Introduction
Preeclampsia (PE), which is predominantly
characterized by hypertension and proteinuria and is a
pregnancy-specific disease that originates in the placenta, is a
serious threat to maternal and perinatal life (1). Although PE is considered a common
obstetric complication, the mechanism of pathogenesis remains to be
fully elucidated. At present, the majority of studies suggest that
abnormal differentiation and apoptosis in trophoblast cells results
in cell dysfunction, changes in infiltration and spiral artery
remodeling in trophoblast cells, which eventually leads to the
occurrence of PE (2,3). Thus, the dysfunction of trophoblast
cells is considered an important cause of PE. MicroRNA (miRNA) are
a class of endogenous and highly conserved non-coding small RNA,
with the length from 18 to 24 nucleotides. The basic function of
miRNA is to bind to the specific pairing bases of target mRNA
[i.e., miRNA-specific binding to the 3′ untranslated region (3′UTR)
of target mRNA], causing degradation or translational repression of
the target mRNA and promoting post-transcriptional gene silencing
(4). Previous studies suggest that
miRNA have important roles in various basic physiological
processes, such as cell growth, differentiation, proliferation and
apoptosis (5–7). Furthermore, previous studies have
indicated a series of expression imbalances between miRNA and
target genes in PE placental tissues, which have resulted in cell
dysfunction in placental tissues; thus, abnormal expression levels
of miRNA have been demonstrated to be closely related to the
occurrence of PE in PE patients (8–10).
miR18b (miR-18b) is involved in the regulation of multiple
diseases. It has been reported that miR-18b has a role as a tumor
suppressor gene by targeting the MDM2-p53 pathway in melanoma
(11). Moreover, miR-18b is
abnormally expressed in gastric cancer (12), breast cancer (13) and nasopharynx cancer (14). The present study indicated that
miR-18b exhibited pathologically low expression levels in PE
placentas; however, the pathological mechanism of miR-18b in the
pathogenesis of PE remains poorly studied (15).
Hypoxia inducible factor-1 (HIF-1) is a key
transcriptional regulatory factor that mediates cellular adaptation
to hypoxia microenvironment (16).
HIF-1 is overexpressed in various cancers and precancerous lesions
and is considered to be the central initiating molecule of tumor
angiogenesis (17). HIF-1 is a
heterodimer that contains two subunits of HIF-1α and HIF-1β
(18). Taylor (19) revealed that, under normoxic
conditions, prolyl hydroxyl-lase domain proteins (PHD) were able to
hydroxylate the proline residues in the oxygen-dependent
degradation domain of the Q subunit and subsequently enhance the
affinity of HIF-1α and tumor suppressor protein (von Hippel-Lindau
protein), ultimately promoting the combination of HIF-1α to a
ubiquitin-dependent proteolytic enzyme complex to enzyme
hydrolysis. In the case of hypoxia, PHD activity decreased and
HIF-1α rapidly gathered in the intracellular space, combined with β
isoforms and was transferred to the nucleus to promote the
transcription of hypoxia-responsive genes. Ietta et al
(20) reported that HIF-1 was the
key molecular component that mediated the regulation of hypoxia
during trophoblast cell-associated invasion and differentiation
processes. A previous study indicated that the expression levels of
HIF-1α are sustained throughout pregnancy (21). HIF-1α expression in villus tissues is
significantly increased in weeks 8 to 10 of pregnancy, the time
point at which the placental oxygen content is lower (22). Additionally, HIF-1α expression has
been revealed to exhibit a clear downward trend in weeks 10 to 12
of pregnancy, the time point at which the placental oxygen content
increases and the invasion ability of trophoblast cells
subsequently peaks as maternal-fetal circulation has been
established (22). These changes
have notable influences on the functions of trophoblast cells and
the entire placenta, indicating that HIF-1α may have a close
relationship with the pathogenesis of PE.
In the present study, the expression levels of
HIF-1α and miR-18b in placental tissues were detected and the roles
of HIF-1α and miR-18b in PE were analyzed. The present findings may
further elucidate the pathogenesis mechanism of PE and provide a
novel theoretical basis for the treatment of PE.
Materials and methods
Tissue specimen collection
A total of 50 pregnant women consisting of 25 PE
patients and 25 normal controls were selected in the Obstetrics and
Gynecology of Qingdao Municipal Hospital (Qingdao, China), between
May 2014 and December 2014. The criteria used for diagnosis of
pre-eclampsia were as follows: i) Systolic blood pressure of ≥140
mm Hg or diastolic blood pressure of ≥90 mm Hg occurring 20 weeks
post-gestation in a woman whose blood pressure was previously
normal; and ii) proteinuria, with excretion of ≥0.3 g in a 24-h
period. All studied pregnant women had given birth via cesarean
section and no significant difference (P>0.05) in age (20–35
years old) and gestational age (35–40 weeks) was exhibited between
the patients of the PE group and the control group. All specimen
collections were approved by the Ethics Committee of Qingdao
Municipal Hospital and all subjects gave their informed written
consent. All cases were confirmed by pathology, following surgery.
Placental tissue samples were collected during cesarean section and
immediately stored at −80°C for detection by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting. The present study was approved by the Ethics
Review Board of Qingdao Municipal Hospital.
Reagents
Normal human trophoblast cell line (HTR-8/SVneo)
cells were purchased from American Type Culture Collection
(Manassas, VA, USA). TRIzol and Lipofectamine 2000 were purchased
from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
miR-18b mimics/inhibitors were purchased from Shanghai GenePharma
Co., Ltd. (Shanghai, China). Rabbit anti-human HIF-1α polyclonal
antibody was purchased from Abcam (ab82832; Cambridge, MA, USA).
