Introduction
Knee osteoarthritis (OA) is characterized by the
progressive destruction of articular cartilage and is the leading
cause of pain and physical disability in the elderly (1). The early stage of OA limits movement
and causes knee discomfort, thereby impairing patients' ability to
perform everyday activities and their quality of life (QOL)
(1). The prevalence of radiographic
knee OA in Japan is increasing and is estimated to affect 30
million Japanese people over the age of 50 (1). According to the treatment guidelines
for OA from the Knee Osteoarthritis Research Society International,
non-steroidal anti-inflammatory agents, selective cyclooxygenase-2
inhibitors and acetaminophen are recommended for treatment of OA
(2). However, these agents have also
been reported to enhance cartilage destruction and promote OA
(3,4). Therefore, novel substances with a
chondroprotective action have been sought. The current study
evaluated Ajuga decumbens (AD) as a candidate that exhibits
a protective action on OA. AD is a natural herb and has long been
used as a medicine for pain relief in Japan (5,6).
Notably, AD has been indicated to have an anti-inflammatory action
in a rat arthritis model (7).
Furthermore, AD promotes the osteoblastic differentiation of
cultured osteoblasts (7) and the
repair of articular cartilage and subchondral bone in a cartilage
damaged model (8). However, these
beneficial effects on the joint have not been studied in humans. In
the current study, the effect of orally administered AD extract
(ADE) was evaluated in subjects who experienced knee discomfort
associated with physical activity, but did not require clinical
treatment. A dietary supplement containing ADE was administered to
the subjects, and the effect on the knee joint was evaluated using
OA scores, including the Japanese Knee Osteoarthritis Measure
(JKOM) (9,10) and the Japan Orthopedic Association
(JOA) criteria (11). In addition,
type II collagen degradation [cross-linked C-telopeptide of type II
collagen (CTX-II) and collagen type II cleavage (C2C)] and
synthesis [procollagen II C-terminal propeptide (PIICP)] markers
and matrix metalloproteinase (MMP)-13 were analyzed to evaluate the
effects of ADE on cartilage metabolism. Furthermore, to evaluate
the effect of ADE in more detail, subgroup analyses of the subjects
with lower levels of knee discomfort [Kellgren-Lawrence (K/L)
grades (12) of 0-I and JOA score
>75 points] were performed.
Materials and methods
Study design
A prospective, randomized, double-blind,
placebo-controlled and parallel-group comparative study was
designed to assess the efficacy and safety of a diet supplemented
with ADE. The present study was conducted from November 2014-June
2015 and involved one medical clinical service organization center
(Umeda Oak Clinic, Osaka, Japan) under the control of one medical
investigator in Japan. The protocol was submitted to and approved
by the institutional ethics committee of Aisei Hospital Ueno Clinic
(Tokyo, Japan) in October 2014 (20141030–2), and the study was
conducted in accordance with The Declaration of Helsinki and
Ethical Guidelines for Epidemiological Research (recognized by the
Japanese Government in 2008). The objective of the present study
was explained to all subjects and written informed consent was
provided prior to enrollment.
Subject enrollment
Male and female Japanese subjects (aged 40–80;
male:female ratio, 65:58) with knee discomfort associated with
physical activity but without radiographic evidence of knee
osteoarthritis (K/L grades 0-II: Grade 0, n=69; grade I, n=32,
grade II, n=22) were enrolled from the database of volunteers in
NEUES Co., Ltd. (Tokyo, Japan). K/L classification of 0 means
‘definite absence of X-ray changes’, I means ‘doubtful absence of
X-ray changes’ and II means ‘minimal absence of X-ray changes’
(12). The major exclusion criteria
were as follows: i) The presence of gout or rheumatoid arthritis
that may cause joint pain; ii) suffering from any other injuries
that may have required the use of anti-inflammatory or other
medications or physiotherapy treatment by an orthopedist; iii)
previous surgical treatment of knee joints; iv) routine use of
dietary supplements or medicines containing ADE, hyaluronic acid,
glucosamine, chondroitin sulfate or collagen peptides, or are rich
in calcium; v) requirement to undergo pharmacological treatments
during the study period; vi) performing hard exercise; vii) history
of osseous or articular diseases within the past year; viii)
diagnosis as having malignant tumor, hypertension, heart disease,
kidney disease, thyroid disorder or other serious illness that may
have required other physiotherapy treatment; ix) pregnancy; x)
nursing mothers or women of childbearing potential; xi)
participation in another clinical study; and xii) presence of any
medical condition judged by the medical investigator to preclude
the subject's inclusion in the study.
Study interventions
The subjects were randomized to the ADE diet group
and placebo group, and evaluated. The ADE diet was manufactured in
the form of a 1.5-g powder. The ingredients were 10 mg ADE (>1%
20-hydroxyecdysone), 360 mg dextrin, 25 mg hydroxypropyl
methylcellulose, 46 mg cellulose, 9 mg calcium stearate and 1,050
mg palatinose. ADE was purchased from Matsuura Yakugyo Co, Ltd.
(Nagoya, Japan). The amount of ADE was 10 mg in the 1.5-g ADE
powder. The placebo diet comprised crystalline cellulose, dextrin
and caramel pigment instead of ADE. The study supplement was
wrapped in a single pack (1.5 g) and provided with a wafer, and the
subject took the supplement after breakfast once a day with two
cups of water for 12 weeks.
Randomization and blinding
Due to the fact that symptoms, especially pain, vary
according to gender in arthritis (13), research coordinators created an
allocation table for males and females, randomly assigned eligible
subjects and granted allocation numbers to test diet. The
allocation table was sealed until the end of the study. All
research staff and subjects were blinded to the treatment
allocation during the test period. Only the statistics experts and
data monitoring committee were unblinded, however, they had no
contact with subjects. Following the study, the subjects' data were
fixed, and the allocation table was made available for reviewing
the study information.
Evaluation of efficacy
The outcomes for the evaluation of efficacy were
based on the changes in subscale scores of the JOA criteria for
osteoarthritic knees; the changes in the subscale scores of the
JKOM criteria; the levels of urinary CTX-II and serum C2C as type
II collagen degradation biomarkers (14,15); the
levels of serum PIICP as a type II collagen synthesis biomarker
(16), the levels of serum MMP-13
(17), the ratio of CTX-II/PIICP and
the ratio of C2C/PIICP. These parameters were measured at the
baseline and at weeks 4, 8 and 12. Serum and second void of morning
urine were collected from the subjects in a fasting state at
baseline, weeks 4, 8 and 12 during the intervention. Serum and
urine samples were immediately used for routine laboratory tests;
sera and urine samples were aliquoted and stored at −80°C until the
assays of CTX-II, C2C, PIICP and MMP-13 using their respective
ELISA assay kits: CTX-II, Urine CartiLaps EIA (AC-10F1,
Immunodiagnostic Systems, Ltd., Tyne & Wear, UK); C2C, Collagen
Type II Cleavage ELISA (60–1001-001; IBEX Technologies, Inc.,
Montreal, QC, Canada,); PIICP, Enzyme-linked Immunosorbent Assay
kit For Procollagen II C-Terminal Propeptide (SEA964Hu; Uscn Life
Sciences, Inc., Wuhan, China); and MMP-13, ELISA Kit For Matrix
Metalloproteinase 13 (SEA099Hu; Uscn Life Sciences, Inc.). Serum
C2C and urinary CTX-II were used as markers for type II collagen
degradation, and serum PIICP was used as a marker for type II
collagen synthesis. The ratios of C2C/PIICP and CTX-II/PIICP were
also analyzed to assess the changes in the balance of type II
collagen degradation and synthesis (cartilage metabolism). The
concentrations of CTX-II were corrected for urinary creatinine (Cr)
and expressed as ng/mmol Cr. Serum MMP-13 was also used as a marker
for cartilage degradation, because MMP-13 is a collagenase that
contributes to cartilage degradation by cleaving type II collagen
triple helix (18).
