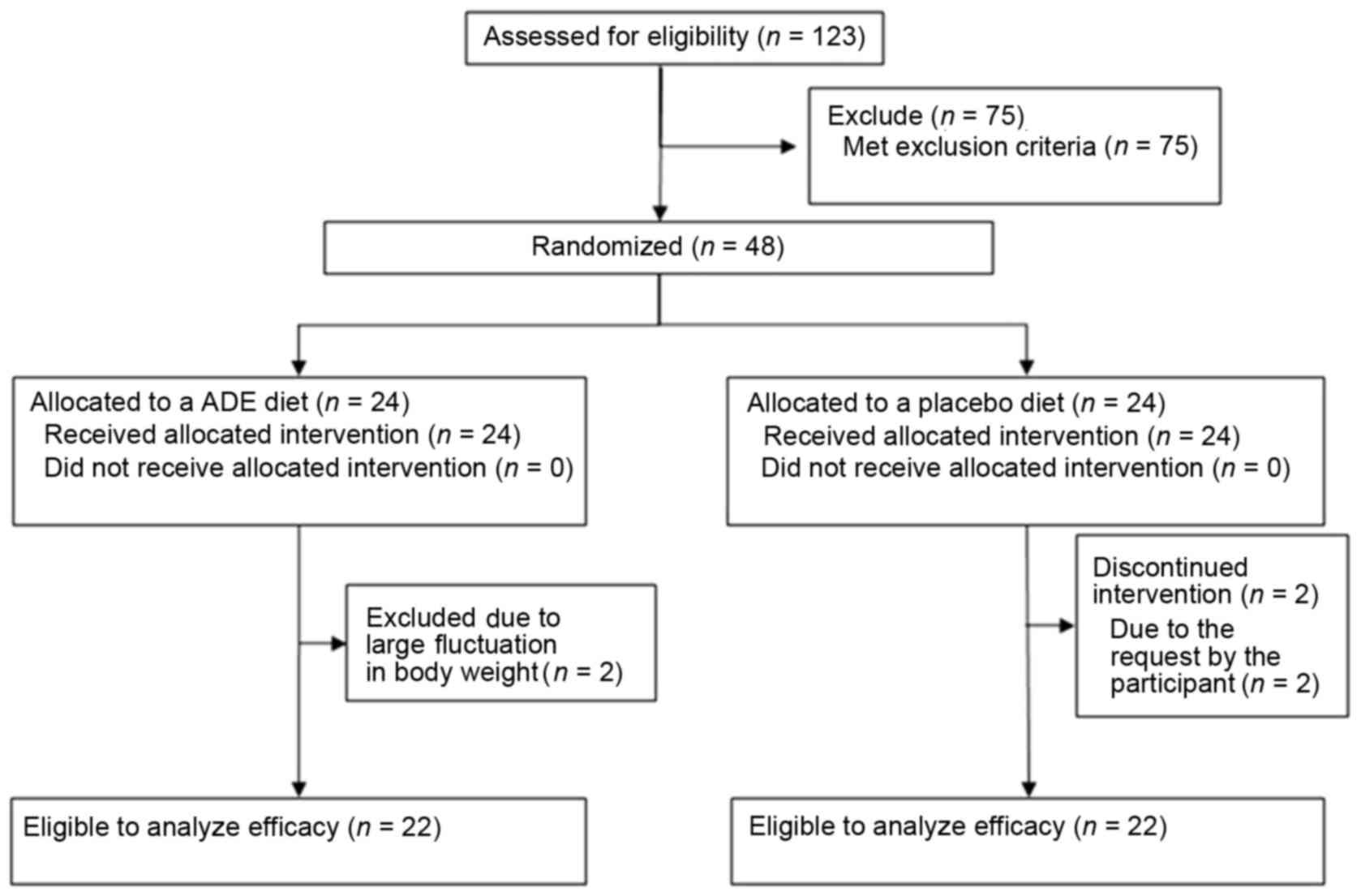
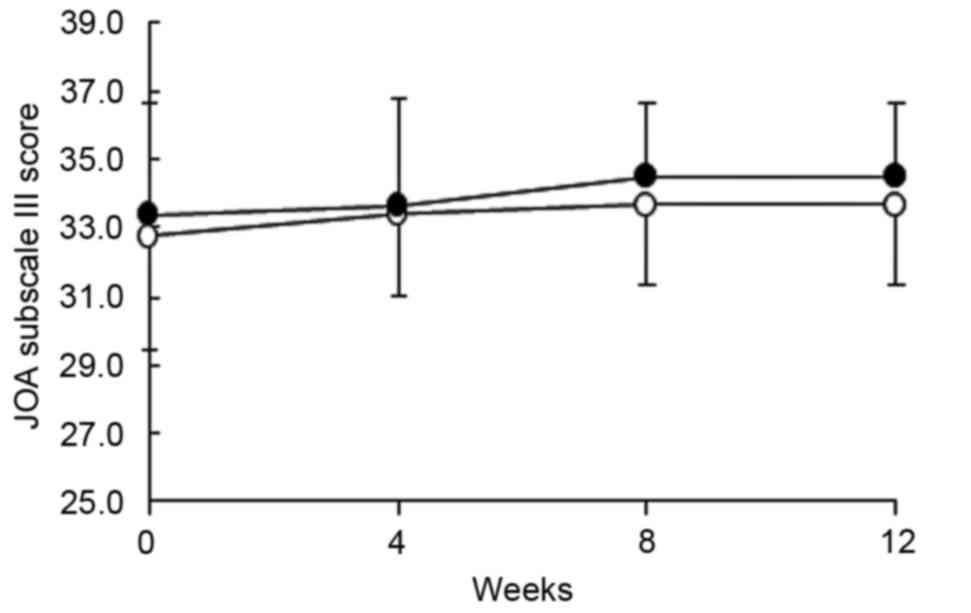
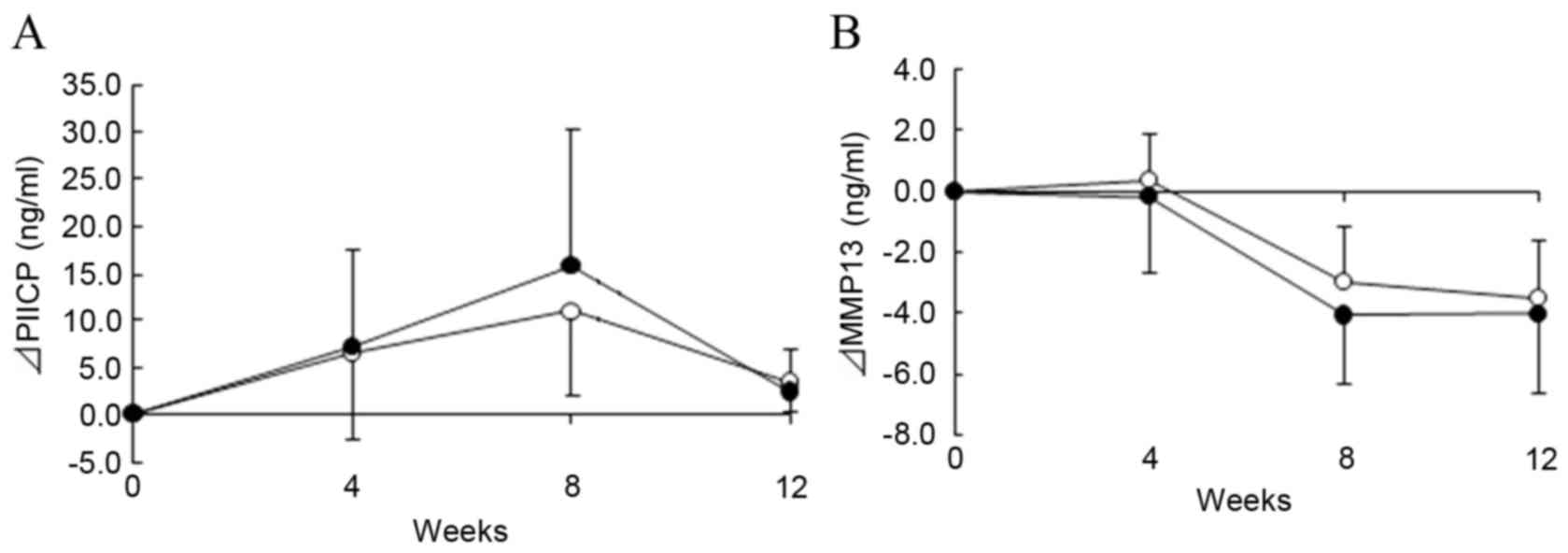
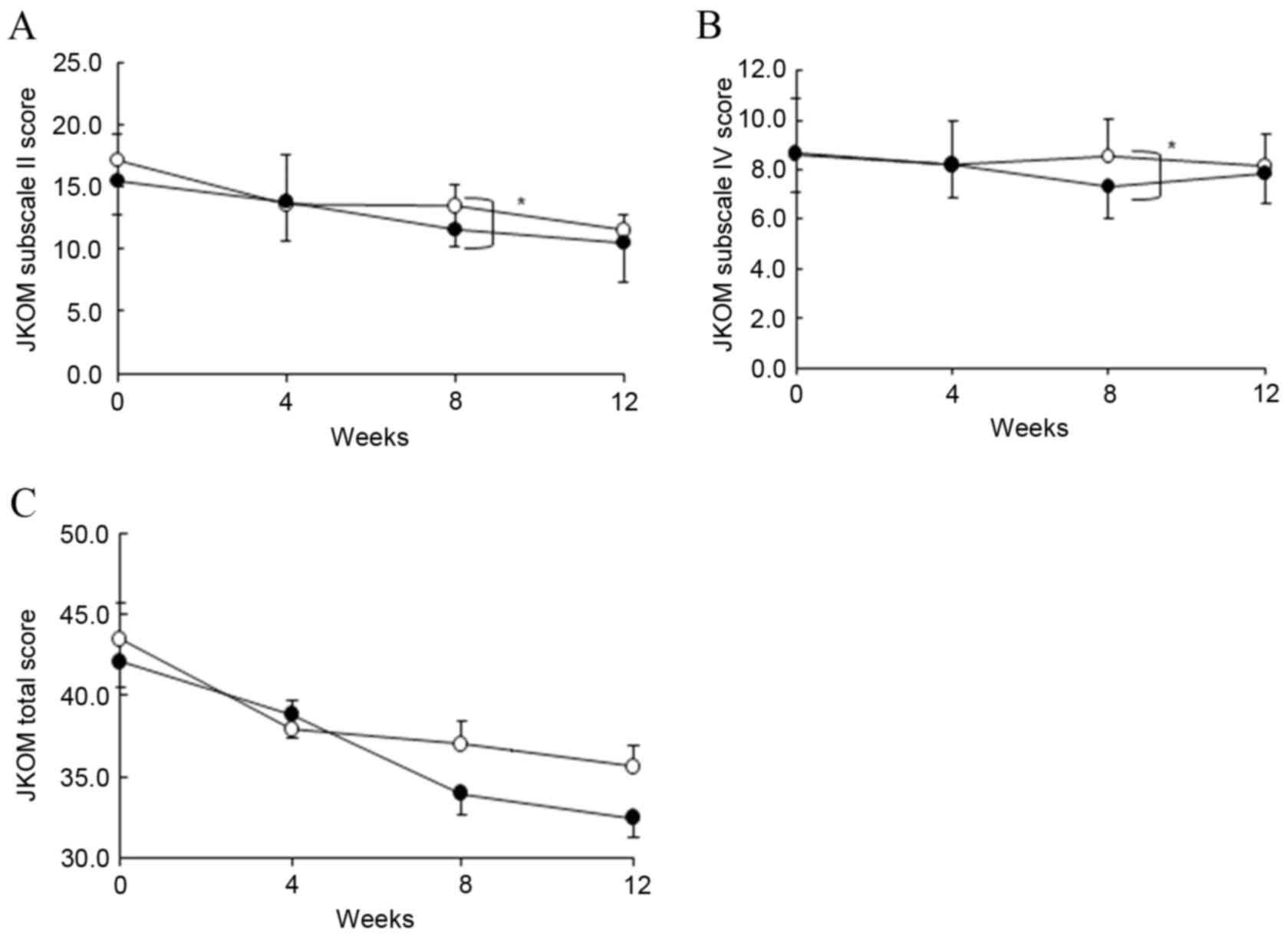
|
1
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McAlindon TE, Bannuru RR, Sullivan MC,
Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y,
Hunter DJ, Kawaguchi H, et al: OARSI guidelines for the
non-surgical management of knee osteoarthritis. Osteoarthritis
Cartilage. 22:363–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu G and Wang Q: Practical Illustrations
of Chinese Materia Medica. Fujian Science & Technology
Publishing House; Fuzhou: pp. 92–93. 2006
|
|
4
|
Ou M: Chinese-English Manual of
Common-Used in Traditional Chinese Medicine. Joint Publishing (Hong
Kong) Co., Ltd.; Hong Kong: pp. 164–165. 1989
|
|
5
|
Rashad S, Low F, Revell P, Hemingway A,
Rainsford K and Walker F: Effect of non-steroidal anti-inflammatory
