies used in this work: Mouse anti-CDK2 (SC-6248,
Santa Cruz Biotechnology), rabbit anti-CDK4 (SC-260, Santa Cruz
Biotechnology), rabbit anti-Cyclin D1 (SC-718, Santa Cruz
Biotechnology), mouse anti-c-Myc (ab32, Abcam), rabbit anti-ERK
(4695S, Cell Signaling Technology), rabbit anti-P-ERK (4370S, Cell
Signaling Technology), rabbit anti-AKT (4685S, Cell Signaling
Technology), rabbit anti-P-AKT (4060L, Cell Signaling Technology),
rabbit anti-GAPDH (47724, Santa Cruz Biotechnology), rabbit
anti-cleaved-Caspase-3 (9661S, Cell Signaling Technology), rabbit
anti-Caspase-3 (9662S, Cell Signaling Technology), rabbit
anti-cleaved-PARP1 (CY5035, Abways Technology), mouse anti-PARP1
(SC-74469X, Santa Cruz Biotechnology), HRP-Ms (7076, Cell Signaling
Technology), and HRP-Rb (7074, Cell Signaling Technology).
Tumor xenografts
Six-week-old NOD-SCID mice were purchased from the
National Resource Centre for Rodent Laboratory Animals of China.
Initially, 1×107 DB cells suspended in 100 µl with 1
part matrigel and 2 part DMEM were injected subcutaneously into the
left and right thighs of the mice. On day 16 after tumor injection,
the mice were injected intraperitoneally with ART (200 mg/kg) every
day until day 25. Then, the mice were sacrificed at day 25
post-injection.
Statistical analysis
Statistical analysis was performed using SPSS 11.0
software, and the results were presented as the mean ± standard
deviation from triplicate experiments. Significance differences
were determined by two-tailed Student's t-test, and P<0.05 was
considered statistically significant.
Results
ART treatment inhibits the growth and
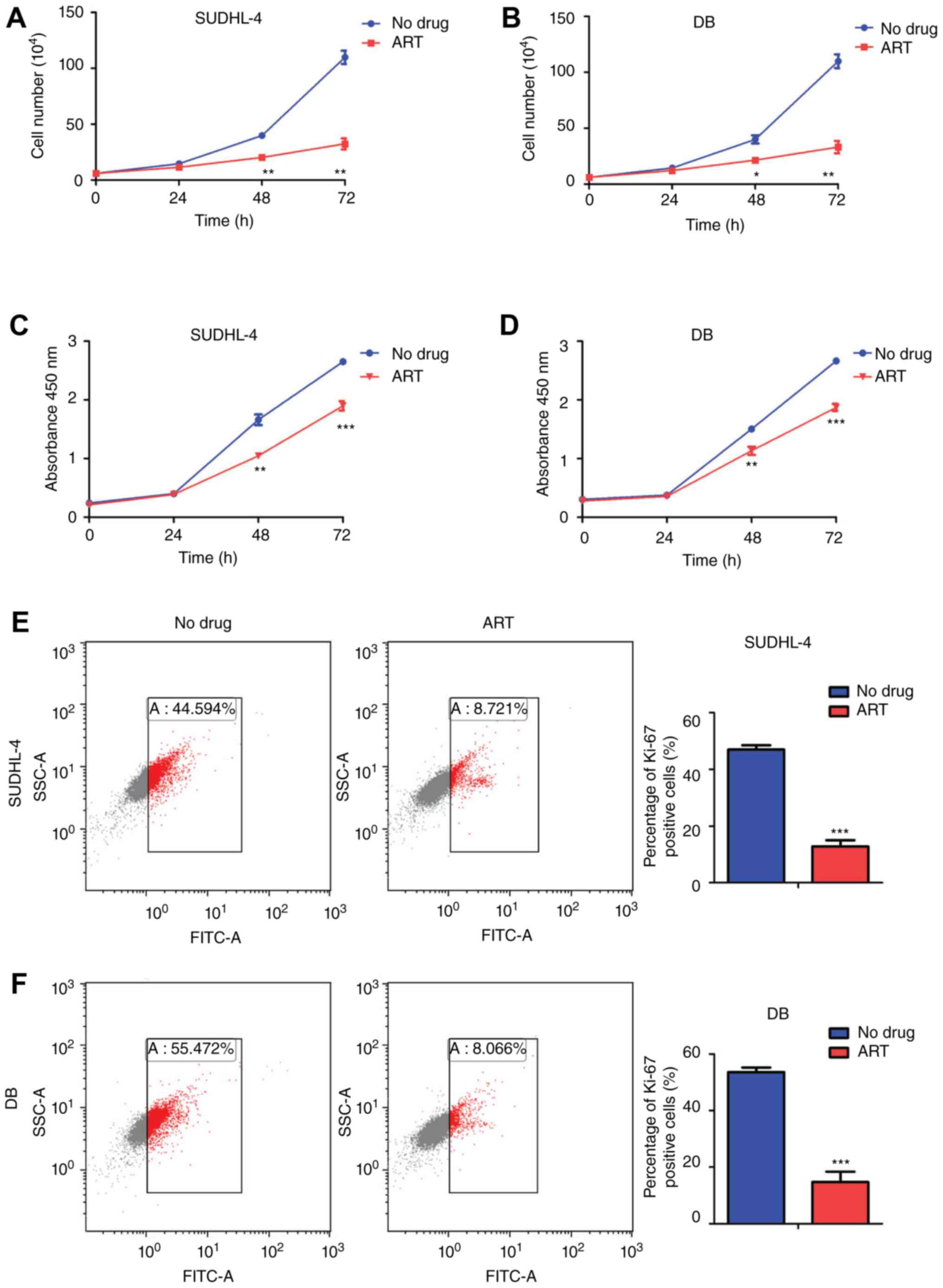
proliferation of SUDHL-4 and DB cells
Preliminary experiments indicated that 0.1 mM ART
treatment for 48 h led to half maximal inhibition of SUDHL-4 and DB
cells. There was no significant abnormality in the growth of
SUDHL-4 and DB cells after ART (0.1 mM) treatment for 24 h.