JOA criteria (10)
are used by physicians for subjectively evaluating knee OA
treatment, based on the following subcategories: I) Pain/walking
function (rated from 0 to 30); II) pain/step-up and -down function
(rated from 0 to 25); III) joint flexion/stiffness (rated from
0–35); IV) swelling (rated from 0–10); and V) aggregated total
symptoms. The maximum scores in each subscale indicate no symptoms
or functional disability, whereas a score of 0 indicates extreme
difficulty in performing daily tasks. The sum of the scores of
subscales I–IV represents the score of subscale V (total symptoms
score).
The JKOM (9,10) is a self-answered questionnaire that
includes five subcategories: I, Pain evaluated by a visual analog
scale (VAS); II, pain and stiffness during the past few days (8
questions); III, activities of daily living during the past few
days (10 questions); IV, general activities during the past month
(5 questions); and V, general health conditions during the past
month (2 questions). VAS values range from 0 (no pain) to 100 (pain
that cannot be tolerated). The responses to each question
(subcategories II–V) are assigned between 1 and 5 points, with 1
point indicating good functional status and 5 points indicating the
worst functional status. The JKOM score is higher in subjects with
more pain and physical disability, and this evaluation modality is
reported to be reliable and valid for studying the clinical
outcomes of knee OA (10). The
outcome of JKOM has been reported to be closely correlated with
that of other arthritis-related scales, such as the Western Ontario
and McMaster Universities Arthritis Index and the Medical Outcomes
Study 36-Item Short-Form Health Survey (10).
Evaluation of safety
Tolerability and safety were evaluated on the basis
of the incidence and severity of dietary supplement-related adverse
events reported throughout the study and by changes in physical
parameters such as body weight and body mass index (BMI), blood
pressure and pulse rates and laboratory test variables, including
hematology, blood biochemistry and urinalysis, which were measured
by LSI Medience Corporation (Tokyo, Japan). Each parameter was
assessed at 4, 8 and 12 weeks from the start of administration. In
addition, throughout the intervention period, changes in physical
condition and joints, and use of pharmaceutical products were
recorded by subjects in a diary.
Sample size
It was estimated that ~50 subjects would be
necessary to accurately assess the efficacy and safety of ADE,
based on a previous study in which a dietary supplement was
administered to individuals with knee discomfort who did not
require clinical treatment (19,20). To
recruit a sufficient number of subjects for analysis, 123 subjects
were screened and 48 subjects without any of the exclusion criteria
were selected. Due to fluctuations in body weight and requests by
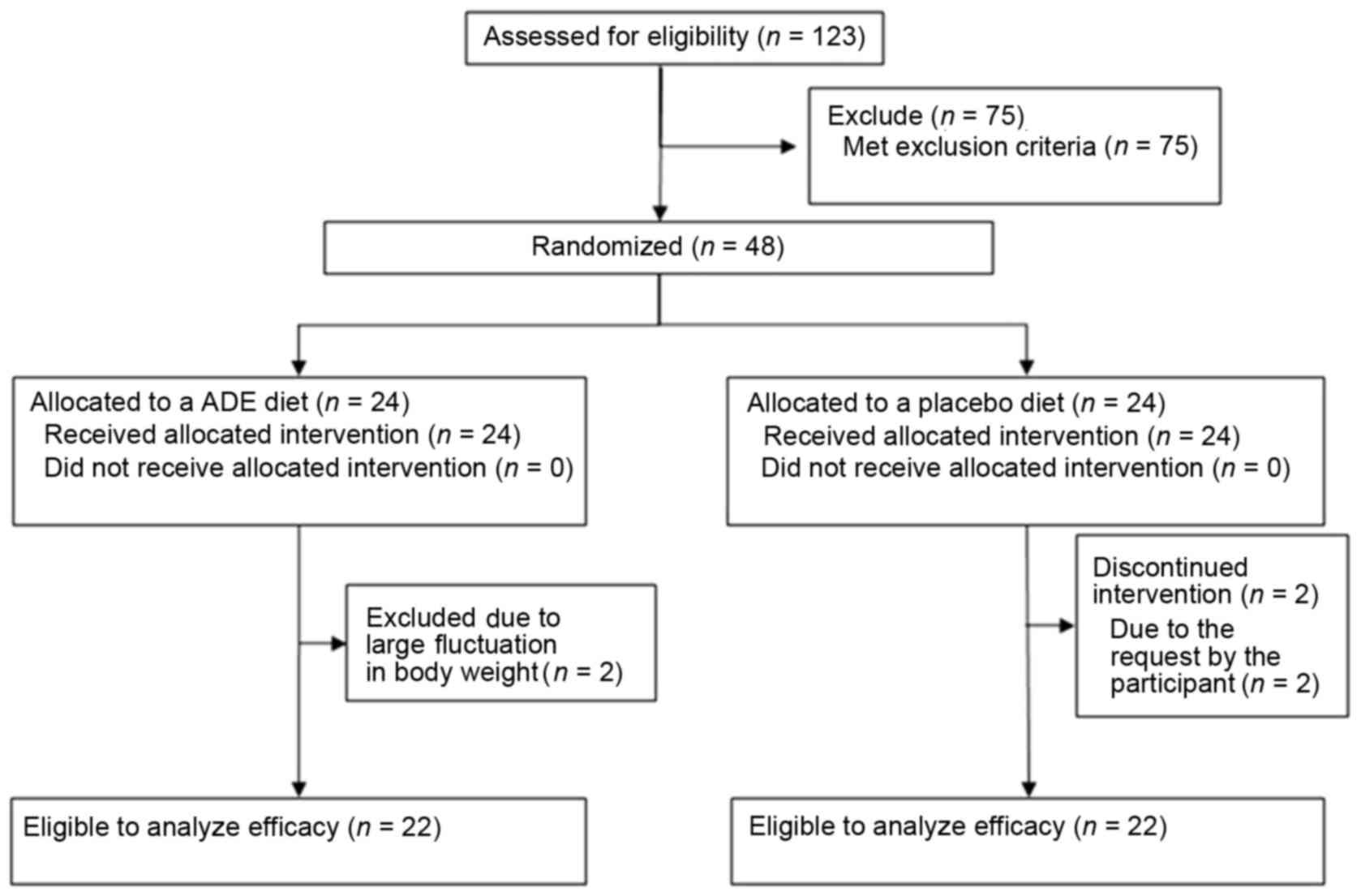
the participants, four subjects were excluded from the trial.
Finally, 22 subjects in the ADE group and 22 subjects in the
placebo group were analyzed (Fig.
1). Furthermore, to evaluate the effect of the test supplement
in more detail, the present analysis focused primarily on subjects
with mild knee discomfort. Therefore, subjects with K/L grades of
II and ≤75 points of JOA score were excluded, and the subjects (ADE
group, n=18; placebo group, n=14) with K/L grades of 0-I and >75
points of JOA score were evaluated.
Statistical analysis
All data are expressed as the mean ± standard
deviation unless otherwise specified. The statistical analysis was
based on a previous study (20). The
baseline characteristics of the entire subject population were
compared between the two groups (ADE diet and placebo) using
Student's t-test (for continuous variables) and the Mann-Whitney U
test (for categorical variables). Changes in the JOA and JKOM
scores, and biomarker levels during the intervention were compared
with the baseline values using the Student's t-test (for
quantitative variables) and the Wilcoxon signed-rank test (for
qualitative variables). Comparisons between the two groups were
performed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Microsoft Excel
2013 (Microsoft Corporation, Redmond, WA, USA) was used for
statistical analysis using the t-test, and SPSS 21 software (IBM
SPSS, Armonk, NY, USA) was used for other tests.