The reverse transcription kit, SYBR PrimeScript miRNA RT-PCR kit
and SYBR-Green real-time PCR reagents were all purchased from
Takara Biotechnology Co., Ltd. (Dalian, China).
Cell culture and transfection
HTR-8/SVneo cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)-F12 complete culture medium
(Hyclone, Logan, UT, USA) supplemented with 20% fetal bovine serum
(FBS; Sijiqing, Hangzhou, China) at 4×105 per well and
maintained at 37°C in a humidified atmosphere of 5% CO2
and 20% O2. For transfection, HTR-8/SVneo cells were
seeded in 6-well plates and cell transfection was performed when
~70% confluency was achieved. Four groups were allocated,
consisting of the hsa-miR-18b mimic transfected group (transfected
with mimic), hsa-miR-18b inhibitor transfected group (transfected
with inhibitor), negative control transfected group (transfected
with negative controls) and non-transfected control group (blank/no
transfection preformed). Lipofectamine 2000 transfection reagents
were used for transfection. Cells were harvested at 48 h following
transfection.
RNA extraction and reverse
transcription
Total RNA were extracted from placental tissues and
HTR-8/SVneo cells using the TRIzol and phenol chloroform method.
RNA was reverse transcribed into cDNA, and the cDNA was stored at
−20°C. For miRNA reverse transcription, the reverse transcription
system consisted of 6 µl RNA, 10 µl miRNA reaction buffer mix (2X),
2 µl BSA (0.1%) and 2 µl miRNA PrimeScript RT Enzyme mix. Reverse
transcription was performed at 37°C for 60 min.
RT-qPCR analysis
mRNA expression levels of miR-18b and HIF-1α in
tissues and HTR-8/SVneo cells were detected by SYBR-Green
quantitative PCR. U6 and GAPDH were used as internal
controls of miR-18b and HIF-1α, respectively. RT-qPCR for miR-18b
was performed in 25 µl of reaction system containing 12.5 µl SYBR
Premix Ex Taq, 0.5 µl forward primer
(5′-UAAGGUGCAUCUAGUGCAGUUAG-3′), 1 µl Uni-miR qPCR primer
(5′-CCAUAAGGUGCAUCUAGUGCAGU-3′), 2 µl template and 8.5 µl
double-distilled H2O. RT-qPCR for miR-18b was performed
using a verititm 96-well thermal cycler (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using the following procedure: 95°C for 30
sec, followed by 40 cycles of 94°C for 5 sec and 60°C for 20 sec.
RT-qPCR for HIF-1α was performed in a 20-µl reaction mixture
containing 10 µl SYBR Premix Ex Taq, 0.5 µl forward primer
(5′-TCAAAGTCGGACAGCCTCAC-3′), 0.5 µl reverse primer
(5′-TAGCTGCATGATCGTCTGGC-3′), 1 µl cDNA template and 8 µl
double-distilled H2O. RT-qPCR for HIF-1α was performed
using the following procedure: 95°C for 10 min, followed by 40
cycles of 94°C for 1 min, 60°C for 40 sec and 72°C for 40 sec. Each
experiment was performed in triplicate. The relative expression of
HIF-1α was calculated using the 2−∆∆Cq method (23).
Western blotting
Total proteins (50 µg) were extracted from placental
tissues and HTR-8/SVneo cells by incubating with pre-cold RIPA
lysate (50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 1%
Triton X-100 and 1% sodium deoxycholate). The concentration of
protein was determined using a BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). Proteins of 10 µl
were subjected to a 12% SDS-PAGE electrophoresis (100 V) and
transferred to a PVDF membrane (Merck Millipore, Darmstadt,
Germany). The membrane was blocked with 5% skimmed milk in
Tris-buffered saline with 0.1% Tween-20 for 1 h at room
temperature. The primary antibodies anti-HIF-1α (ab113642; 1:1,000;
Abcam) and anti-GAPDH (ab8245; 1:2,000; Abcam) for detecting the
expression of endogenous GAPDH as an internal reference were
incubated at 4°C overnight. After washing with PBS with Tween-20
for 15 min three times, the secondary antibodies horseradish
peroxidase-conjugated goat anti-rabbit antibodies (ab6789, 1:1,000,
Abcam) were added and incubated for 1 h at room temperature and
then washed by PBS with Tween-20 for 15 min three times.
Chemiluminescence reagent (EMD Millipore, Billerica, MA, USA) was
used for color development. The protein was placed in the ECL
luminescent solution and the image signal was obtained and analyzed
by the image lab software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The gray value of the target protein band was compared
with that of the GAPDH. The ratio of the gray value of the band is
the relative content of the target protein.
Cell migration
Effects of miR-18b on the migration of HTR-8/SVneo
cells were measured using a Transwell chambers assay (Corning Inc.,
Corning, NY, USA). Transfected cells were trypsinized, resuspended
to 5×105 cells/ml in DMEM supplemented with 0.1% bovine
serum albumin (BSA). Cells (200 µl with the density of
5×105 cells/ml) were added to the upper compartment of
the chamber. To the lower compartment, DMEM supplemented with 20%
FBS was added as the chemotactic factor. Once incubated at 37°C for
4 h, cells inside the upper compartment of the chamber were wiped
and the cells migrated through to the lower compartment of the
chamber were fixed with 100% methanol. Evaluation of migrated cells
was performed under a light microscope at a magnification of ×400
following 0.1% crystal violet cell staining.
