Introduction
Cardiovascular disease is the leading cause of
mortality globally (1). Arteries are
the primary vessels affected in cardiovascular disease, thus
studies investigating the mechanisms of arterial growth and repair
are very important. Although successful therapies such as
percutaneous coronary intervention (2) and coronary artery bypass graft
(3) exist to reduce plaque formation
and restore blood flow in patients suffering from ischemic
cardiovascular disease (1), a
significant proportion of patients do not benefit from such
treatment options. It is known that patients with coronary heart
disease can recruit collateral vessels, thus experiencing an
improvement in symptoms (1). The
postnatal vascular system is critical for maintaining homeostasis
and adapts readily to environmental cues, and physiological or
pathological conditions (4). Two
different responses, angiogenesis and arteriogenesis (5), occur during this adaptation.
Vascular function is regulated by intercellular
communication (6,7). In the vessel wall, cell-to-cell
communication occurs by extracellular diffusion and convection of
humoral factors, or by the intercytoplasmic exchange of small
signaling molecules (<1 kDa), metabolites and ions across gap
junctions (6). Endothelial gap
junctions are channels that allow but strictly regulate
communication between endothelial cells, adjacent smooth muscle and
circulating blood cells, as well as throughout the endothelial
monolayer (6). Gap junction protein
α 5 (Gja5, also known as Connexin-40) is the constitutive vascular
gap junction protein across species and the vascular bed, and
serves an important role in coupling between cells in the vascular
wall. Gja5, is essential for a number of physiological processes,
particularly in the response to changes in the metabolic demands of
tissues (8,9). However, the mechanism of the arterial
specific regulation of connexins remains unknown (10,11).
It has been hypothesized that arterial Gja5
expression serves a functional role in flow driven arteriogenesis.
Therefore, the current study used Gja5 mutant mice and set up a
model of femoral artery occlusion (FAO). Subsequently, blood flow
was assessed using Laser Doppler blood flow (LDF) imaging.
Furthermore, genetic evidence of mice demonstrating the functional
importance of Gja5 in acute ischemic cardiovascular disease was
obtained.
Materials and methods
Experimental animals
To determine the function of Gja5 in mouse
arteriogenesis, CX40 enhanced-green fluorescent protein (EGFP)
knock in reporter mice and Gja5 mutant mice from the Max Delbrück
Center for Molecular Medicine (Berlin, Germany) were used in this
study. The present study used 20 reporter mice and 168 Gja5 mutant
mice. The reporter mice are CX40 enhanced-green fluorescent protein
(EGFP) knock in mice. This mutant mouse has a CX40 promoter,
following with an EGFP gene, which means that whenever there is
CX40 gene expression (such as in the arterial endothelial cells),
there is GFP expression as well. These mutant mice were described
by Chadjichristos et al (12)
and Miquerol et al (13),
respectively. A total of 168 experimental mice were divided into
three groups: Gja5-/− group (Cx40 knockdown), Gja5+/− group (Cx40
het) and Gja5+/+ group (wild type; wt). Each group contained 56
mice. All experiments involving animals were performed according to
institutional and National Institutes of Health guidelines (Using
Animals in Intramural Research) (14) and the protocol was approved by the
local ethics committee (Zhejiang Provincial People's Hospital,
Hangzhou, China).
Femoral artery occlusion (FAO)
model
FAO results in flow driven formation of an arterial
collateral network, which increases blood flow to the ischemic
hindlimb (15). Occlusion of the
right femoral artery in 12 week-old mice was performed as
previously described (16).
Mice were anesthetized with an intraperitoneal
injection of 100 mg/kg ketamine (100 mg/ml; Pharmacia; Pfizer, New
York, NY, USA) and 10 mg/kg xylazine (20 mg/ml; Bayer Vital GmbH,
Leverkusen, Germany) and placed in a supine position. The right
inguinal area was shaved and disinfected with 70% ethanol. The
femoral artery was then exposed and separated from the vein and
nerve. The two ligations required for the FAO were conducted
according to Hoefer's method (16).