Following ART (0.1 mM) treatment for 48 h, the number of SUDHL-4
cells was (20.26±2.58) ×104 and the number of DB cells
was (21.44±2.70) ×104, which were much lower compared
with those of the negative controls, (39.99±2.38) ×104
for SUDHL-4 cells and (40.11±6.36) ×104 for DB cells.
These results showed that ART treatment for 48 h significantly
inhibited the growth of SUDHL-4 and DB cells. The percentages of
growth inhibition in SUDHL-4 and DB cells were 49.35±5.33% and
46.37±1.69%, respectively (Fig. 1A and
B). For the 72 h treatment, the growth inhibition percentages
were 70.63±5.53% for SUDHL-4 cells and 70.05±6.22% for DB cells,
showing more significant inhibitory effects on DLBCL cell growth
(Fig. 1A and B). CCK8 analysis
indicated that ART significantly inhibited the proliferation of
DLBCL cells after 48 and 72 h treatments (Fig. 1C and D). The DLBCL cells exposed to
ART treatment had significantly decreased Ki-67 expression, which
confirmed the inhibition of DLBCL cell proliferation (Fig. 1E and F). These results indicated that
treatment with ART had a significant inhibitory effect on cell
growth and proliferation in DLBCL cells.
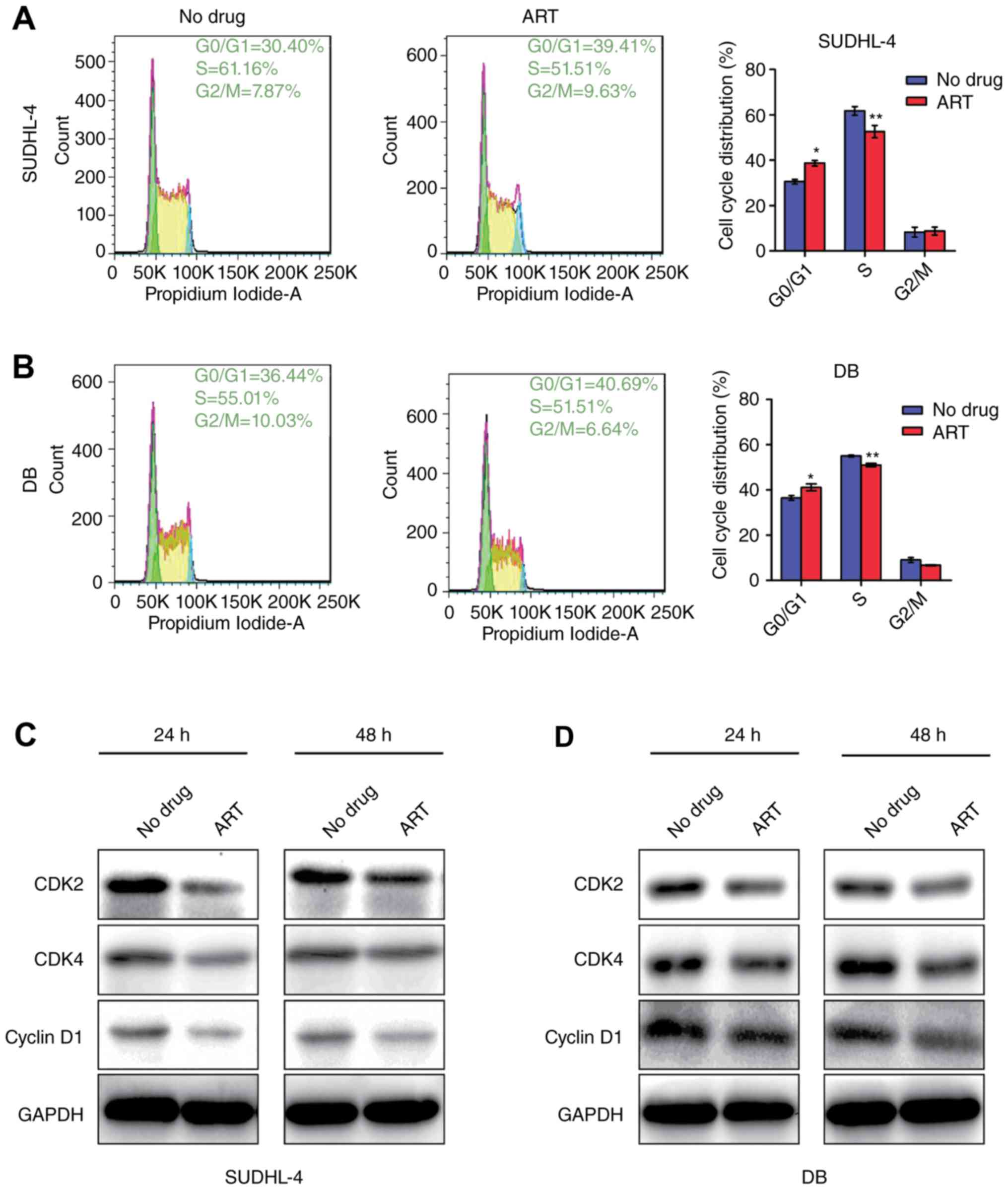
ART treatment results in G0/G1 phase
arrest of DLBCL cells and down-regulates cyclin expression
To determine whether the growth delay was due to
arrest in any specific cell cycle phase, we used flow cytometry to
compare the cell cycle distribution of untreated cells vs. that of
cells treated with 0.1 mM ART. Our data recorded severe arrest in
G0/G1 phase of SUDHL-4 cells (38.73±1.25%) and DB cells
(41.12±1.56%) after 0.1 mM ART treatment for 48 h compared with