MTT assay
The effect of miR-18b on total cellular metabolic
activity of HTR-8/SVneo cells was studied using an MTT Cell
Viability Assay kit (Beyotime Institute of Biotechnology).
HTR-8/SVneo cells were cultured in DMEM supplemented with 10% BSA
at 37°C in a humidified atmosphere containing 5% CO2.
Subsequently, cells were transfected with hsa-miR-18b mimic,
hsa-miR-18b inhibitors, negative control vector or not transfected
(blank used for comparison) in 96-well plates at a density of 3,000
cells/well using Lipofectamine 2000, according to the
manufacturer's instructions. For the MTT assay, each sample was
provided with three parallel wells and 15 µl of MTT was added to
each well at 24, 48 and 72 h following transfection. Following 4-h
incubation at 37°C, the supernatant was removed, dimethyl sulfoxide
(10 µl/well) was added and incubated at 37°C for 4 h to dissolve
the purple formazan and the absorbance value of each well was read
at a wavelength of 492 nm.
Cell invasion assay
The invasive ability of HTR-8/SVneo cells (ATCC)
seeded in 24-well plates was examined using growth factor-depleted
matrigel invasion chambers (BD Biosciences, San Jose, CA, USA) and
Transwell inserts (Corning, Inc., NY, USA). A total of 500 µl
serum-free DMEM (HyClone) was added to Matrigel chambers, incubated
at room temperature for 1 h to hydrate matrix glue and the
remaining medium was removed. Subsequently, 750 µl DMEM
supplemented with 20% FBS (Sijiqing, Hangzhou, China) was added to
the lower chamber. Cells were transfected with hsa-miR-18b mimic,
hsa-miR-18b inhibitors, negative control vector or not transfected
(blank). Transfected cells were trypsinized, centrifugally
collected and resuspended to 4×105 cells/ml in DMEM
supplemented with 0.1% BSA. Cells (500 µl with the density of
4×105 cells/ml) were added to the upper compartment of
the chamber. Following incubation at 37°C in a humidified
atmosphere containing 5% CO2 for 18 h, the cells inside
the upper compartment of the chamber were wiped using a cotton swab
and the cells invaded through to the lower compartment of the
chamber with 100% methanol were fixed. Evaluation of migrated cells
was performed under a light microscope following 0.1% crystal
violet (Beyotime Institute of Biotechnology) cell staining.
Statistical analysis
All statistical analyses were performed using
Statistical Package for Social Sciences software for Windows
(version 16.0; SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated in triplicate (n=3). Data are presented as mean ± standard
deviation. All data were analyzed with a normality test. Variance
analysis was applied for multiple sets of measurement data analysis
and Student's t-test was applied for two sets of data analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of miR-18b and
HIF-1α in PE and normal placental tissues detected by RT-qPCR
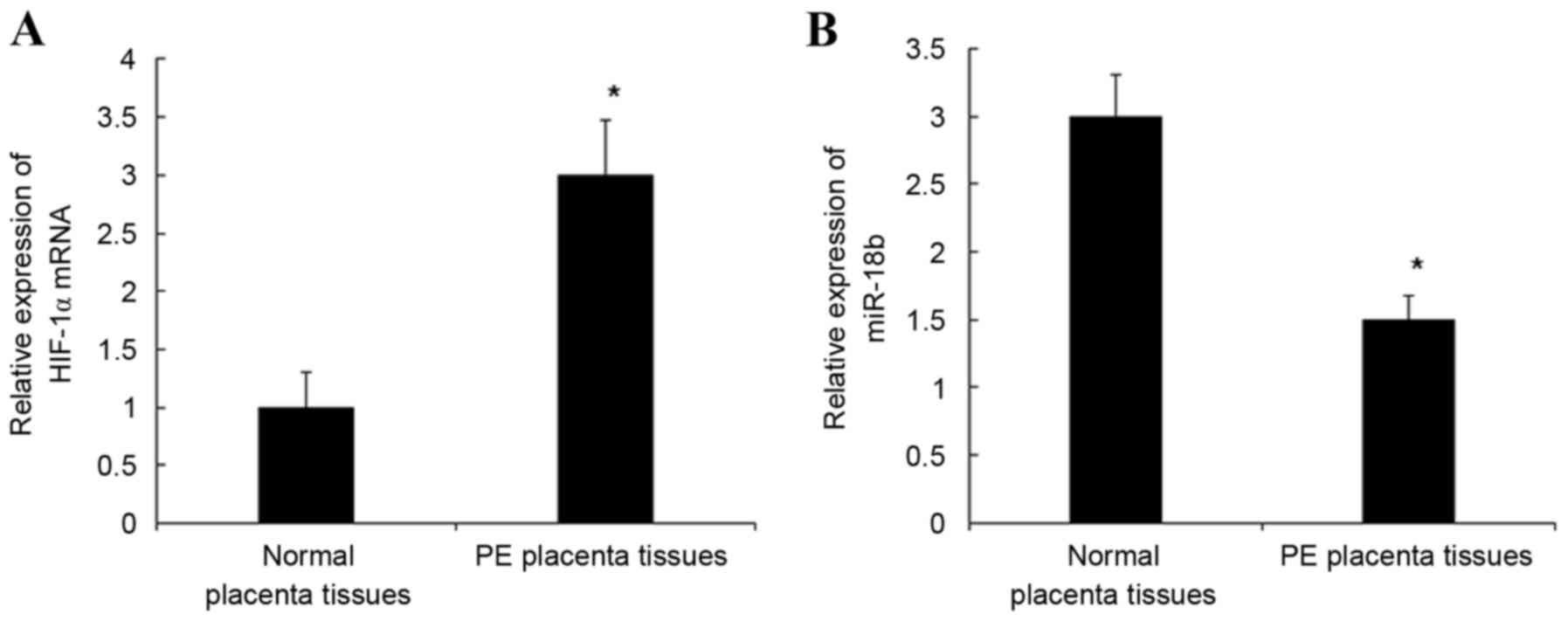
mRNA expression levels of miR-18b and HIF-1α in PE
and normal placental tissues were detected by RT-qPCR. As indicated
in Fig. 1, the mRNA expression
levels of HIF-1α mRNA in PE placental tissues were significantly
increased when compared with normal placental tissues (P<0.05;
Fig. 1A) and the mRNA expression
levels of miR-18b in PE placental tissues were significantly
decreased when compared with normal placental tissues (P<0.05;
Fig. 1B). These results indicate
that the downregulated mRNA expression levels of miR-18b may be
related to the presence of HIF-1α in placental tissues.