The proximal circumflex femoral artery is very closely connected to
the lateral caudal femoral artery. Therefore, the upper ligation
was performed proximally to both branches and the second ligation
was conducted below both branches. The femoral artery was then
split into the saphenous and popliteal artery. The second ligation
was placed proximally to this position and the wounds were
subsequently closed.
Assessment of blood flow with Laser
Doppler Flow (LDF) imaging
For repeated assessment of hindlimb blood flow
following FAO, the non-invasive LDF imaging technique was used
(15). The Doppler signal is
linearly proportional to perfusion of the upper 200–300 µm of the
skin (17). Tissue perfusion is
quantified in regions of interest, defined in the limbs relative to
the contralateral, non-ligated side, and the results are presented
as color-coded images (18). Laser
Doppler Imaging measurements were taken from the feet, as these
measurements correlate with other measures of limb perfusion
(19). Following anaesthesia with an
intraperitoneal injection of 100 mg/kg ketamine (100 mg/ml;
Pharmacia; Pfizer) and 10 mg/kg xylazine (20 mg/ml; Bayer Vital
GmbH) of animals, perfusions of both hindlimbs were obtained
separately prior to FAO, immediately following FAO, and 1, 3, 7, 14
and 21 days after FAO using a scanning Laser Doppler Flow Imager
(model LDI2-HR, Moor Instruments, Axminster, UK).
Demonstration for the collaterals in
wt mice 7 days post FAO
The collaterals in wt mice 7 days post FAO were
demonstrated through using a Leica Fluorescent microscope at a
magnification of ×7.5 (Leica Microsystems, GmbH, Watzlar,
Germany).
In vitro experiment
To measure Gja5 mRNA expression in gastrocnemicus
(GC) muscle, 8 mice from each group: Gja5+/+ and Gja5+/− were
sacrificed on day 7 after FAO by cervical dissociation. The
gastrocnemicus muscles were selected as it is related to the
femoral artery (20). Skin and
fasciae were removed from the thighs and lower limbs of the ligated
and non-ligated sides of the animal. The GC muscle was isolated and
excised, and immediately frozen in liquid nitrogen at −80°C. Total
RNA of the GC muscle was isolated using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
following the manufacturers protocol. Reverse transcription was
performed using the ThermoScript™ RT-PCR System for First-Strand
cDNA Synthesis (catalogue no. 11146024; Thermo Fisher Scientific,
Inc.). Subsequently, quantitative polymerase chain reaction (qPCR;
Eurogentec, San Diego, CA, USA) was performed using TaqMan
probe-based chemistry. Primers were as follows: Forward primer (For
Gja5 gene, 5′-3′): CAG CCT GGC TGA ACT CTA CCA, reverse primer: CTG
CCG TGA CTT GCC AAA G and probes: TaqMan probe, CGC TGT CGG ATC TTC
TTC CAG CCC AG. Primers were designed using the Primer Express 2.0
software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Real-time PCR amplification reaction was performed on a Sequence
Detection System (7900 HT; Applied Biosystems, Foster City, USA)
using the Taqman gene expression Master Mix Plus (Eurogentec,
Liege, Belgium). The thermal cycling conditions were as follows:
50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C
for 10 sec and 60°C for 1 min. PCR was completed according to
manufacturer's instructions with 2X TaqMan universal PCR master
mix, 900 nM primers and 250 nM probe. Reactions were performed in
triplicate. Data were collected and analyzed with the Sequence
Detection System 2.3 software (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The relative amount of mRNA was calculated
following normalization to glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; forward primer: GAA GGT GAA GGT CGG AGT C, reverse primer:
GAA GAT GGT GAT GGG ATT TC and TaqMan probe: CAA GCT TCC CGT TCT
CAG CC). The comparative CT Method (−2ΔΔCq Method) was
used as described in the User Bulletin 2: ABI PRISM 7700 Sequence
Detection System.