that of the untreated cells (30.67±1.45% for SUDHL-4 cells and
36.46±1.05% for DB cells) (Fig. 2A and
B). Additionally, the percentages of S-phase cells were
decreased by 9.12±0.82% (SUDHL-4) and 4.03±1.13% (DB) (P<0.05)
(Fig. 2A and B). Taken together, the
results showed that ART treatment arrested cells in G0/G1 phase,
leading to failure to enter S-phase.
CDK2, CDK4 and Cyclin D1 play important roles in
G1/S transition, which is positively related to cell proliferation.
Reduction of these cyclins indicates that cells are arrested in G1
phase and cell proliferation is restrained. To determine how ART
affected cell cycle distribution of DLBCL cells, we measured the
CDK2, CDK4 and Cyclin D1 levels and found that these proteins were
substantially down-regulated with ART treatment for 24 and 48 h
(Fig. 2C and D). These results were
consistent with the cell cycle detection by flow cytometry.
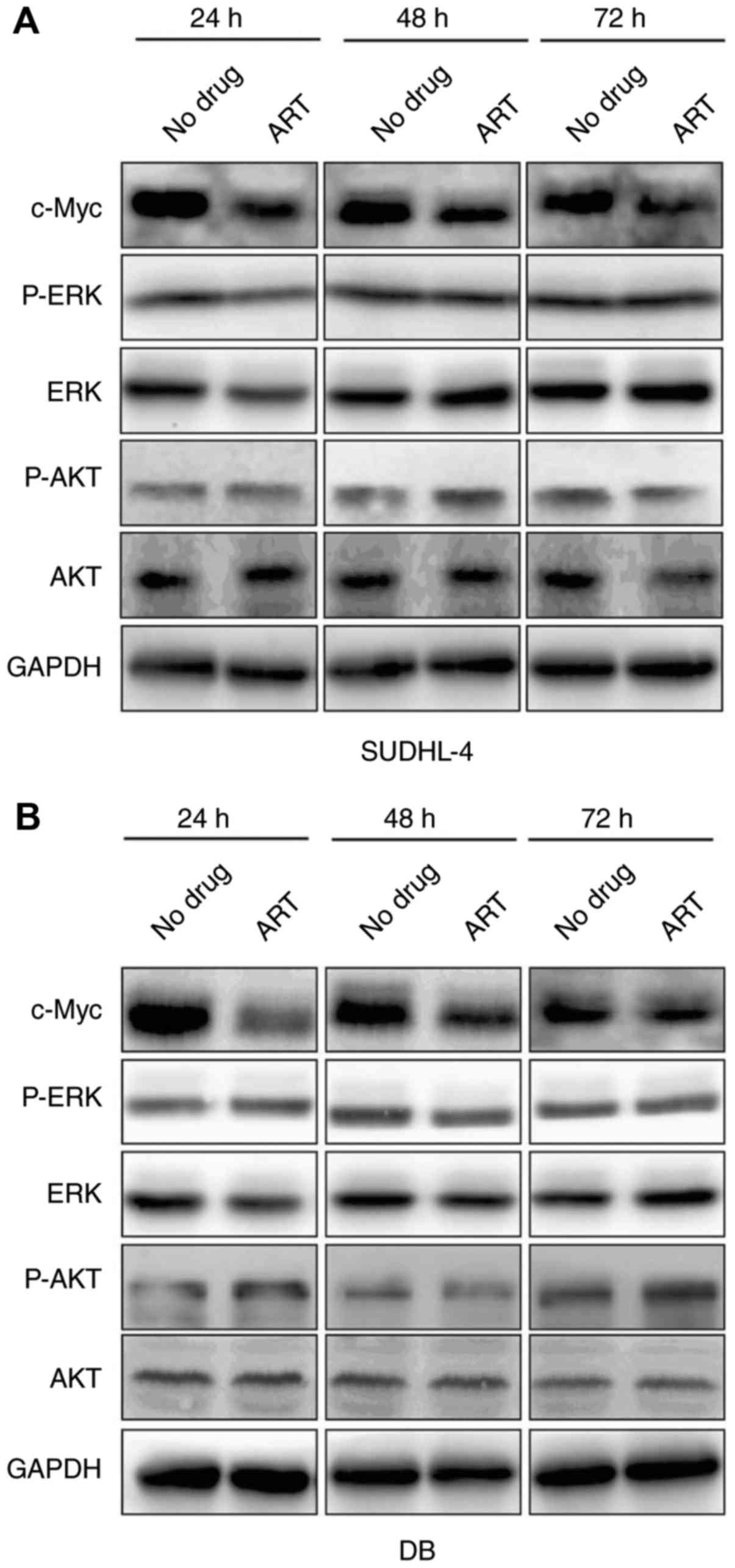
ART specifically inhibits the
expression of c-Myc
c-Myc is a proto-oncogene that is involved in many
malignant behaviors of cancers including proliferation, invasion
and activation of cancer signaling pathways (14). ERK and AKT are important kinases in
MAPK signaling and PI3K signaling respectively, which are important
for tumor progression (15,16). Our results showed that c-Myc
expression was dramatically down-regulated after SUDHL-4 and DB
cells were treated for different durations (24, 48 and 72 h)
(Fig. 3A and B). However, ART
treatment had no significant effect on the expression of P-ERK/ERK
and P-AKT/AKT at 24, 48 or 72 h (Fig. 3A
and B), suggesting that ART-mediated inhibition of cell