Prediction of hsa-miR-18b target
genes
Target genes of hsa-miR-18b were predicted using
TargetScan software (http://www.targetscan.org/) as described previously
(24). Results indicated a targeted
regulatory relationship between HIF-1α and miR-18b and the specific
regulatory binding sequences were shown in Fig. 2. The present finding suggests that
HIF-1α is one of the target genes of miR-18b.
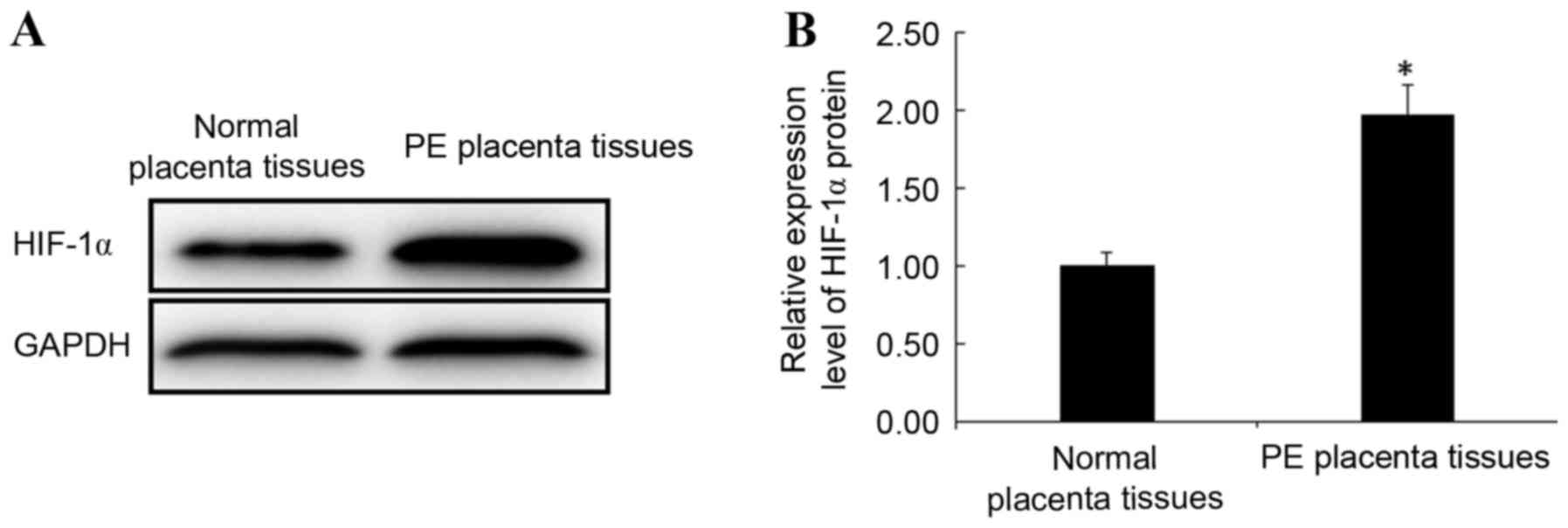
Protein expression of HIF-1α in
placental tissues
Protein expression levels of HIF-1α in PE and normal
placental tissues were detected by western blotting. Consistent
with the trend observed for HIF-1α mRNA expression, the protein
expression levels of HIF-1α in PE placental tissues were
significantly increased when compared with normal placental tissues
(P<0.05; Fig. 3). This result
indicates that the abnormal expression of HIF-1α is closely related
to the occurrence of PE.