Results
Study population
A flow chart of subjects included in the present
study is shown in Fig. 1A total of
123 candidates were screened based on K/L grades and JOA score by
orthopedists. In addition, a lifestyle questionnaire was
administered and body measurements, physical examinations, JKOM
assessments and laboratory test variables were performed. The 44
candidates without any of the exclusion criteria were finally
selected and randomized to the ADE diet group (n=22) and placebo
group (n=22).
Baseline characteristics in the study
population
Table I shows the
baseline characteristics of the study population in the ADE diet
(n=22) and placebo (n=22) groups. There were no significant
differences in any of the baseline characteristics between the ADE
diet and placebo groups. The baseline characteristics included
demographic characteristics (age and male/female ratio),
physiological characteristics (body height, body weight, BMI,
systolic blood pressure, diastolic blood pressure and pulse rate),
distribution of K/L grades, aggregated JOA scores, VAS and total
scores of JKOM, and the levels of biomarkers for type II collagen
metabolism (CTX-II, C2C, PIICP and MMP-13).
 | Table I.Baseline characteristics of the study
population in the ADE diet and placebo groups. |
Table I.
Baseline characteristics of the study
population in the ADE diet and placebo groups.
| Variable | ADE diet (n=22) | Placebo (n=22) | P-value |
|---|
| Age (years) |
51.8±7.3 |
53.5±8.6 | 0.488 |
| Male:female (n) | 9:13 | 10:12 | 1.000 |
| Height (cm) | 165.0±9.2 | 161.0±8.4 | 0.135 |
| Body weight (kg) |
62.6±9.1 |
60.3±10.1 | 0.426 |
| Body mass index
(kg/m2) |
23.0±2.6 |
23.2±3.2 | 0.747 |
| Body fat mass
(%) |
26.4±9.2 |
27.0±7.25 | 0.787 |
| Systolic blood
pressure (mmHg) |
113.6±11.3 |
116.9±18.1 | 0.475 |
| Diastolic blood
pressure (mmHg) |
72.8±7.6 |
75.5±11.7 | 0.371 |
| Pulse rate
(beats/min) |
68.0±7.2 |
71.5±8.5 | 0.148 |
| Kellgren-Lawrence
grades 0:I:II (n) | 13:6:3 | 10:6:6 | 0.528 |
| Aggregate scores of
JOA criteria |
84.8±8.5 |
81.8±9.1 | 0.235 |
| JKOM (I) VAS score
(mm) |
56.4±16.8 |
54.1±14.8 | 0.636 |
| JKOM (II–V) total
score (points) |
47.8±16.7 |
46.5±11.4 | 0.776 |
| CTX-II (ng/mmol
Cr) |
170.4±106.2 |
192.8±100.4 | 0.488 |
| C2C (ng/ml) |
292.9±42.9 |
290.9±34.0 | 0.235 |
| PIICP (ng/ml) |
9.3±2.8 |
9.7±3.5 | 0.636 |
| MMP-13 (ng/ml) |
9.6±4.0 |
8.7±4.3 | 0.424 |
Study population assessment based on
JOA subscale scores
Table II shows the
changes in the individual subscale and aggregate scores of the JOA
criteria in the ADE diet and placebo groups during the 12-week
intervention. In the ADE diet and placebo groups, three subscale
scores, I) Pain/walking function, II) Pain/step up and down
function and V) Aggregated total symptom scores, were increased
relative to the baseline values during the 12-week intervention
(all P<0.01 at 12 weeks, except placebo group subscale II,
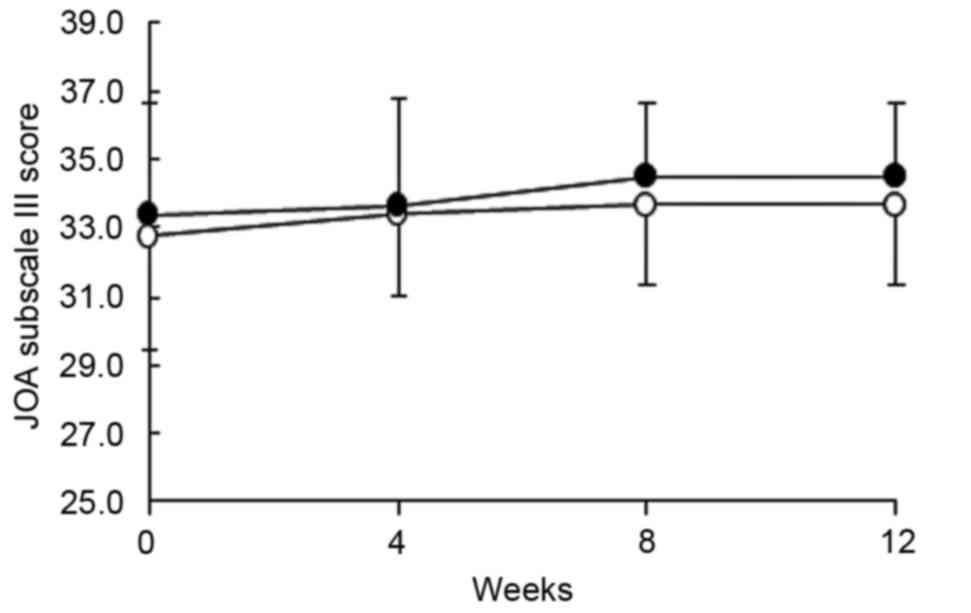
P<0.05). Fig. 2 shows the change
in subscale III (joint flexion/stiffness) over the intervention
period. The baseline scores were not different between the two
groups; however, the score markedly increased in the ADE diet group
compared with the placebo group at 8 and 12 weeks (P=0.095 at both
timepoints), suggesting that an ADE-containing diet may improve
joint flexion and stiffness.
 | Table II.Individual and aggregate scores of JOA
criteria during the intervention period in the ADE diet (n=22) and
placebo (n=22) groups. |
Table II.
Individual and aggregate scores of JOA
criteria during the intervention period in the ADE diet (n=22) and
placebo (n=22) groups.
| JOA criteria
(points) | Group | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| I. Pain/walking
function | ADE diet | 21.4±5.2 |
25.2±4.5a |
25.5±4.3a |
26.4±3.8a |
|
| Placebo | 20.5±5.3 |
25.7±4.4a |
25.9±2.9a |
28.0±3.0a |
| II. Pain/step up
and down function | ADE diet | 20.0±3.5 | 20.5±3.4 | 20.9±3.7 |
22.5±3.0a |
|
| Placebo | 19.1±2.9 |
21.1±2.1b |
21.1±2.1b |
21.8±2.9b |
| III. Joint
flexion/stiffness | ADE diet | 33.4±3.2 | 33.6±3.2 | 34.5±2.1 | 34.5±2.1 |
|
| Placebo | 32.7±3.4 | 33.4±2.4 | 33.6±2.3 | 33.6±2.3 |
| IV. Swelling | ADE diet | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 |
|
| Placebo | 9.5±1.5 | 10.0±0.0 | 10.0±0.0 | 9.8±1.1 |
| V. Aggregate total
symptoms | ADE diet | 84.8±8.5 |
89.3±8.2a |
90.9±8.5a |
93.4±7.3a |
|
| Placebo | 81.8±9.1 |
90.2±6.3a |
90.7±5.8a |
93.2±6.5b |
Study population assessment based on
JKOM subscale scores
Table III shows the
changes in the five subscale scores of the JKOM responses during
the 12-week intervention. In the subscale scores (I–V) and total
score, there were no significant differences between the ADE diet
and placebo groups at the baseline. Notably, in the ADE diet group,
subscale scores I (pain evaluated by VAS) and V (general health
conditions) were significantly decreased at 4, 8 and 12 weeks
compared with the baseline (all P<0.01, except subscale V at
week 4, P<0.05). Furthermore, in the ADE diet group, subscale
score IV (general activity) was significantly decreased at 8 weeks
compared with the baseline (P<0.05), and subscale scores II
(pain and stiffness during the past few days), III (activities of
daily living during the past few days) and total score were
significantly decreased at 8 and 12 weeks compared with the
baseline (all P<0.01 except subscale III at week 8, P<0.05).