proliferation was predominantly regulated by decreasing the
expression of c-Myc, rather than the other two key signaling
pathways involved in cell proliferation.
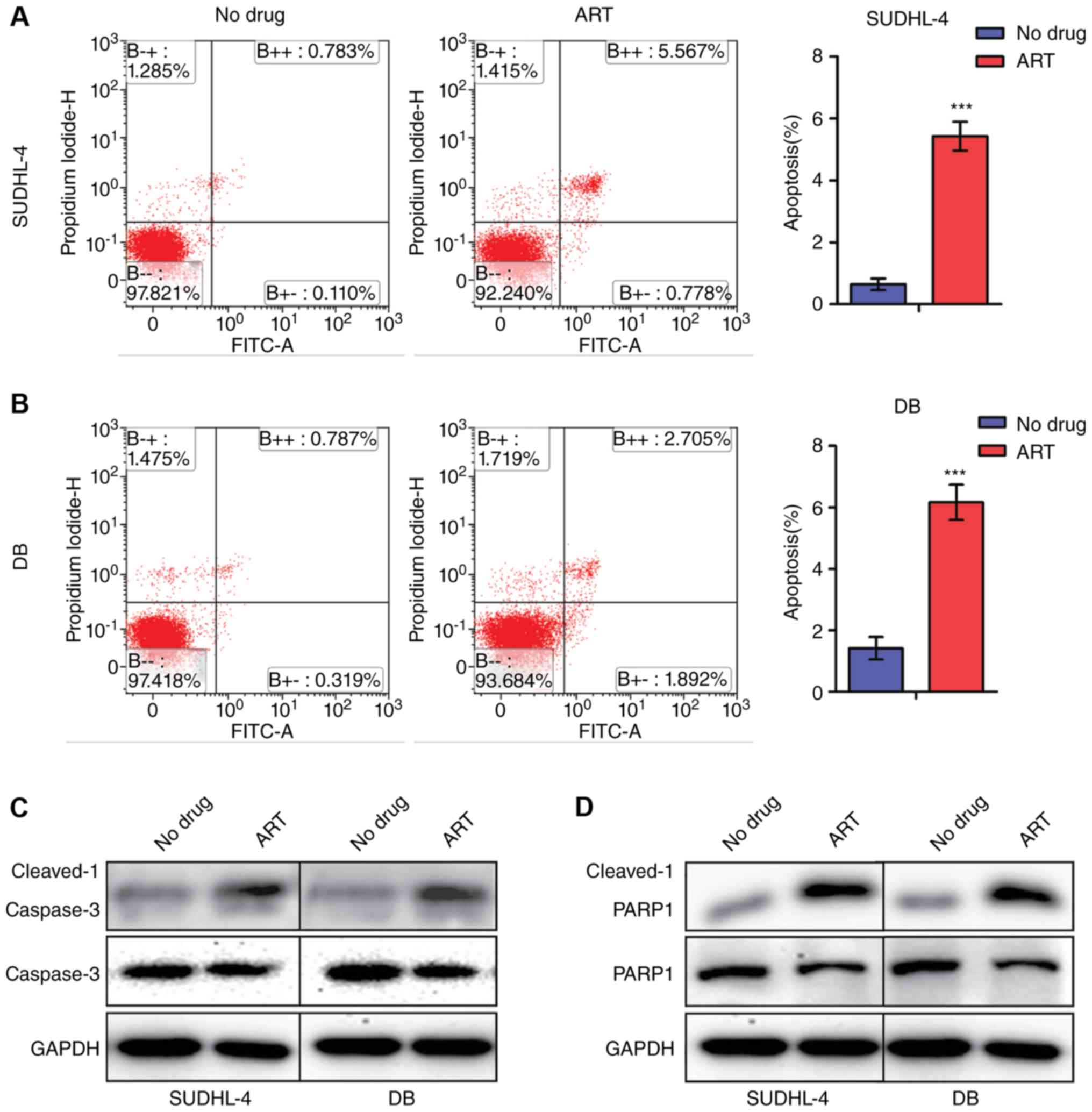
High-concentration ART treatment
induces apoptosis of DLBCL cells by activating the Caspase-3/PARP1
axis
To determine whether ART affected DLBCL cell
apoptosis, we evaluated the intensity of apoptosis by Annexin
V-FITC and PI staining using flow cytometry. Treating SUDHL-4 and
DB cells with ART (0.1 mM) did not make any notable difference on
the percentages of apoptotic cells, compared with the negative
controls (data not shown). While the percentages of apoptotic cells
were significantly increased to 5.03±0.59% in SUDHL-4 cells and
6.83±1.08% in DB cells after treatment with ART (0.3 mM)
(P<0.05) (Fig. 4A and B).
Furthermore, our results showed that the active forms of Caspase-3
and PARP1 (cleaved-Caspase-3 and cleaved-PARP1) were significantly
increased after ART treatment, suggesting that ART promoted the
cleavage of Caspase-3 and PARP1 (Fig. 4C
and D). These results indicated that ART induced apoptosis by
activating the Caspase-3/PARP1 axis.
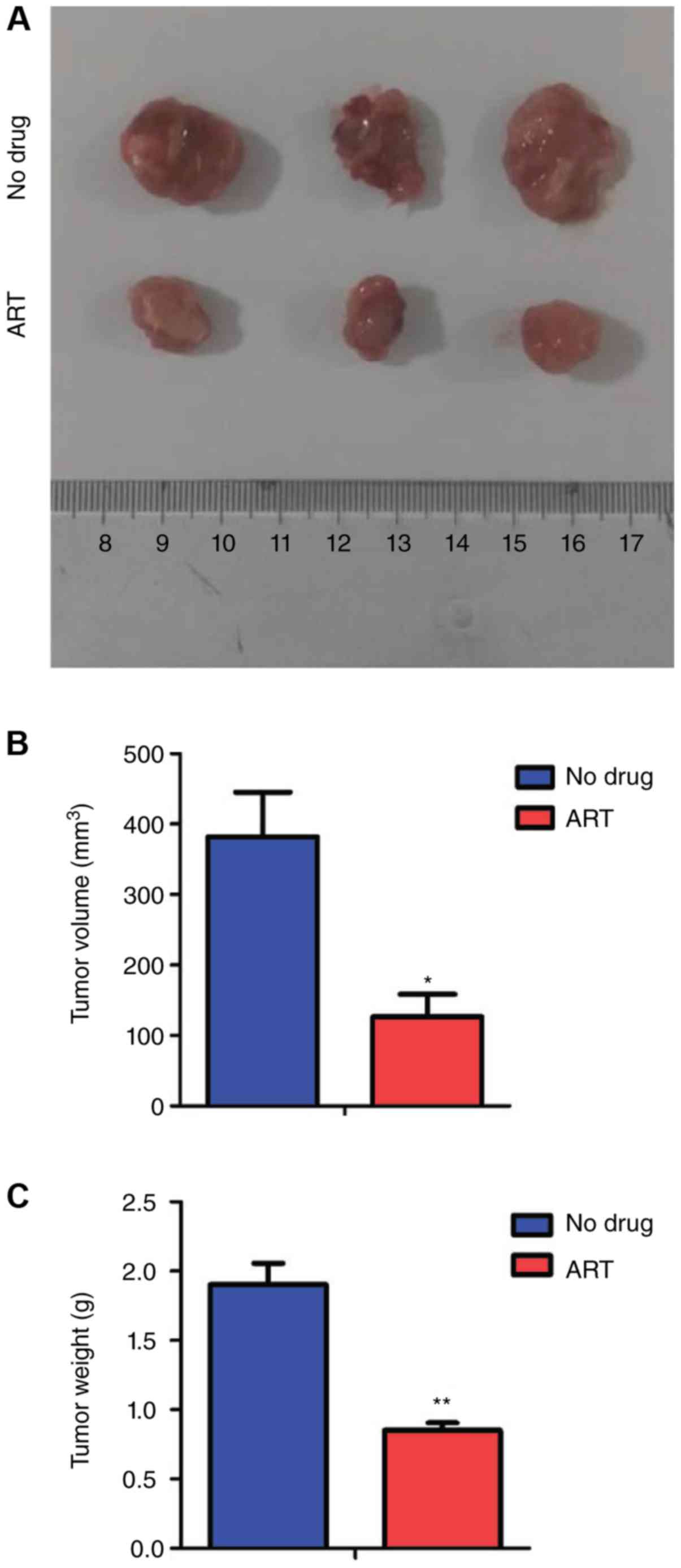
ART treatment inhibits DLBCL cell
growth in vivo
To further determine the in vivo effects of
ART, we constructed transplanted tumor models using six-week-old
NOD-SCID mice. Equal amounts of DB cells were injected
subcutaneously into left and right thighs of mice. Then, the mice
were injected intraperitoneally with ART (200 mg/kg) once a day
from day 16 to day 25 post-injection. Our results showed that the