Statistical analysis
Graphpad Prism 5 software (GraphPad, Inc., La Jolla,
CA, USA) was used to analyze the data. Data are presented as mean ±
standard error of the mean. P-values were calculated using paired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Gja5 (Connexin-40) is expressed in
mouse arteries
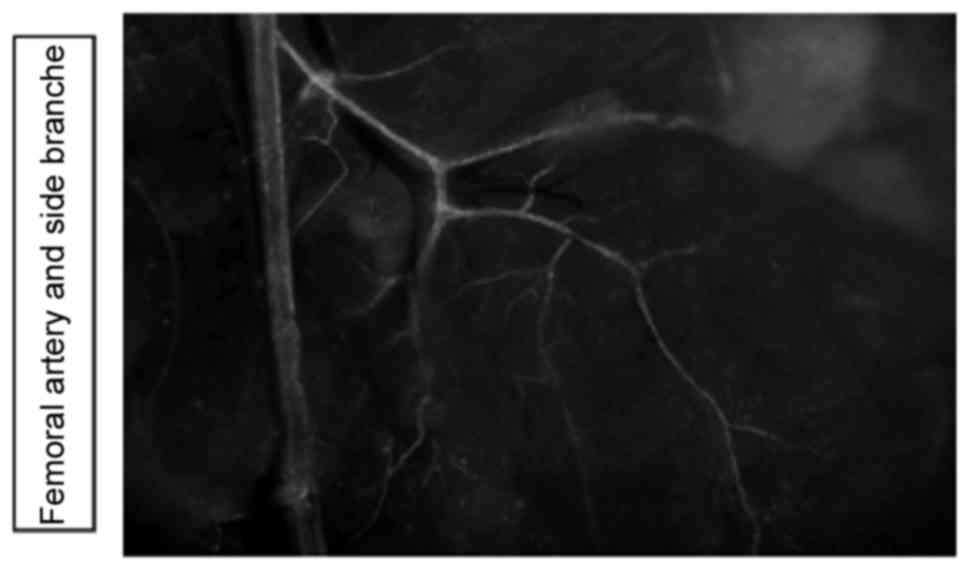
Using CX40EGFP reporter animals, it was demonstrated
that the Gja5 was expressed in the hindlimb arteries, assessed
using the Leica fluorescent microscope (Fig. 1).
Greater reduction of hindlimb
perfusion in Gja5 deficient mice compared with Gja5+/+ mice
following FAO
To determine whether Gja5 serves a functional role
in the flow mediated adaptive remodelling of arteries, the hindlimb
femoral artery occlusion model was used and Gja5 mutant mice were
investigated. To measure hindlimb perfusion following FAO, hindlimb
perfusion with LDF imaging was measured at the following
time-points: Prior to FAO, immediately following FAO and 1, 3, 7,
14 and 21 days following FAO. LDF imaging demonstrated that there
was a greater reduction of hindlimb perfusion in Gja5 deficient
mice compared with Gja5+/+ mice following FAO (P<0.05; Table I).
 | Table I.Hindlimb perfusion with Laser Doppler
Blood Flow imaging were compared among Gja5+/+, Gja5+/− and Gja5-/−
mice. |
Table I.
Hindlimb perfusion with Laser Doppler
Blood Flow imaging were compared among Gja5+/+, Gja5+/− and Gja5-/−
mice.
|
| Time of analysis |
|---|
|
|
|
|---|
| Group | Before | Immediately
after | Day 1 | Day 3 | Day 7 | Day 14 | Day 21 |
|---|
| Gja5-/− |
894.95±27.46 |
39.02±1.66 |
38.21±1.54b |
52.33±3.23b |
112.17±19.4b |
263.96±36.2a |
374.53±61.07a |
| Gja5+/− |
878.9±33.38 |
45.85±4.45 |
53.9±9.8 |
77.22±16.49 |
295.93±12.85 |
398.7±40.00 |
552.68±25.25 |
| Gja5+/+ |
932.19±17.25 |
37.73±1.16 |
56.81±5.47 |
85.12±8.22 |
240.26±17.18 |
396.96±27.97 |
500.45±57.18 |
There was no significant difference in hindlimb
perfusion among Gja5+/+, Gja5+/− and Gja5-/− mice prior to FAO and
acutely following FAO (P>0.05); there was a significant
reduction in hindlimb perfusion in Gja5-/− mice compared with
Gja5+/+ mice 1, 3, 7, 14 and 21 days following FAO (P<0.05), but
there was no difference between Gja5+/− mice and Gja5+/+ mice
(P>0.05; Table I).