In the placebo group, subscale scores I, II and III and total score
were significantly decreased relative to the baseline (all
P<0.01); however, subscales IV and V were not significantly
changed.
 | Table III.Changes in the individual and total
scores of JKOM response during the intervention period in the ADE
diet (n=22) and placebo (n=22) groups. |
Table III.
Changes in the individual and total
scores of JKOM response during the intervention period in the ADE
diet (n=22) and placebo (n=22) groups.
| JKOM criteria | Group | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| I: VAS score,
mm | ADE diet | 56.4±16.8 |
35.5±24.0b |
25.5±21.2b |
19.5±18.5b |
|
|
|
| (−36.9±38.3) | (−53.5±36.8) |
(−64.9±31.8b) |
|
| Placebo | 54.1±14.8 |
26.2±19.2b |
25.1±18.1b |
18.3±23.0b |
|
|
|
| (−51.2±33.1) | (−53.0±30.8) |
(−62.5±47.6b) |
| II: Pain and
stiffness in the knees, points | ADE diet | 17.5±5.9 | 16.1 ± 6.8 |
14.0±6.5b |
13.0±6.3b |
|
|
|
|
(−8.1±18.0c) | (−20.3±18.0) | (−26.5±16.9) |
|
| Placebo | 18.3±4.6 |
13.6±3.4b |
13.7±3.8b |
13.1±6.2b |
|
|
|
| (−22.8±22.2) | (−21.6±26.0) | (−26.9±31.2) |
| III: Condition in
daily life, points | ADE diet | 16.4±6.7 | 16.1±7.8 |
14.1±6.2a |
13.3±6.0b |
|
|
|
|
(−1.5±19.0d) | (−11.9±17.2) | (−15.5±19.8) |
|
| Placebo | 15.6±4.5 |
12.5±2.8b |
12.5±3.1b |
13.2±6.0b |
|
|
|
| (−15.8±25.8) | (−17.3±18.1) | (−8.9±56.4) |
| IV: General
activities, points | ADE diet | 9.7±3.5 | 9.3±3.9 |
8.5±3.7a | 8.9±3.4 |
|
|
|
| (−3.5±21.2) | (−11.6±22.9) | (−6.8±20.0) |
|
| Placebo | 8.7±2.3 | 8.2±1.8 | 8.2±1.7 | 8.1±1.4 |
|
|
|
| (−1.7±23.1) | (−0.9±26.9) | (−0.3±31.7) |
| V: Health
conditions, points | ADE diet | 4.2±1.8 |
3.5±1.3a |
3.2±1.1b |
3.4±1.4b |
|
|
|
| (−10.9±27.0) | (−16.6±29.1) | (−13.6±28.9) |
|
| Placebo | 4.0±1.7 | 3.5±1.2 | 3.4±1.1 | 3.3±1.3 |
|
|
|
| (0.9±49.5) | (−5.1±37.0) | (−3.9±57.5) |
| Total score | ADE diet | 47.8±16.7 | 45.0±18.1 |
39.8±16.0b |
38.6±16.0b |
|
|
|
|
(−5.9±14.4d) | (−15.8±15.9) | (−18.2±15.7) |
|
| Placebo | 46.5±11.4 |
37.8±7.5b |
37.8±7.6b |
37.7±12.8b |
|
|
|
| (−16.4±17.6) | (−16.2±17.7) | (−15.8±31.4) |
In terms of percentage change from the baseline,
subscales II and III and total score were significantly decreased
in the placebo and ADE diet group at 4 weeks following intervention
(P<0.05 and P<0.01, respectively). However, subscale scores
I, III, IV and V and total score were markedly more decreased in
the ADE diet group compared with the placebo group at 8 or 12 weeks
following intervention. In particular, the subscales of III, IV and
V exhibited a greater decrease in the ADE diet group at 12 weeks
compared with the placebo group: −15.5±19.8% in the ADE diet group
vs. −8.9±56.4% in the placebo group for subscale III; −6.8±20.0% in
the ADE diet group vs. −0.3±31.7% in the placebo group for subscale
IV; −13.6±28.9% in the ADE diet group vs. −3.9±57.5% in the placebo
group for subscale V.
Study population assessment based on
type II collagen metabolism
To evaluate the effect of the ADE diet on type II
collagen metabolism, urine and serum samples were used to evaluate
levels of cartilage metabolism markers (CTX-II, C2C, PIICP) and a
collagenase (MMP-13), as shown in Table
IV. CTX-II (a type II collagen degradation marker) was
significantly increased relative to the baseline in both the ADE
and placebo groups at 8 weeks (P<0.05 and P<0.01,
respectively), whereas C2C (another type II collagen degradation
marker) was not significantly changed during the intervention
period. PIICP (a type II collagen synthesis marker) was
significantly increased compared with the baseline in both groups
at 4, 8 and 12 weeks (all P<0.01 except ADE diet group at 12
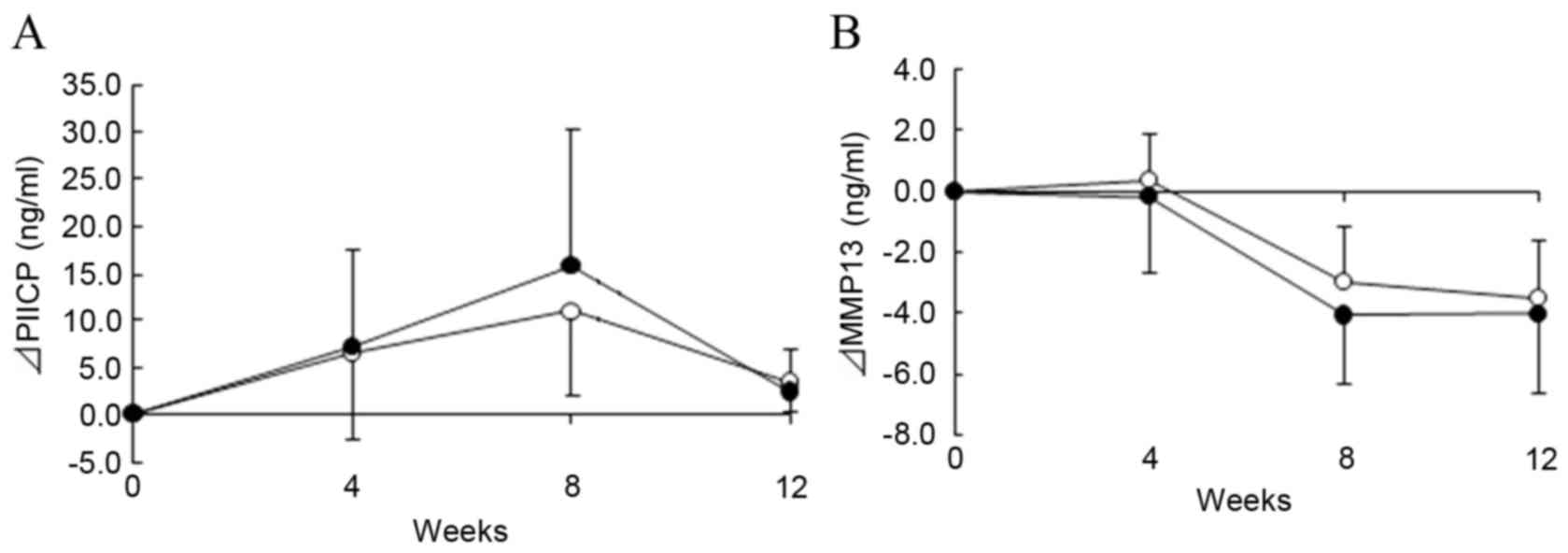
weeks, P<0.05). Notably, PIICP was markedly increased at 8 weeks
in the ADE diet group compared with the placebo group (Fig. 3A), suggesting that an ADE-containing
diet may enhance type II collagen synthesis. Furthermore, MMP-13
was significantly reduced at 8 and 12 weeks in both groups relative
to the baseline (Fig. 3B;
P<0.01).