ART-treated groups presented smaller tumor volumes (Fig. 5A and B) and lighter tumor weights
(Fig. 5C) than those of the control
groups, which suggested that ART alleviated the tumor burden of
mice in vivo.
Discussion
Recent studies have shown that artemisinin drugs
exhibited significant cytotoxicity and inhibitory effects on cancer
cells, including leukemia, stomach cancer, breast cancer, and
pancreatic cancer cells (3–6). The anti-tumor effects and mechanisms of
ART on lymphoma remain unexplored. Our results showed that ART
significantly inhibited the proliferation of DLBCL cells and
arrested these cells in G0/G1 phase. Moreover, increased
concentrations of ART induced apoptosis of DLBCL cells. Together,
our data first indicated that ART treatment significantly inhibited
proliferation, promoted G0/G1 phase arrest and induced apoptosis of
DLBCL cells, suggesting that ART is a potential drug to DLBCL
treatment.
Artemisinin has been reported to inhibit tumor
angiogenesis by suppressing VEGF expression or to treat
P53-independent tumors by blocking the apoptosis pathway (13), but the mechanisms of ART-mediated
inhibition of lymphoma cell proliferation remain unclear. CDK2 is a
crucial cyclin-dependent kinase and essential for G1/S transition.
This protein maintains Rb phosphorylation in late G1 phase to
ensure cells enter S phase (17) and
is thus considered a potential target for anti-tumor treatments
(18). Cyclin D1/CDK4 can act on
Cyclin D1-pRb to regulate the G1/S transition (19,20). Our
results showed that ART-treated DLBCL cells had decreased
expression of three cell cycle-dependent proteins (CDK2, CDK4, and