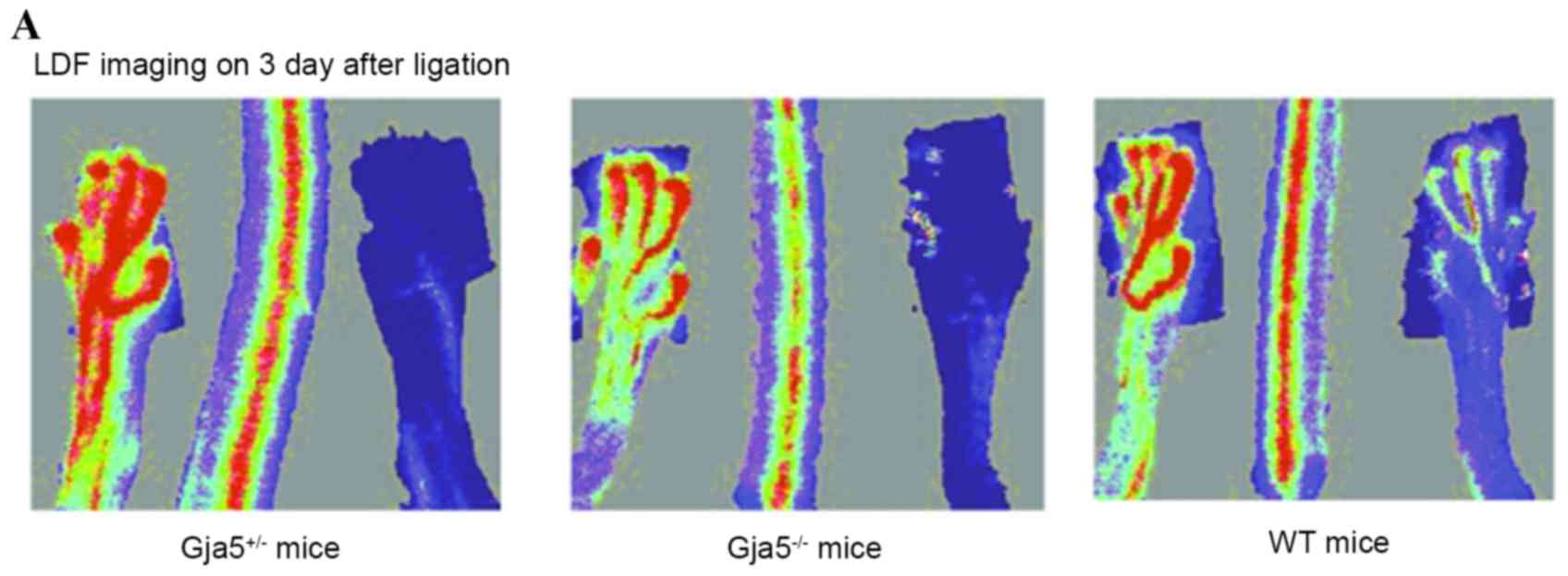
LDF imaging 3 days following FAO indicated that
hindlimb perfusion remained reduced in Gja5-/− mice compared with
Gja5+/+ mice (P=0.000187313), but there was no difference between
Gja5+/− mice and Gja5+/+ mice when compared to Gja5+/+ mice or
Gja5+/− mice. LDF imaging 7 days following FAO demonstrated that
hindlimb perfusion remained reduced in Gja5-/− mice compared with
Gja5+/+ mice (P=0.00036731). However, there were no differences
between Gja5+/− mice and Gja5+/+ mice (Fig. 2).