 | Table IV.Levels of biomarkers for CII
metabolism and the ratio of CII destruction to synthesis during the
intervention period in the ADE diet (n=22) and placebo (n=22)
groups. |
Table IV.
Levels of biomarkers for CII
metabolism and the ratio of CII destruction to synthesis during the
intervention period in the ADE diet (n=22) and placebo (n=22)
groups.
| Variable | Group | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| CTX-II (ng/mmol
Cr) | ADE diet | 170.4±106.2 | 156.2±59.2 |
222.8±107.1a | 203.8±85.8 |
|
| Placebo | 192.8±100.4 | 180.6±147.9 |
274.2±172.6b | 236.0±152.8 |
| C2C (ng/ml) | ADE diet | 292.8±42.9 | 293.2±39.1 | 286.9±55.4 | 295.6±38.1 |
|
| Placebo | 290.9±34.0 | 283.8±30.5 | 283.8±33.2 | 294.0±46.4 |
| PIICP (ng/ml) | ADE diet | 9.3±2.8 |
18.0±9.5b |
26.0±12.8b |
11.4±4.7a |
|
| Placebo | 9.7±3.5 |
15.6±9.2b |
19.6±9.1b |
12.5±4.1b |
| MMP-13 (ng/ml) | ADE diet | 9.6±4.0 | 9.6±4.0 |
5.9±3.6b |
5.6±2.9b |
|
| Placebo | 8.7±4.3 | 9.2±4.2 |
5.5±3.4b |
5.1±3.3b |
| CTX-II/PIICP | ADE diet | 37.2±26.2 |
20.9±10.4a |
14.6±9.5b | 29.3±10.5 |
|
| Placebo | 38.2±28.5 |
24.4±12.1a |
17.8±8.3b |
26.2±9.2a |
| C2C/PIICP | ADE diet | 23.0±24.1 |
11.4±7.6a | 12.6±15.4 | 19.8±9.4 |
|
| Placebo | 29.5±37.1 | 17.9±18.7 | 17.8±16.1 | 21.1±15.1 |
To further determine the balance of type II collagen
metabolism (degradation and synthesis), the ratios of CTX-II and
C2C to PIICP was evaluated. In the ADE diet group, the CTX-II/PIICP
ratio was significantly reduced at weeks 4 and 8 (P<0.05 and
P<0.01, respectively), and C2C/PIICP ratio was significantly
reduced at week 4 relative to the baseline (P<0.05). Conversely,
the C2C/PIICP ratio was not significantly changed in the placebo
group, although the CTX-II/PIICP ratio was significantly reduced at
4, 8 and 12 weeks relative to the baseline in this group
(P<0.05, P<0.01 and P<0.05, respectively).
Baseline characteristics for subgroup
analysis
To determine the ADE effect on subjects with mild
knee discomfort, the subjects with lower K/L grades (0-I) and
higher JOA score (>75) were selected, and the subgroup analysis
was performed. The baseline data are shown in Table V. The ADE diet and placebo groups
contained 18 and 14 subjects, respectively. There were no
significant differences in any of the baseline characteristics
between the two groups.
 | Table V.Baseline characteristics of the
subgroup participants with mild knee pain in the ADE diet and
placebo groups. |
Table V.
Baseline characteristics of the
subgroup participants with mild knee pain in the ADE diet and
placebo groups.
| Variable | ADE diet
(n=18) | Placebo (n=14) | P-value |
|---|
| Age (years) |
51.4±7.1 |
54.1±7.2 | 0.311 |
| Male:female
(n) | 7:11 | 8:6 | 0.476 |
| Height (cm) | 164.5±9.4 | 162.3±8.7 | 0.511 |
| Body weight
(kg) |
62.1±8.5 |
59.4±9.3 | 0.400 |
| Body mass index
(kg/m2) |
22.9±2.6 |
22.5±2.4 | 0.601 |
| Body fat mass
(%) |
27.4±7.9 |
22.3±8.2 | 0.164 |
| Systolic blood
pressure (mmHg) |
111.6±11.3 |
116.0±16.7 | 0.389 |
| Diastolic blood
pressure (mmHg) |
71.5±7.3 | 74.43±8.2 | 0.295 |
| Pulse rate
(beats/min) |
68.4±7.5 |
70.5±8.3 | 0.460 |
| Kellgren-Lawrence
grades, 0:I:II (n) | 12:6:0 | 8:6:0 | 0.718 |
| Aggregate scores of
JOA criteria |
86.4±7.0 |
85.0±5.5 | 0.363 |
| JKOM (I) VAS score
(mm) |
53.1±15.2 |
59.0±13.7 | 0.265 |
| JKOM (II–V) total
score (points) |
42.1±9.7 |
43.5±9.7 | 0.554 |
| CTX-II (ng/mmol
Cr) |
166.2±110.8 |
169.6±86.2 | 0.924 |
| C2C (ng/ml) |
286.2±43.3 |
286.1±26.9 | 0.998 |
| PIICP (ng/ml) |
9.8±2.2 |
9.0±3.8 | 0.463 |
| MMP-13 (ng/ml) |
9.5±4.1 |
8.4±4.5 | 0.463 |
Subgroup assessment based on JOA
subscale score
The JOA subscales for the subgroup analysis are
shown in Table VI. In the ADE diet
and placebo groups, subscale scores I and V were significantly
increased at 4, 8 and 12 weeks (P<0.01), and the subscale II
score was significantly increased at 12 weeks relative to the
baseline (P<0.01 in ADE diet group, P<0.05 in placebo group).
However, there were no significant differences in these subscale
scores between the two groups. Notably, subscale III was markedly
increased and reached the maximum score (35 points, indicating no
symptoms) at weeks 8 and 12 in the ADE diet group, whereas the
subscale score only increased minimally in the placebo group.
 | Table VI.Individual scores and aggregate
scores of JOA criteria during the intervention period for subgroup
participants with mild knee pain in the ADE diet (n=18) and placebo
(n=14) groups. |
Table VI.