Cyclin D1), indicating that ART treatment arrested DLBCL cells in
G1 phase and inhibited proliferation by suppressing the expression
of cell cycle proteins. A previous report showed that inhibition of
miR-34a abolished the ART-mediated CDK4 down-regulation and cell
cycle arrest (21). Moreover,
transcription factors including mTOR, NF-κB, and CREB are reported
to be involved in the ART-mediated inhibition of proliferation
(22–24). Thus, miRNAs and these transcription
factors may be key mediators for ART in down-regulating cell
cycle-related gene expression in DLBCL.
The proto-oncogene c-Myc plays major roles in the
cell proliferation, cell growth regulation, protein synthesis, and
cell adhesion of tumor cells (14).
Previous reports have indicated that artemisinin showed potent
anti-cancer activity in cells overexpressing c-Myc (25) and induced cell cycle arrest and
apoptosis in prostate cancer cells by inhibiting c-Myc (26). ERK signaling, a key signal pathway
from surface receptors to the nucleus, is related to progression of
various neoplastic diseases (15,27–29). AKT
signaling is also involved in the regulation of cell proliferation,
differentiation, apoptosis and migration by regulating its
downstream target proteins Bad, Caspase9, NF-κB, GSK-3 and others
via phosphorylation (16,30–32). We
also found that ART treatment significantly decreased the
expression level of c-Myc in DLBCL. However, ART treatment did not
affect the expression and the phosphorylation of two key kinases,
ERK and AKT. These results further confirmed that c-Myc was a key
downstream factor of ART in inhibiting DLBCL cell proliferation.