drugs on course of osteoarthritis. Lancet. 2:11491989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herman JH and Hess EV: Nonsteroidal
anti-inflammatory drugs and modulation of cartilaginous changes in
osteoarthritis and rheumatoid arthritis. Clinical implications. Am
J Med. 77:16–25. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono Y, Fukaya Y, Imai S and Yamakuni T:
Beneficial effects of Ajuga decumbens on osteoporosis and
arthritis. Biol Pharm Bull. 31:1199–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawada Y, Sugimoto A, Fukuda K, Osaki T
and Minami S: Oral administration of Ajuga decumbens extract has a
synergetic effect with glucosamine on cartilaginous injury in a
rabbit osteoarthritis model. J Chitin Chitosan Sci. 2:191–196.
2014. View Article : Google Scholar
|
|
9
|
Akai M, Iwaya T, Kurosawa H, Doi T, Nasu
T, Hyashi K and Fujino K; JKOM (Japanese Knee Osteoarthritis
Measure), : Development of new disease-specific QOL measure for
patients with knee osteoarthritis: Japanese Knee Osteoarthritis
Measure (JKOM). J Physical Medicine. 16:55–62. 2005.(In
Japanese).
|
|
10
|
Akai M, Doi T, Fujino K, Iwaya T, Kurosawa
H and Nasu T: An outcome measure for Japanese people with knee
osteoarthritis. J Rheumatol. 32:1524–1532. 2005.PubMed/NCBI
|
|
11
|
Koshino T and Niwa J: Assessment criteria
for knee diseases and treatments. J Jpn Orthop Assoc. 62:900–904.
1988.(In Japanese).
|
|
12
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muraki S, Oka H, Akune T, Mabuchi A, En-yo
Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, et al:
Prevalence of radiographic knee osteoarthritis and its association
with knee pain in the elderly of Japanese population-based cohorts:
The ROAD study. Osteoarthritis Cartilage. 17:1137–1143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reijman M, Hazes JM, Bierma-Zeinstra SM,
Koes BW, Christgau S, Christiansen C, Uitterlinden AG and Pols HA:
A new marker for osteoarthritis: Cross-sectional and longitudinal
approach. Arthritis Rheum. 50:2471–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nelson F, Dahlberg L, Laverty S, Reiner A,
Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC,
et al: Evidence for altered synthesis of type II collagen in
patients with osteoarthritis. J Clin Invest. 102:2115–2125. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Wei X, Zhou J, Zhang J, Li K, Chen
Q, Terek R, Fleming BC, Goldring MB, Ehrlich MG, et al:
Identification of α2-macroglobulin as a master inhibitor of
cartilage-degrading factors that attenuates the progression of
posttraumatic osteoarthritis. Arthritis Rheum. 66:1843–1853. 2014.