Individual scores and aggregate
scores of JOA criteria during the intervention period for subgroup
participants with mild knee pain in the ADE diet (n=18) and placebo
(n=14) groups.
| JOA criteria
(points) | Group | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| I. Pain/walking
function | ADE diet | 22.5±4.6 |
26.1±4.0a |
26.7±3.4a |
27.5±3.1a |
|
| Placebo | 21.8±4.6 |
25.4±5.0a |
26.4±2.3a |
28.9±2.1a |
| II. Pain/step up
and down function | ADE diet | 20.3±3.6 | 20.8±3.5 | 21.4±3.8 |
23.1±3.0a |
|
| Placebo | 19.3±2.7 | 20.7±1.8 | 21.4±2.3 |
22.1±2.6b |
| III. Joint
flexion/stiffness | ADE diet | 33.6±2.9 | 33.9±2.7 | 35.0±0.0 | 35.0±0.0 |
|
| Placebo | 33.9±2.1 | 33.9±2.1 | 34.3±1.8 | 34.3±1.8 |
| IV. Swelling | ADE diet | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 |
|
| Placebo | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 |
| V. Aggregate total
symptoms | ADE diet | 86.4±7.9 |
90.8±6.7a |
93.1±6.4a |
95.6±5.7a |
|
| Placebo | 85.0±5.5 |
90.0±6.5a |
92.1±4.7a |
95.4±5.0a |
Subgroup assessment based on JKOM
subscale scores
Table VII shows the
JKOM subscale scores for the subgroup during the 12-week
intervention. In the ADE diet group, subscale scores (I–V) and
total score decreased relative to the baseline at 8 or 12 weeks
(all P<0.01, except subscales IV and V, P<0.05). Furthermore,
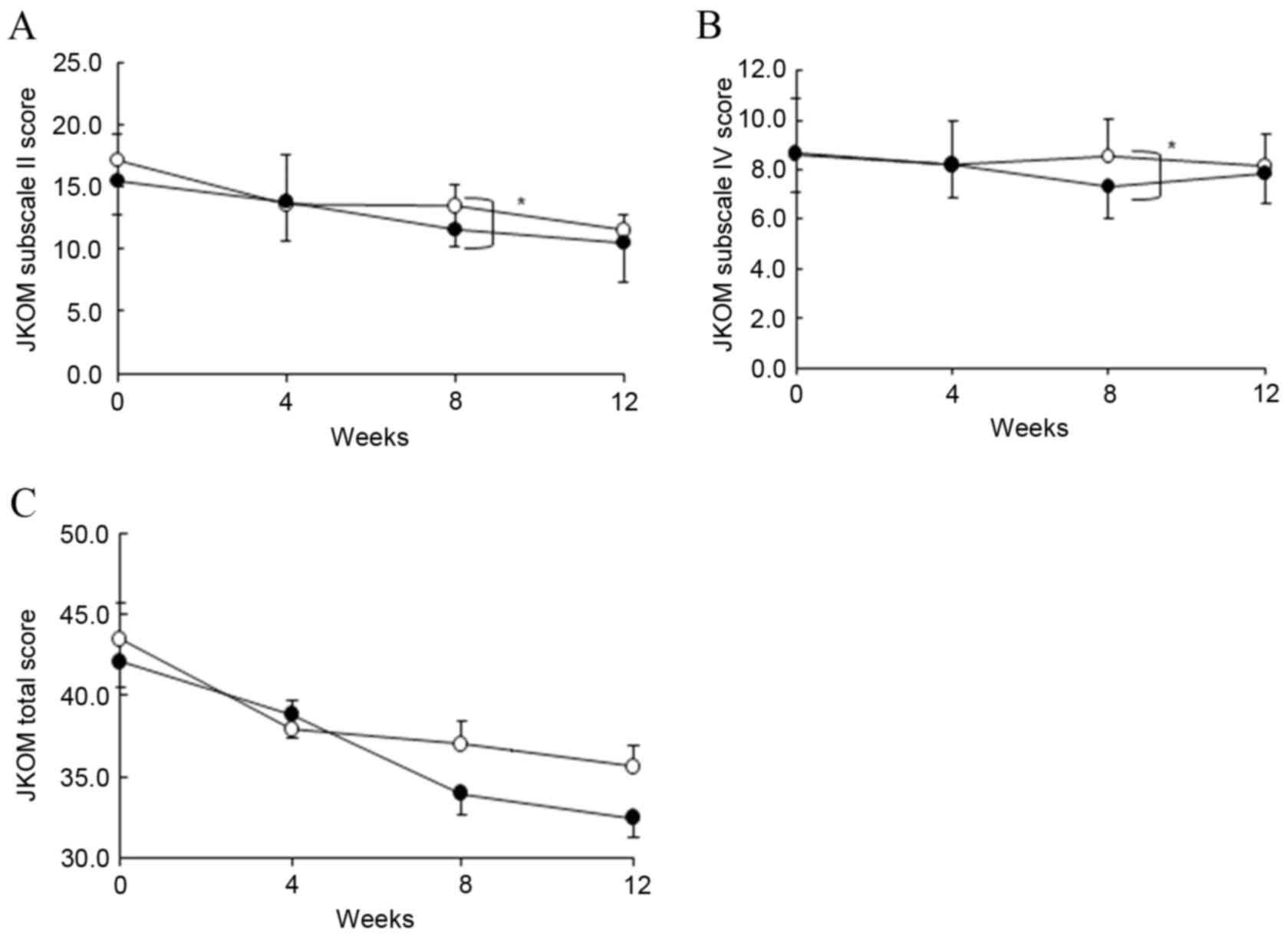
subscale scores II and IV significantly decreased in the ADE diet
group compared with the placebo group at 8 weeks (Fig. 4A and B; P<0.05). Total score was
markedly decreased in the ADE diet group compared with the placebo
group at 8 weeks (Fig. 4C).
Furthermore, in terms of percentage change from the baseline, the
subscale scores I, II, IV and V and total score exhibited a greater
decrease in the ADE diet group compared with the placebo group at 8
weeks (Table VII).
 | Table VII.Individual and total scores of JKOM
response during the intervention for subgroup participants with
mild knee pain in the ADE diet (n=18) and placebo (n=14)
groups. |
Table VII.
Individual and total scores of JKOM
response during the intervention for subgroup participants with
mild knee pain in the ADE diet (n=18) and placebo (n=14)
groups.
| JKOM criteria | Group | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| I: VAS, mm | ADE diet | 53.1±15.2 |
28.3±19.0b |
19.3±17.8b |
14.1±13.7b |
|
|
|
|
(−44.9±35.5b) |
(−60.0±37.4b) |
(−71.9±28.1b) |
|
| Placebo | 60.4±12.8 |
31.5±20.5b |
25.1±20.9b |
13.2±17.0b |
|
|
|
|
(−47.7±30.1b) |
(−57.4±34.9b) |
(−74.7±71.9b) |
| II: Pain and
stiffness in the knees, points | ADE diet | 15.4±3.8 |
13.7±3.8a |
11.6±3.6b,c |
10.4±2.4b |
|
|
|
|
(−9.7±18.0a) |
(−23.4±17.2b) |
(−30.7±12.3b) |
|
| Placebo | 17.1±4.3 |
13.5±2.9b |
13.4±3.2a |
11.4±4.1a |
|
|
|
|
(−17.2±24.5b) |
(−16.9±29.5a) |
(−31.1±21.9b) |
| III: Condition in
daily life, points | ADE diet | 14.2±4.3 | 13.6±4.5 |
12.1±3.4b |
11.2±1.5b |
|
|
|
| (−2.8±19.7) |
(−12.3±15.4b) |
(−16.7±16.7b) |
|
| Placebo | 14.1±3.7 | 12.6±2.8 |
11.6±2.1a | 12.8±6.8 |
|
|
|
| (−7.1±27.7) |
(−14.3±19.5a) | (−2.5±67.8) |
| IV: General
activities, points | ADE diet | 8.7±1.6 | 8.2±1.4 |
7.3±1.3a,c | 7.8±1.2 |
|
|
|
| (−2.9±21.1) |
(−12.6±24.2a) | (−7.0±20.9) |
|
| Placebo | 8.6±2.2 | 8.2±1.8 | 8.6±1.5 | 8.1±1.3 |
|
|
|
| (−2.2±20.1) | (4.0±28.9) | (−0.3±31.0) |
| V: Health
conditions, points | ADE diet | 3.8±1.5 | 3.3±0.8 |
3.0±0.8a |
3.1±0.8a |
|
|
|
| (−8.1±26.5) | (−13.7±31.3) | (−12.3±31.3) |
|
| Placebo | 3.5±1.3 | 3.6±1.3 | 3.4±1.3 | 3.3±1.3 |
|
|
|
| (10.4±55.5) | (0.5±40.9) | (3.3±66.6) |
| Total score | ADE diet | 42.1±9.7 | 38.8±9.4 |
34.0±7.2b |
32.5±4.0b |
|
|
|
| (−6.7±15.2) |
(−17.2±15.8b) |
(−20.3±13.1b) |
|
| Placebo | 43.8±9.7 |
37.7±7.2a |
36.5±5.7a |
35.9±11.9a |
|
|
|
|
(−10.8±17.8a) |
(−12.3±18.6a) | (−15.3±31.4) |
Safety assessment
The safety of 48 subjects allocated to the ADE diet
and placebo groups was evaluated. A total of 11 (45.8%) subjects in
the placebo group (n=24) and 13 (54.2%) subjects in the ADE diet
group (n=24) reported adverse events. Major adverse events reported
were respiratory symptoms (sore throat, cough, rhinorrhea and/or
fever) and joint pain (knee, hip, shoulder or elbow). All adverse
events were of mild intensity and judged by the medical
investigator to be unrelated to the intervention. Furthermore,
among the physical measurement parameters (body weight and BMI),
physiological examinations (systolic and diastolic blood pressures
and pulse rate) and laboratory tests (urinalysis, hematology and
blood chemistry), BMI and some parameters in hematology and blood
chemistry indicated marked changes (data not shown) during the
intervention in a small number of subjects; however, these changes
were within reference values. Based on these findings, it was
concluded that ADE-containing food did not induce adverse
events.