Moreover, the data indicated that ART induced DLBCL cell apoptosis
by activating the cleavage of Caspase-3 and PARP1. In summary, we
elucidated the critical mechanisms underlying proliferation
inhibition and apoptosis induction by ART in DLBCL cells,
indicating ART may be an alternative anti-cancer drug for DLBCL
treatment.
Drug resistance and relapse of DLBCL are major
challenges to clinical treatment. Hematopoietic stem cell
transplant (HSCT) may improve the outcome of patients with relapsed
or refractory DLBCL. However, HSCT availability is often limited by
patient age, treatment-related morbidities, and poor performance
status in many cases (33).
Therefore, novel targeted therapies are urgently needed. Natural
pharmaceuticals from plants have been increasingly tested due to
their multiple targets and few side effects. Recent reports showed
that ART could inhibit angiogenesis and reverse chemoresistance
(5,34). Our results demonstrated that the
natural botanical ART significantly inhibited the proliferation and
induced apoptosis of DLBCL cells, suggesting that ART might be a
promising combined pharmaceutical with conventional
chemotherapeutics to combat chemoresistance and relapse of
DLBCL.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (81570179 and 81170499 to J. C).
Glossary
Abbreviations
Abbreviations:
|
ART
|
artemether
|
|
DLBCL
|
diffuse large B cell lymphoma
|
|
NHL
|
non-Hodgkin lymphoma
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Feugier P, Van Hoof A, Sebban C,
Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E,
Tilly H, Morschhauser F, et al: Long-term results of the R-CHOP
study in the treatment of elderly patients with diffuse large
B-cell lymphoma: A study by the Groupe d'Etude des Lymphomes de
l'Adulte. J Clin Oncol. 23:4117–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grandesso F, Nabasumba C, Nyehangane D,
Page AL, Bastard M, De Smet M, Boum Y and Etard JF: Performance and
time to become negative after treatment of three malaria rapid
diagnostic tests in low and high malaria transmission settings.
Malar J. 15:4962016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia G, Kong R, Ma ZB, Han B, Wang YW, Pan
SH, Li YH and Sun B: The activation of c-Jun
NH2-terminal kinase is required for
dihydroartemisinin-induced autophagy in pancreatic cancer cells. J
Exp Clin Cancer Res. 33:82014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahbazfar AA, Zare P, Ranjbaran M,
Tayefi-Nasrabadi H, Fakhri O, Farshi Y, Shadi S and Khoshkerdar A:
A survey on anticancer effects of artemisinin, iron, miconazole,
and butyric acid on 5637 (bladder cancer) and 4T1 (Breast cancer)
cell lines. J Cancer Res Ther. 10:1057–1062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu ZP, Gao CW, Wu YG, Zhu QS, Yan Chen,
Xin Liu and Chuen Liu: Inhibitive effect of artemether on tumor
growth and angiogenesis in the rat C6 orthotopic brain gliomas
model. Integr Cancer Ther. 8:88–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerhardt T, Jones R, Park J, Lu R, Chan
HW, Fang Q, Singh N and Lai H: Effects of antioxidants and
pro-oxidants on cytotoxicity of dihydroartemisinin to Molt-4 human
leukemia cells. Anticancer Res. 35:1867–1871. 2015.PubMed/NCBI
|
|
7
|
Krishna S, Ganapathi S, Ster IC, Saeed ME,
Cowan M, Finlayson C, Kovacsevics H, Jansen H, Kremsner PG, Efferth
T and Kumar D: A randomised, double blind, placebo-controlled pilot
study of oral artesunate therapy for colorectal cancer.
EBioMedicine. 2:82–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaelsen FW, Saeed ME, Schwarzkopf J and
Efferth T: Activity of Artemisia annua and artemisinin derivatives,
in prostate carcinoma. Phytomedicine. 22:1223–1231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shterman N, Kupfer B and Moroz C:
Comparison of transferrin receptors, iron content and isoferritin
profile in normal and malignant human breast cell lines.
Pathobiology. 59:19–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Efferth T, Benakis A, Romero MR, Tomicic
M, Rauh R, Steinbach D, Häfer R, Stamminger T, Oesch F, Kaina B and
Marschall M: Enhancement of cytotoxicity of artemisinins toward
cancer cells by ferrous iron. Free Radic Biol Med. 37:998–1009.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pang Y, Qin G, Wu L, Wang X and Chen T:
Artesunate induces ROS-dependent apoptosis via a Bax-mediated
intrinsic pathway in Huh-7 and Hep3B cells. Exp Cell Res.