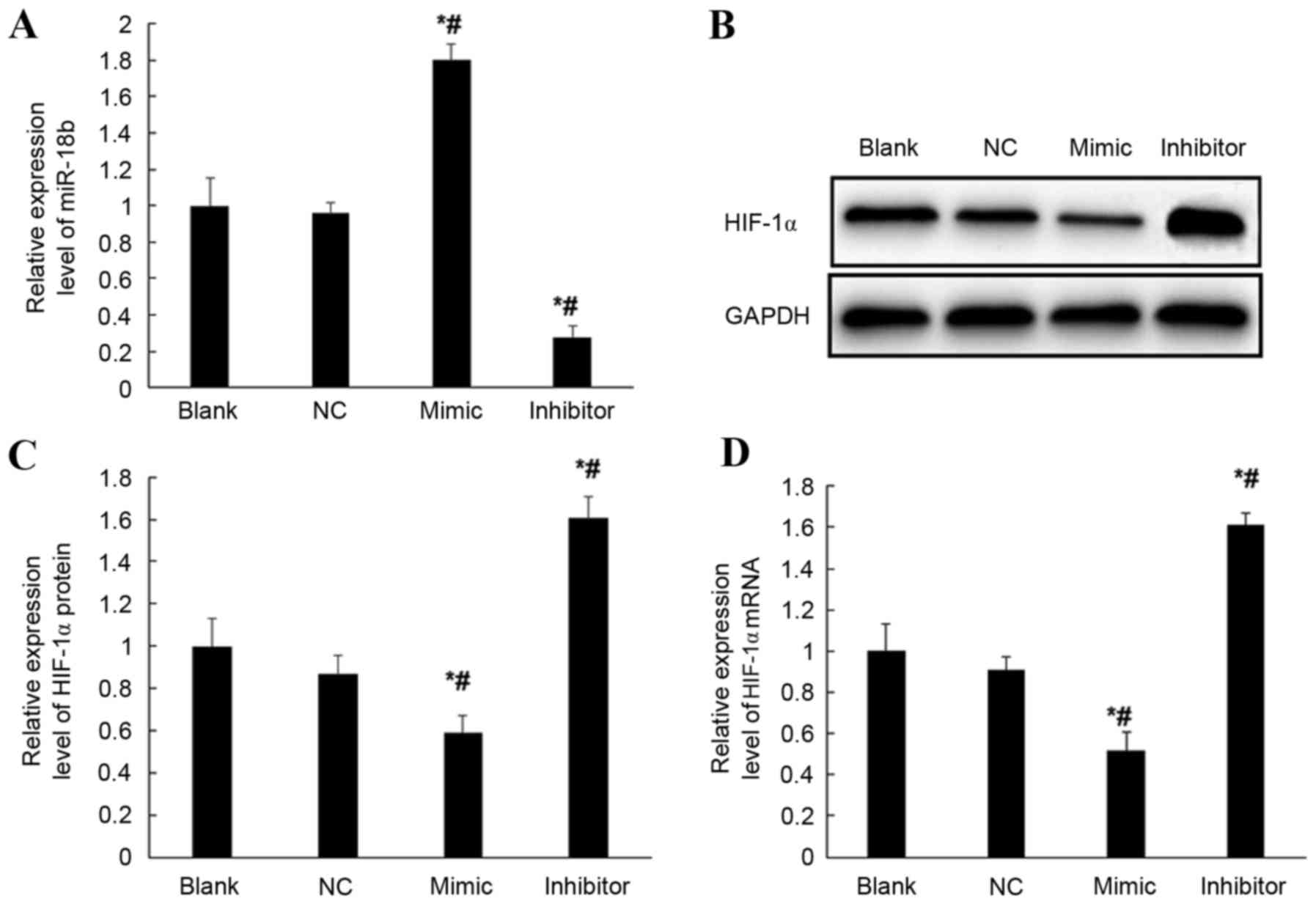
Expression of HIF-1α in transfected
HTR-8/SVneo cells
To verify the regulation of miR-18b on the
expression of HIF-1α gene, mRNA expression levels of HIF-1α in
hsa-miR-18b mimic-transfected, hsa-miR-18b inhibitor-transfected
and negative control vector-transfected HTR-8/SVneo cells (NC) and
normal HTR-8/SVneo cells (blank) were compared by RT-qPCR. mRNA
expression levels of miR-18b were significantly increased in
miR-18b mimic transfected HTR-8/SVneo cells, which were
>1.7-fold higher when compared with the NC and blank groups
(P<0.05; Fig. 4A). Furthermore,
the mRNA expression levels of miR-18b were significantly decreased
in miR-18b inhibitor-transfected HTR-8/SVneo cells, which were
<30% of the mRNA expression levels exhibited by miR-18b in the
NC and blank groups (P<0.05; Fig.
4A). Moreover, the mRNA and protein expression levels of HIF-1α
gene were compared. When compared with the expression levels
exhibited in the NC and blank groups, overexpression of miR-18b
resulted in significantly decreased expression levels of HIF-1α in
HTR-8/SVneo cells at protein (P<0.05; Fig. 4B and C) and mRNA level (P<0.05;
Fig. 4D). Conversely, inhibited
expression of miR-18b resulted in significantly increased
expression levels of HIF-1α in HTR-8/SVneo cells at protein
(P<0.05; Fig. 4B and C) and mRNA
level (P<0.05; Fig. 4D). These
results suggest that overexpression of miR-18b may inhibit the
transcription and translation of HIF-1α gene.