Discussion
In this randomized, double-blind, placebo-controlled
clinical trial, the effects of oral administration of ADE were
evaluated in subjects with knee discomfort associated with physical
activity. The effectiveness of ADE was assessed primarily on the
basis of the JOA and JKOM scores. The majority of JOA criteria were
improved from the baseline values in both groups. Subscale III
(joint flexion/stiffness) was markedly improved in the ADE diet
group compared with the placebo group at weeks 8 and 12 after the
intervention. Notably, it has previously been reported that ADE
extract promotes the repair of articular cartilage in a rabbit
cartilage damaged model (8).
Furthermore, cartilage defects have been reported to correlate with
the disturbance of knee extension during the progression of OA
(21,22). Therefore, it may be speculated that
the improvement of joint flexion/stiffness may be due to the
potential of ADE to repair injured cartilage in the knee joint.
Conversely, subscales I (pain/walking function) and II
(pain/step-up and down function) of the JOA criteria increased in
both the ADE and the placebo group during the intervention, and
there was no significant difference between the two groups. The
high scores in the placebo group may be due to the characteristics
of these pain-associated subscales, which depend on self-reporting
and subjective measurements. Subscale IV (swelling) did not
significantly change during the intervention in the ADE or placebo
group. This may be explained by the fact that no subjects
participating in the present study exhibited swelling in knee
joints, as evidenced by the high scores (full score of 10 points)
at the baseline and during the intervention.
In the assessment of JKOM scores, the percentages of
change from the baseline exhibited a greater decrease in the ADE
diet group compared with the placebo group at 12 weeks in the
subscales III (condition in daily life), IV (general activities)
and V (health conditions). The questions for subscale III evaluate
the ability to perform daily routines during the last few days,
such as ‘How difficult is ascending or descending stairs?’ and ‘How
long can you walk on a flat surface without taking a rest?’. The
questions for subscale IV evaluate the general activities over the
previous month, such as ‘Have you gone to an event or to a
department store during the last month?’ and ‘Were things that you
usually do (some kind of lesson and meeting friends) difficult
because of knee pain during the last month?’. The questions for
subscale V evaluate general health during the last month, such as
‘Do you think your health during the last month has been average?’
and ‘Do you think that knee pain has been affecting your health
badly during the last month?’. Thus, the current results suggest
that an ADE-supplemented diet may improve the ability to perform
daily routines, general activities and health conditions.
Furthermore, to evaluate the effects of ADE more
clearly, subjects with minimal cartilage damage (K/L 0-I and >75
points of JOA criteria) were selected and analyzed. Magnetic
resonance imaging classification previously demonstrated that
irregularity and thinning but no defects were observed on the
articular surface of the knee joint in subjects with >75 points
of JOA criteria (23). The current
results indicated that subscales II (pain and stiffness in knees)
and IV (general activities) of the JKOM criteria were significantly
improved in the ADE diet group at 8 weeks compared with the placebo
group. In addition, the total score of JKOM was markedly improved
at 8 weeks in the ADE diet group compared with the placebo group
and continued to decrease until 12 weeks in the ADE diet group. As
ADE improved both subscale II (pain and stiffness in knees) of JKOM
and subscale II (flexion and stiffness) of JOA, an ADE diet may
alleviate stiffness in subjects with physical activity-associated
knee discomfort. In addition, it may improve the ability to perform
general activities in these subjects.
It has previously been reported that biomarkers for
cartilage metabolism, particularly type II collagen metabolism, may
be used for evaluating the pathogenesis of joint destruction and
monitoring structure-modifying agents or therapies (24). Type II collagen degradation
biomarkers such as CTX-II, C1, 2C and C2C, were used to estimate
the efficacy of chondroprotective agents, glucosamine (25,26) and
chondroitin sulfate (27).
Furthermore, type II collagen synthesis biomarkers such as PIICP
have been used alone or in combination with type II collagen
degradation biomarkers (e.g., CTX-II and C2C) for the assessment of
chondroprotective agents such as glucosamine (28–30). The
current study used CTX-II and C2C as type II collagen degradation
markers, PIICP as a type II collagen synthesis marker, and MMP-13
as a major collagen-degrading enzyme. In addition, the ratios of
the synthesis and degradation of type II collagen (C2C/PIICP and
CTX-II/PIICP) were analyzed. In the ADE diet group, the levels of
PIICP increased markedly at week 8 compared with the placebo group.
ADE has been reported to promote the repair of damaged cartilage
(8) and enhance the synthesis of
collagen in false aged model rats (7). Based on these findings, ADE may have
enhanced the synthesis of collagen, especially type II collagen, in
the subjects involved in the present study, as evidenced by the
increase of PIICP, which is a type II collagen synthesis marker.
Furthermore, the levels of MMP-13 were significantly reduced in
both groups compared with the baseline; however the changes of
MMP-13 levels from baseline were more substantially reduced at
weeks 4, 8 and 12 in the ADE diet group compared with the placebo
group, although the difference was not significant. It has been
reported that MMP-13 may be induced by inflammatory cytokines
(18), whereas ADE exhibits an
anti-inflammatory action on an arthritis model (7). Therefore, the anti-inflammatory action
of ADE may be associated with the suppression of MMP-13. Together,
these observations suggest that the chondroprotective and
anti-inflammatory actions of ADE may contribute to improving knee
joint function (as evidenced by subscale III of JOA and subscale II
of JKOM), and general activities and QOL (as evidenced by subscale
IV and total score of JKOM) in the subjects with mild knee
discomfort.
In conclusion, in the current study, the
administration of ADE-containing diet was confirmed to be safe and
improved the scores concerning joint flexion and general activities
in the subjects with mild knee discomfort. Notably, the management
of knee discomfort is more effective at an early stage for
maintaining knee function; thus, ADE could be a promising candidate
as a functional food that is beneficial for joint health.