View Article : Google Scholar
|
|
18
|
Poole AR, Nelson F, Dahlberg L, Tchetina
E, Kobayashi M, Yasuda T, Laverty S, Squires G, Kojima T, Wu W and
Billinghurst RC: Proteolysis of the collagen fibril in
osteoarthritis. Biochem Soc Symp. 115–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braham R, Dawson B and Goodman C: The
effect of glucosamine supplementation on people experiencing
regular knee pain. Br J Sports Med. 37:45–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagaoka I, Suzuki A, Kurokawa M, Tamonaga
A, Fukagawa M, Watanabe K and Yamamoto T: Effect of a dietary
supplement containing collagen peptide on symptoms and biomarkeres
in individuals with knee pain. Glucosamine Res. 9:40–47. 2013.(In
Japanese).
|
|
21
|
Watanabe H, Urabe K, Kamiya K, Hamazaki N,
Miida K, Suda K, Hendona T, Fujita M, Aikawa J, Itoman M and Futami
T: Relationship between quality of life using the Japan Knee
Osteoarthritis Measure (JKOM) and physical function in patients
with osteoarthritis of the knee. Jpn Physical Therapy Association.
34:67–73. 2007.(In Japanese).
|
|
22
|
Watanabe H, Urabe K, Takahira N, Ikeda N,
Fujita M, Obara S, Hendona T, Aikawa J and Itoman M: Quality of
life, knee function, and physical activity in Japanese elderly
women with early-stage knee osteoarthritis. J Orthop Surg (Hong
Kong). 18:31–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nozaki H, Takezawa Y, Suguro T, Igata L,
Kudo Y and Motegi M: MRI of articular cartilaginous lesions: MRI
findings in osteoarthritis of the knee joint. Jpn j rheum and joint
surg. 13:341–352. 1994.
|
|
24
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Curr Opin Rheumatol. 15:641–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christgau S, Henrotin Y, Tankó LB, Rovati
LC, Collette J, Bruyere O, Deroisy R and Reginster JY:
Osteoarthritic patients with high cartilage turnover show increased
responsiveness to the cartilage protecting effects of glucosamine
sulphate. Clin Exp Rheumatol. 22:36–42. 2004.PubMed/NCBI
|
|
26
|
Cibere J, Thorne A, Kopec JA, Singer J,
Canvin J, Robinson DB, Pope J, Hong P, Grant E, Lobanok T, et al:
Glucosamine sulfate and cartilage type II collagen degradation in
patients with knee osteoarthritis: Randomized discontinuation trial
results employing biomarkers. J Rheumatol. 32:896–902.
2005.PubMed/NCBI
|
|
27
|
Mazieres B, Hucher M, Zaïm M and Garnero
P: Effect of chondroitin sulphate in symptomatic knee
osteoarthritis: A multicentre, randomised, double-blind,
placebo-controlled study. Ann Rheum Dis. 66:639–645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshimura M, Sakamoto K, Tsuruta A,
Yamamoto T, Ishida K, Yamaguchi H and Nagaoka I: Evaluation of the
effect of glucosamine administration on biomarkers for cartilage
and bone metabolism in soccer players. Int J Mol Med. 24:487–494.
2009.PubMed/NCBI
|
|
29
|
Momomura R, Naito K, Igarashi M, Watari T,
Terakado A, Oike S, Sakamoto K, Nagaoka I and Kaneko K: Evaluation
of the effect of glucosamine administration on biomarkers of
cartilage and bone metabolism in bicycle racers. Mol Med Rep.
7:742–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagaoka I: Recent Aspects of the
chondroprotective and anti-inflammatory actions of glucosamine, a
functional food. Juntendo Med J. 60:580–587. 2014. View Article : Google Scholar
|