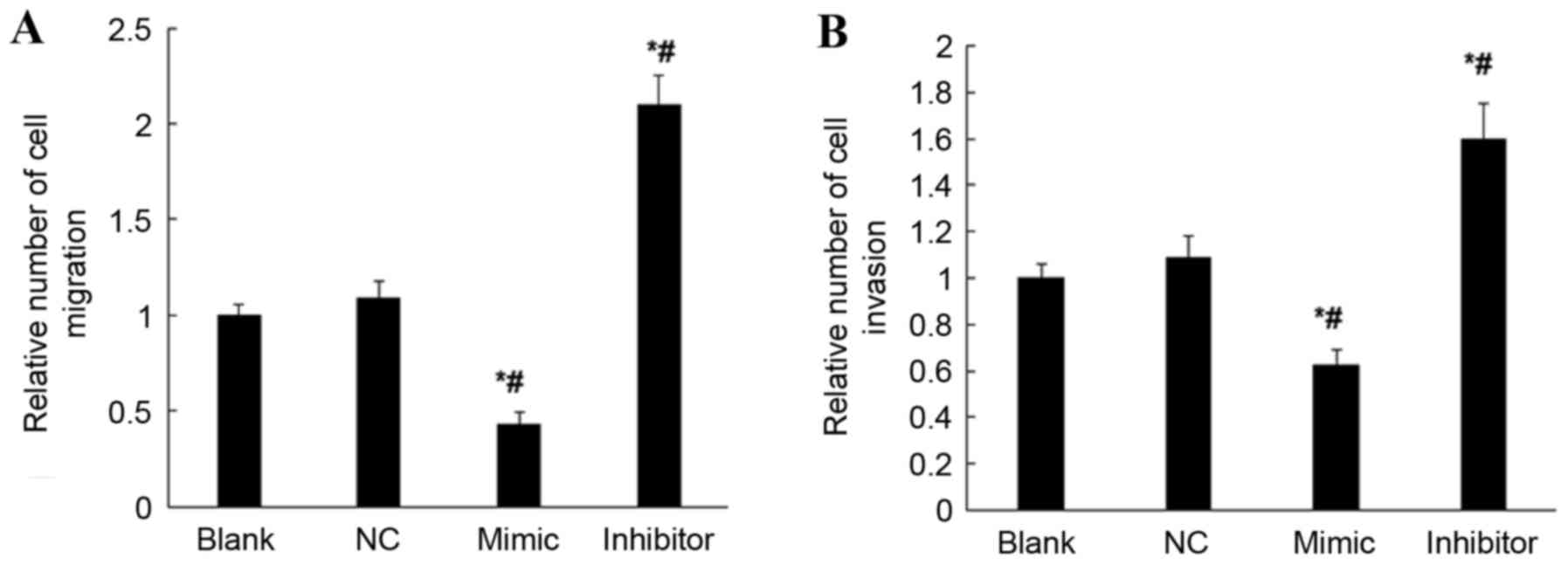
miR-18b inhibits cell migration and
invasion ability of HTR-8/SVneo cells
Possible effects of miR-18b on the cell migration
and invasion ability of HTR-8/SVneo cells were studied using the
cell migration and invasion assay. When compared with the NC and
blank groups, overexpression of miR-18b resulted in significantly
decreased cell migration and invasion abilities of HTR-8/SVneo
cells (P<0.05; Fig. 5); however,
inhibited expression of miR-18b resulted in significantly increased
cell migration and invasion abilities of HTR-8/SVneo cells
(P<0.05; Fig. 5). These results
suggest that miR-18b may inhibit the cell migration and invasion
abilities of HTR-8/SVneo cells.
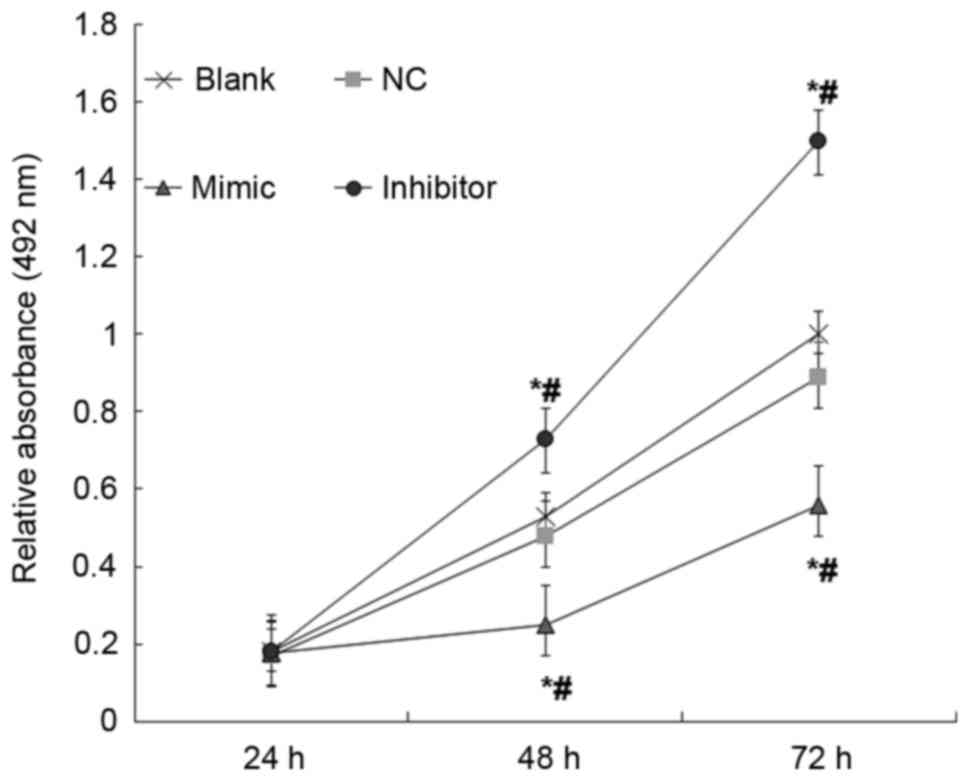
miR-18b inhibits total cellular
metabolic activity of HTR-8/SVneo cells
Possible effects of miR-18b on the total cellular
metabolic activity of HTR-8/SVneo cells were studied using MTT
assays. When compared with the NC and blank groups, overexpression
of miR-18b resulted in significantly decreased total cellular
metabolic activity of HTR-8/SVneo cells; however, inhibited
expression of miR-18b resulted in significantly increased total
cellular metabolic activity of HTR-8/SVneo cells (P<0.05;
Fig. 6). These results indicate that
miR-18b may inhibit the total cellular metabolic activity of
HTR-8/SVneo cells.