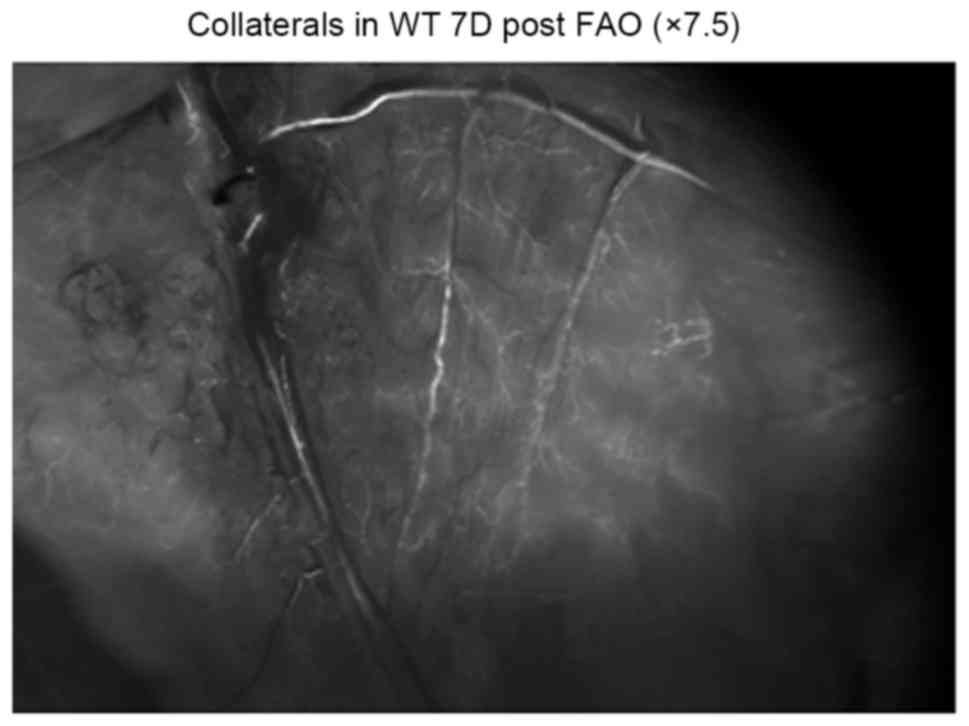
Fig. 3 demonstrates
the collaterals in wt mice 7 days post FAO (7.5× Fluorescence
microscopy; Leica Microsystems AG, Heerbrugg, Switzerland) through
Leica Fluorescent microscope. No significant difference was
observed between Gja5+/− mice and Gja5+/+ mice. The collaterals in
wt mice, 7 days post FAO was more than the collaterals in wt mice 3
days post FAO. LDF imaging on day 7 after FAO demonstrated that
hindlimb perfusion recovered partly, compared with LDF imaging on
day 3.
Expression of Gja5 in the GC muscle
following FAO
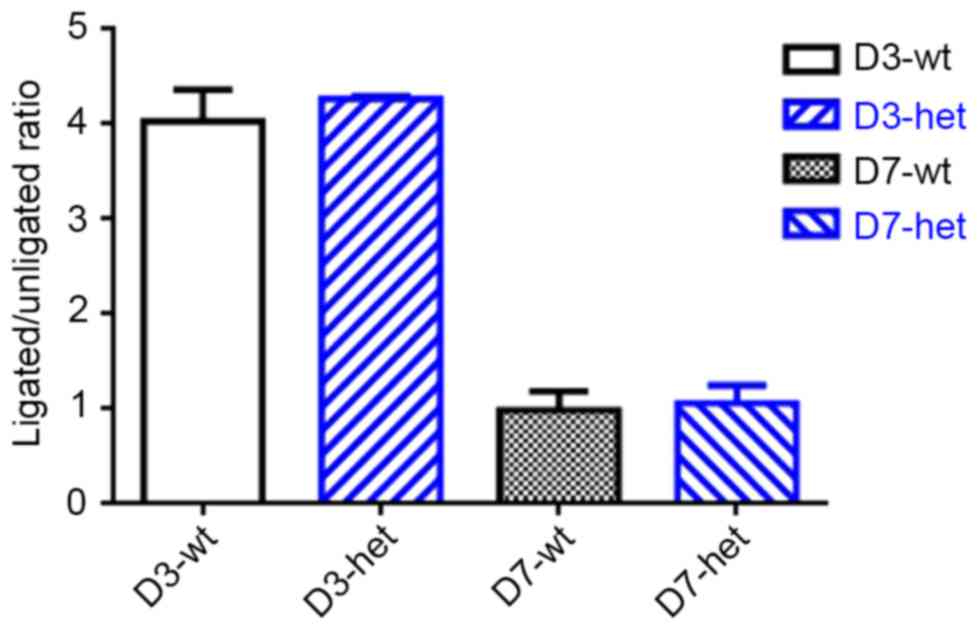
Using RT-qPCR, expression of Gja5 mRNA was measured
in the experimental animals (Fig.