Acknowledgements
The authors would like to thank Professor Yamamoto
(Total Technological Consultant Co., Ltd., Tokyo, Japan) for his
valuable advice and support and Dr Nakagawa (Total Technological
Consultant Co., Ltd.) for his statistical analysis of the data and
preparation of the manuscript. This study was funded by Asahi Food
& Healthcare Company Ltd. (Tokyo, Japan). However, Asahi Food
& Healthcare did not have any role in the design and conduction
of the study, subject recruitment, collection, management or
analysis of the data.
References
|
1
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McAlindon TE, Bannuru RR, Sullivan MC,
Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y,
Hunter DJ, Kawaguchi H, et al: OARSI guidelines for the
non-surgical management of knee osteoarthritis. Osteoarthritis
Cartilage. 22:363–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu G and Wang Q: Practical Illustrations
of Chinese Materia Medica. Fujian Science & Technology
Publishing House; Fuzhou: pp. 92–93. 2006
|
|
4
|
Ou M: Chinese-English Manual of
Common-Used in Traditional Chinese Medicine. Joint Publishing (Hong
Kong) Co., Ltd.; Hong Kong: pp. 164–165. 1989
|
|
5
|
Rashad S, Low F, Revell P, Hemingway A,
Rainsford K and Walker F: Effect of non-steroidal anti-inflammatory
drugs on course of osteoarthritis. Lancet. 2:11491989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herman JH and Hess EV: Nonsteroidal
anti-inflammatory drugs and modulation of cartilaginous changes in
osteoarthritis and rheumatoid arthritis. Clinical implications. Am
J Med. 77:16–25. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono Y, Fukaya Y, Imai S and Yamakuni T:
Beneficial effects of Ajuga decumbens on osteoporosis and
arthritis. Biol Pharm Bull. 31:1199–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawada Y, Sugimoto A, Fukuda K, Osaki T
and Minami S: Oral administration of Ajuga decumbens extract has a
synergetic effect with glucosamine on cartilaginous injury in a
rabbit osteoarthritis model. J Chitin Chitosan Sci. 2:191–196.
2014. View Article : Google Scholar
|
|
9
|
Akai M, Iwaya T, Kurosawa H, Doi T, Nasu
T, Hyashi K and Fujino K; JKOM (Japanese Knee Osteoarthritis
Measure), : Development of new disease-specific QOL measure for
patients with knee osteoarthritis: Japanese Knee Osteoarthritis
Measure (JKOM). J Physical Medicine. 16:55–62. 2005.(In
Japanese).
|
|
10
|
Akai M, Doi T, Fujino K, Iwaya T, Kurosawa
H and Nasu T: An outcome measure for Japanese people with knee
osteoarthritis. J Rheumatol. 32:1524–1532. 2005.PubMed/NCBI
|
|
11
|
Koshino T and Niwa J: Assessment criteria
for knee diseases and treatments. J Jpn Orthop Assoc. 62:900–904.
1988.(In Japanese).
|
|
12
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muraki S, Oka H, Akune T, Mabuchi A, En-yo
Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, et al:
Prevalence of radiographic knee osteoarthritis and its association
with knee pain in the elderly of Japanese population-based cohorts:
The ROAD study. Osteoarthritis Cartilage. 17:1137–1143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reijman M, Hazes JM, Bierma-Zeinstra SM,
Koes BW, Christgau S, Christiansen C, Uitterlinden AG and Pols HA:
A new marker for osteoarthritis: Cross-sectional and longitudinal
approach. Arthritis Rheum. 50:2471–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nelson F, Dahlberg L, Laverty S, Reiner A,
Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC,
et al: Evidence for altered synthesis of type II collagen in
patients with osteoarthritis. J Clin Invest. 102:2115–2125. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Wei X, Zhou J, Zhang J, Li K, Chen
Q, Terek R, Fleming BC, Goldring MB, Ehrlich MG, et al:
Identification of α2-macroglobulin as a master inhibitor of
cartilage-degrading factors that attenuates the progression of
posttraumatic osteoarthritis. Arthritis Rheum. 66:1843–1853. 2014.
View Article : Google Scholar
|
|
18
|
Poole AR, Nelson F, Dahlberg L, Tchetina
E, Kobayashi M, Yasuda T, Laverty S, Squires G, Kojima T, Wu W and
Billinghurst RC: Proteolysis of the collagen fibril in
osteoarthritis. Biochem Soc Symp. 115–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braham R, Dawson B and Goodman C: The
effect of glucosamine supplementation on people experiencing
regular knee pain. Br J Sports Med. 37:45–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagaoka I, Suzuki A, Kurokawa M, Tamonaga
A, Fukagawa M, Watanabe K and Yamamoto T: Effect of a dietary
supplement containing collagen peptide on symptoms and biomarkeres
in individuals with knee pain. Glucosamine Res. 9:40–47. 2013.(In
Japanese).
|
|
21
|
Watanabe H, Urabe K, Kamiya K, Hamazaki N,
Miida K, Suda K, Hendona T, Fujita M, Aikawa J, Itoman M and Futami
T: Relationship between quality of life using the Japan Knee
Osteoarthritis Measure (JKOM) and physical function in patients
with osteoarthritis of the knee. Jpn Physical Therapy Association.
34:67–73. 2007.(In Japanese).
|
|
22
|
Watanabe H, Urabe K, Takahira N, Ikeda N,
Fujita M, Obara S, Hendona T, Aikawa J and Itoman M: Quality of
life, knee function, and physical activity in Japanese elderly
women with early-stage knee osteoarthritis. J Orthop Surg (Hong
Kong). 18:31–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nozaki H, Takezawa Y, Suguro T, Igata L,
Kudo Y and Motegi M: MRI of articular cartilaginous lesions: MRI
findings in osteoarthritis of the knee joint. Jpn j rheum and joint
surg. 13:341–352. 1994.
|
|
24
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Curr Opin Rheumatol. 15:641–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christgau S, Henrotin Y, Tankó LB, Rovati
LC, Collette J, Bruyere O, Deroisy R and Reginster JY:
Osteoarthritic patients with high cartilage turnover show increased
responsiveness to the cartilage protecting effects of glucosamine
sulphate. Clin Exp Rheumatol. 22:36–42. 2004.PubMed/NCBI
|
|
26
|
Cibere J, Thorne A, Kopec JA, Singer J,
Canvin J, Robinson DB, Pope J, Hong P, Grant E, Lobanok T, et al:
Glucosamine sulfate and cartilage type II collagen degradation in
patients with knee osteoarthritis: Randomized discontinuation trial
results employing biomarkers. J Rheumatol. 32:896–902.
2005.PubMed/NCBI
|
|
27
|
Mazieres B, Hucher M, Zaïm M and Garnero
P: Effect of chondroitin sulphate in symptomatic knee
osteoarthritis: A multicentre, randomised, double-blind,
placebo-controlled study. Ann Rheum Dis. 66:639–645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshimura M, Sakamoto K, Tsuruta A,
Yamamoto T, Ishida K, Yamaguchi H and Nagaoka I: Evaluation of the
effect of glucosamine administration on biomarkers for cartilage
and bone metabolism in soccer players. Int J Mol Med. 24:487–494.
2009.PubMed/NCBI
|
|
29
|
Momomura R, Naito K, Igarashi M, Watari T,
Terakado A, Oike S, Sakamoto K, Nagaoka I and Kaneko K: Evaluation
of the effect of glucosamine administration on biomarkers of
cartilage and bone metabolism in bicycle racers. Mol Med Rep.
7:742–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagaoka I: Recent Aspects of the
chondroprotective and anti-inflammatory actions of glucosamine, a
functional food. Juntendo Med J. 60:580–587. 2014. View Article : Google Scholar
|