Discussion
It has been reported that abnormalities of placental
trophoblast cells may be associated with the pathogenesis of PE
(25,26). Invasion disorder of trophoblast cells
is a central link in the development of PE and placenta ischemia,
and hypoxia of PE has been indicated to further aggravate the
invasion disorder of trophoblast cells, subsequently forming a
vicious cycle (27).
HIF-1α is able to regulate the transcription of
various hypoxia-sensitive factor genes (28). Studies have indicated that the 401 to
603 amino acid residues of HIF-1α have an oxygen-dependent
degradation domain (29,30). Under normoxic conditions, HIF-1α is
degraded and therefore it is difficult to detect the presence of
HIF-1α. However, in the case of hypoxia, the degradation of HIF-1α
is blocked and HIF-1α accumulates in the nucleus (31). The accumulated HIF-1α combines with
HIF-1β in the nucleus to form HIF, which further activates the
transcription and expression of hypoxia-sensitive factor genes by
binding to the hypoxia response elements (32). HIF-1 is able to regulate the
expression of various factors, such as the vascular endothelial
growth factor (VEGF), VEGF receptors (33) and glucose transporters, which have
important roles in erythropoiesis, cell proliferation and
differentiation (34). In the
present study, mRNA and protein expression levels of HIF-1α in PE
placental tissues were significantly higher than that in the normal
population.
It remains unclear whether specific miRNA may
participate in the pathogenesis of PE. Mature miRNA combine with
other proteins to form miRNA-induced silencing complexes, recognize
and bind to target genes by complete or incomplete complementary
binding to the 3′-UTR of the target mRNA and regulate the
expression of target genes (35).
Pineles et al (36) reported
that differential expression of miR-182 and miR-210 was exhibited
in the placentas of PE patients and normal individuals. In
addition, Zhu et al (37)
indicated that the expression of 34 miRNA was disordered in PE
placentas, including 23 miRNA upregulated and 11 downregulated.
Further functional investigation in their study indicated that
these miRNA have important roles in the process of placenta
development and PE occurrence and development (37). Wang et al revealed that
miR-18b was highly expressed in non-metastatic colon cancer
specimens by miRNA chip detection (38). Furthermore, a previous study
demonstrated that the expression of miR-18b was elevated in human
basal cell carcinoma, suggesting that miR-18b may be involved in
the occurrence and development of basal cell carcinoma (39). It has been suggested that in
different tissues, the function and expression of miR-18b may
differ (40).
The villous trophoblast bilayer covering the
placental villi consists of the underlying cytotrophoblast layer
and outer syncytiotrophoblast layer. A population of cells,
extravillous trophoblasts (EVTs), detach from placental villi in
the first 20 weeks of pregnancy and invade the uterine wall,
remodeling the maternal uterine arteries in normal pregnancy
(41). HTR-8/SVneo cells are
non-cancerous cells isolated from EVT (42). In the present study, HTR-8/SVneo
cells were used to investigate the function of miR-18b in placenta
trophoblast cells. miR-18b mimic and miR-18b inhibitor were
transfected into HTR-8/SVneo cells and the changes of related
cellular functions were studied. RT-qPCR results showed that the
mRNA expression levels of miR-18b were significantly increased in
miR-18b mimic-transfected cells and significantly decreased in
miR-18b inhibitor-transfected cells, suggesting that miR-18b mimic
and inhibitor were able to regulate the expression of miR-18b.
Using RT-qPCR and western blotting, the present study revealed that
overexpression of miR-18b significantly inhibited the expression of
HIF-1α, whereas inhibition of miR-18b significantly increased the
expression of HIF-1α in HTR-8/SVneo cells. Subsequently, the
effects of target regulation of miR-18b on HIF-1α with respect to
cell biological functions were further investigated and the
mechanism of abnormal expression of miR-18b exhibited in the
development of PE was explored. The present results indicated that,
in miR-18b-overexpressing HTR-8/SVneo cells, the total cellular
metabolic activity, migration and invasion ability were
significantly decreased, whereas these abilities were significantly
increased when the expression of miR-18b was inhibited. These
results suggest that miR-18b may be involved in the development of
PE through targeted regulation of HIF-1α, subsequently affecting
the cell migration, invasion and viability of HTR-8/SVneo
cells.
The present study exhibited some limitations. One
limitation was that the HTR8-SVneo cell line, a model of primary
EVT used in the present study, is proliferative and may behave
differently to primary cells. Furthermore, miR-18b expression in
human placental tissue was obtained at the end of pregnancy and not
at the first 20 weeks of pregnancy, during which uterine spiral
artery remodeling occurs. Therefore, whether altered miR-18b
expression was the cause or the consequence of pre-eclampsia is
unknown. Moreover, we did not further investigate the clinical
implication of in vivo reduced miR-18b expression or
evaluate the clinical benefits. Further studies are required to
overcome these limitations.
In conclusion, we believe that the present study
indicated that miR-18b may act as an inhibitory factor in the
pathogenesis of PE, and that miR-18b and HIF-1α have a critical
role in the development of PE. We suggest that designing targeted
therapies towards miR-18b and HIF-1α and clinically monitoring the
expression of miR-18b and HIF-1α in placental tissue of PE patients
may be helpful in improving the diagnosis and prognosis of PE.
Acknowledgements
The authors would like to thank Professor Xianghong
Ji (chief physician; Qingdao Municipal Hospital, Oingdao, China)
for her valuable help to the present study.