347:251–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Che N, Ma Y and Xin Y: Protective role of
fucoidan in cerebral ischemia-reperfusion injury through inhibition
of MAPK signaling pathway. Biomol Ther (Seoul). 25:272–278. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo JR, Li W, Wu Y, Wu LQ, Li X, Guo YF,
Zheng XH, Lian XL, Huang HF and Chen YZ: Hepatocyte growth factor
promotes proliferation, invasion, and metastasis of myeloid
leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am
J Transl Res. 8:3630–3644. 2016.PubMed/NCBI
|
|
17
|
Roskoski R Jr: Cyclin-dependent protein
kinase inhibitors including palbociclib as anticancer drugs.
Pharmacol Res. 107:249–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shapiro GI: Cyclin-dependent kinase
pathways as targets for cancer treatment. J Clin Oncol.
24:1770–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park YG, Park S, Lim SO, Lee MS, Ryu CK,
Kim I and Cho-Chung Y: Reduction in cyclin D1/Cdk4/retinoblastoma
protein signaling by CRE-decoy oligonucleotide. Biochem Biophys Res
Comm. 281:1213–1219. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chohan TA, Qian H, Pan Y and Chen JZ:
Cyclin-dependent kinase-2 as a target for cancer therapy: Progress
in the development of CDK2 inhibitors as anti-cancer agents. Curr
Med Chem. 22:237–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hargraves KG, He L and Firestone GL:
Phytochemical regulation of the tumor suppressive microRNA,
miR-34a, by p53-dependent and independent responses in human breast
cancer cells. Mol Carcinog. 55:486–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Odaka Y, Xu B, Luo Y, Shen T, Shang C, Wu
Y, Zhou H and Huang S: Dihydroartemisinin inhibits the mammalian
target of rapamycin-mediated signaling pathways in tumor cells.
Carcinogenesis. 35:192–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu W, Chen SS, Zhang JL, Lou XE and Zhou
HJ: Dihydroartemisinin induces autophagy by suppressing NF-κB
activation. Cancer Lett. 343:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HG, Yang JH, Han EH, Choi JH, Khanal
T, Jeong MH, Jeong TC and Jeong HG: Inhibitory effect of
dihydroartemisinin against phorbol ester-induced cyclooxygenase-2
expression in macrophages. Food Chem Toxicol. 56:93–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu JJ, Meng LH, Shankavaram UT, Zhu CH,
Tong LJ, Chen G, Lin LP, Weinstein JN and Ding J:
Dihydroartemisinin accelerates c-MYC oncoprotein degradation and
induces apoptosis in c-MYC-overexpressing tumor cells. Biochem
Pharmacol. 80:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morrissey C, Gallis B, Solazzi JW, Kim BJ,
Gulati R, Vakar-Lopez F, Goodlett DR, Vessella RL and Sasaki T:
Effect of artemisinin derivatives on apoptosis and cell cycle in
prostate cancer cells. Anticancer Drugs. 21:423–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung TW, Choi H, Lee JM, Ha SH, Kwak CH,
Abekura F, Park JY, Chang YC, Ha KT, Cho SH, et al: Oldenlandia
diffusa suppresses metastatic potential through inhibiting matrix
metalloproteinase-9 and intercellular adhesion molecule-1
expression via p38 and ERK1/2 MAPK pathways and induces apoptosis
in human breast cancer MCF-7 cells. J Ethnopharmacol. 195:309–317.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Youn DH, Park J, Kim HL, Jung Y, Kang JW,
Jeong MY, Sethi G, Ahn KS and Um JY: Chrysophanic acid reduces
testosterone-induced benign prostatic hyperplasia in rats by
suppressing 5α-reductase and extracellular signal-regulated kinase.
Oncotarget. 8:9500–9512. 2017.PubMed/NCBI
|
|
29
|
Zuo WH, Zeng P, Chen X, Lu YJ, Li A and Wu
JB: Promotive effects of bone morphogenetic protein 2 on
angiogenesis in hepatocarcinoma via multiple signal pathways. Sci
Rep. 6:374992016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng X, Jiang J, Shi S, Xie H, Zhou L and
Zheng S: Knockdown of miR-25 increases the sensitivity of liver
cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad
signaling pathway. Int J Oncol. 49:2600–2610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal
Res. 62:2017.doi: 10.1111/jpi.12380. View Article : Google Scholar
|
|
32
|
Wang L, Zhang S, Cheng H, Lv H, Cheng G
and Ci X: Nrf2-mediated liver protection by esculentoside A against
acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free
Radic Biol Med. 101:401–412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Philip T, Guglielmi C, Hagenbeek A, Somers
R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY,
Harousseau JL, et al: Autologous bone marrow transplantation as
compared with salvage chemotherapy in relapses of
chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med.
333:1540–1545. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reungpatthanaphong P and Mankhetkorn S:
Modulation of multidrug resistance by artemisinin, artesunate and
dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines.
Biol Pharm Bull. 25:1555–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|