4). Gja5 expression is presented as a ratio from expression in
the ischemic hindlimb and the control hindlimb, using the GC muscle
of Gja5+/+ and Gja5+/− mice (n=8 animals per group). Levels of Gja5
expression in Gja5+/− and Gja5+/+ mice were 4-fold higher in the
ischemic hindlimb 3 days following FAO. No significant difference
was observed in Gja5 expression in gastrocnemius muscle between
Gja5+/− mice and Gja5+/+ mice (Fig.
4). Subsequently, levels of Gja5 returned to baseline values at
7 days after FAO.
Discussion
Arteriogenesis, the growth of collateral vessels, is
triggered by fluid shear stress (FSS) that occurs due to arterial
occlusion (21–23). It is an important focus of current
cardiovascular research as it may provide novel therapeutic
opportunities such as percutaneous coronary intervention and
coronary artery bypass graft (24,25).
Patients with coronary heart disease are able to recruit collateral
vessels, thus experiencing an improvement in symptoms. However, the
molecular mechanisms responsible for arteriogenesis remain unknown
(26,27).
Connexins, a type of gap junction protein, are a
family of structurally related transmembrane proteins that assemble
in vertebrates to form gap junctions. Gap junctions are formed by a
pair of hemichannels called connexions, each contributed by one of
two neighbouring cells. Gap junctions serve a multifaceted role in
the vasculature and are essential for controlling gene expression,
vascular development and vascular function. Gap junction function
in the vasculature depends on molecular selectivity or the
permeability of different vascular connexin isoforms. It has been
determined that the processes of angiogenesis and neurogenesis
share common molecules and mediators (28), including electrical coupling that
involves gap junction proteins or connexions (11).
To determine the potential role of Gja5 in
arteriogenesis, Gja5 mutant mice were investigated using a
previously established flow driven arteriogenesis model known as
the FAO model (15). Surgical
ligation of the femoral artery at a specific site triggers the
arteriogenesis of small, pre-existing collateral arteries into
functional conduit vessels proximally and ischemic angiogenesis
distally (15). The vascular
response to hindlimb ischemia can be readily evaluated by laser
Doppler-based perfusion measurements and histological
quantification of arteriogenesis. The current study demonstrated
that Gja5 was expressed in the femoral artery. A Laser Doppler Flow
imager was used to measure blood flow and the results indicated
significantly reduced perfusion between days 1 and 21 after FAO in
Gja5-/− mice compared with Gja5+/+ mice. However, there were no
significant differences in perfusion reduction following FAO in
Gja5+/− mice compared with Gja5+/+ mice. It was observed that FAO
induced Gja5 expression in the ischemic hindlimb to a similar
extent in Gja5+/− mice and Gja5+/+ mice. At 3 days after occlusion,
expression was elevated 4-fold in the ischemic hindlimb in Gja5+/−
and Gja5+/+ mice. However, levels of Gja5 expression returned to
baseline values 7 days following occlusion. Notably, Pipp et
al (29) demonstrated the
importance of FSS in arteriogenesis using a porcine ischemic
hindlimb model with high levels of collateral flow and FSS.