References
|
1
|
Maynard SE, Min JY, Merchan J, Lim KH, Li
J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et
al: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may
contribute to endothelial dysfunction, hypertension, and
proteinuria in preeclampsia. J Clin Invest. 111:649–658. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caniggia I, Mostachfi H, Winter J,
Gassmann M, Lye SJ, Kuliszewski M and Post M: Hypoxia-inducible
factor-1 mediates the biological effects of oxygen on human
trophoblast differentiation through TGFbeta(3). J Clin Invest.
105:577–587. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allaire AD, Ballenger KA, Wells SR,
McMahon MJ and Lessey BA: Placental apoptosis in preeclampsia.
Obstet Gynecol. 96:271–276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
5
|
Rácz Z, Kaucsár T and Hamar P: The huge
world of small RNAs: Regulating networks of microRNAs (review).
Acta Physiol Hung. 98:243–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy MS, Casselman RC, Tayade C and
Smith GN: Differential expression of plasma microRNA in
preeclamptic patients at delivery and 1 year postpartum. Am J
Obstet Gynecol. 213:367.e1–e9. 2015. View Article : Google Scholar
|
|
9
|
Akehurst C, Small HY, Sharafetdinova L,
Forrest R, Beattie W, Brown CE, Robinson SW, McClure JD, Work LM,
Carty DM, et al: Differential expression of microRNA-206 and its
target genes in preeclampsia. J Hypertens. 33:2068–2074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enquobahrie DA, Abetew DF, Sorensen TK,
Willoughby D, Chidambaram K and Williams MA: Placental microRNA
expression in pregnancies complicated by preeclampsia. Am J Obstet
Gynecol. 204:178.e12–e21. 2011. View Article : Google Scholar
|
|
11
|
Dar AA, Majid S, Rittsteuer C, de Semir D,
Bezrookove V, Tong S, Nosrati M, Sagebiel R, Miller JR III and
Kashani-Sabet M: The role of miR-18b in MDM2-p53 pathway signaling
and melanoma progression. J Natl Cancer Inst. 105:433–442. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimoto N, Toyama T, Takahashi S,
Sugiura H, Endo Y, Iwasa M, Fujii Y and Yamashita H: Distinct
expressions of microRNAs that directly target estrogen receptor α
in human breast cancer. Breast Cancer Res Treat. 130:331–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang
E, Marincola FM, Mai C, Chen Y, Wei H, et al: Loss of connective
tissue growth factor as an unfavorable prognosis factor activates
miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in
nasopharyngeal carcinoma. Cell Death Dis. 4:e6342013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Li Q, Ren N, Li C, Wang X, Xie M,
Gao Z, Pan Z, Zhao C, Ren C and Yang W: Placental miR~106a-363
cluster is dysregulated in preeclamptic placenta. Placenta.
36:250–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: HIF-1, O(2), and the 3 PHDs:
How animal cells signal hypoxia to the nucleus. Cell. 107:1–3.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor CT: Mitochondria and cellular
oxygen sensing in the HIF pathway. Biochem J. 409:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ietta F, Wu Y, Winter J, Xu J, Wang J,
Post M and Caniggia I: Dynamic HIF1A regulation during human
placental development. Biol Reprod. 75:112–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom
J, Many A, Zamudio S and Caniggia I: Increased expression of sFlt-1
in in vivo and in vitro models of human placental hypoxia is
mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol.
291:R1085–R1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aplin JD: Hypoxia and human placental
development. J Clin Invest. 105:559–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldman-Wohl D and Yagel S: Preeclampsia-a
placenta developmental biology perspective. J Reprod Immunol.
82:96–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah DM: Preeclampsia: New insights. Curr
Opin Nephrol Hypertens. 16:213–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Granger JP, Alexander BT, Llinas MT,
Bennett WA and Khalil RA: Pathophysiology of preeclampsia: Linking
placental ischemia/hypoxia with microvascular dysfunction.
Microcirculation. 9:147–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang
YJ, Zhao L, Chen FH, Wang XT, You QD and Guo QL: HIF-1α is critical
for hypoxia-mediated maintenance of glioblastoma stem cells by
activating Notch signaling pathway. Cell Death Differ. 19:284–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the ubiquitin-proteasome
pathway. Proc Natl Acad Sci USA. 95:7987–7992. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weidemann A and Johnson RS: Biology of
HIF-1alpha. Cell Death Differ. 15:621–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wenger RH, Stiehl DP and Camenisch G:
Integration of oxygen signaling at the consensus HRE. Sci STKE.
2005:re122005.PubMed/NCBI
|
|
33
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
34
|
Liu W, Shen SM, Zhao XY and Chen GQ:
Targeted genes and interacting proteins of hypoxia inducible
factor-1. Int J Biochem Mol Biol. 3:165–178. 2012.PubMed/NCBI
|
|
35
|
Williams PJ and Pipkin Broughton F: The
genetics of pre-eclampsia and other hypertensive disorders of
pregnancy. Best Pract Res Clin Obstet Gynaecol. 25:405–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pineles BL, Romero R, Montenegro D, Tarca
AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P,
et al: Distinct subsets of microRNAs are expressed differentially
in the human placentas of patients with preeclampsia. Am J Obstet
Gynecol. 196:261.e1–e6. 2007. View Article : Google Scholar
|
|
37
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–e7. 2009. View Article : Google Scholar
|
|
38
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harris LK: Review: Trophoblast-vascular
cell interactions in early pregnancy: how to remodel a vessel.
Placenta. 31 Suppl:93–98. 2010. View Article : Google Scholar
|
|
42
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells
extended with lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|