Normally, during the later phases of arteriogenesis, FSS decreases
as the collateral diameter increases, resulting in the
stabilization of FSS. This drop in FSS acts as a signal to arrest
proliferation and as a result, prevents further collateral growth
prior to optimal adaptation. Therefore, the tendency towards a
change in Gja5 expression following FAO is the same as that
regarding the change of FSS in arteriogenesis. This suggests that
FSS is a dominant morphogenic factor in collateral growth and that
Gja5 serves a functional role in arteriogenesis, however further
research is necessary to determine the association between Gja5
expression and FSS following FAO. Taken together, the results of
the current study indicate that Gja5 may function as a positive
modulator in arteriogenesis and serve as a general arterial
marker.
However, the mechanism underlying arterial specific
regulation of connexins remains unknown. Therefore, the present
study hypothesizes that numerous genes may be involved in the
differential regulation of arteriogenesis, and that a combination
analysis of gene expression may help the patient recover from
cardiovascular disease.
In conclusion, the current study demonstrated that
arterial Gja5 expression serves a functional role in acute ischemic
cardiovascular disease and that reduced Gja5 expression in arterial
endothelial cells impairs arteriogenesis.
Acknowledgements
The present study was supported by the Foundation of
Zhejiang Provincial Medical Research (no. 2013ZDA003) and Zhejiang
Provincial Qianjiang talent plan D (no. QJD1202017).
References
|
1
|
Hamawy AH, Lee LY, Crystal RG and
Rosengart TK: Cardiac angiogenesis and gene therapy: A strategy for
myocardial revascularization. Curr Opin Cardiol. 14:515–522. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pursnani S, Korley F, Gopaul R, Kanade P,
Chandra N, Shaw RE and Bangalore S: Percutaneous coronary
intervention versus optimal medical therapy in stable coronary
artery disease: A systematic review and meta-analysis of randomized
clinical trials. Circ Cardiovasc Interv. 5:476–490. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olearchyk AS: Coronary revascularization:
Past, present and future. J Ukr Med Assoc North Am. 1:3–34.
1988.
|
|
4
|
Prior BM, Yang HT and Terjung RL: What
makes vessels grow with exercise training? J Appl Physiol (1985).
97:1119–1128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haefliger JA, Nicod P and Meda P:
Contribution of connexins to the function of the vascular wall.
Cardiovasc Res. 62:345–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross R: Cell biology of atherosclerosis.
Annu Rev Physiol. 57:791–804. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lodish HF, Rodriguez RK and Klionsky DJ:
Points of view: Lectures: Can't learn with them, can't learn
without them. Cell Biol Educ. 3:202–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sohl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miquerol L, Meysen S, Mangoni M, Bois P,
van Rijen HV, Abran P, Jongsma H, Nargeot J and Gros D:
Architectural and functional asymmetry of the His-Purkinje system
of the murine heart. Cardiovasc Res. 63:77–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chadjichristos CE, Scheckenbach KE, van
Veen TA, Sarieddine Richani MZ, de Wit C, Yang Z, Roth I, Bacchetta
M, Viswambharan H, Foglia B, et al: Endothelial-specific deletion
of connexin40 promotes atherosclerosis by increasing CD73-dependent
leukocyte adhesion. Circulation. 121:123–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chadjichristos CE, Scheckenbach KE, van
Veen TA, Sarieddine Richani MZ, de Wit C, Yang Z, Roth I, Bacchetta
M, Viswambharan H, Foglia B, et al: Endothelial-specific deletion
of connexin40 promotes atherosclerosis by increasing CD73-dependent
leukocyte adhesion. Circulation. 121:123–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miquerol L, Meysen S, Mangoni M, Bois P,
van Rijen HV, Abran P, Jongsma H, Nargeot J and Gros D:
Architectural and functional asymmetry of the His-Purkinje system
of the murine heart. Cardiovasc Res. 63:77–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
United States Department of Agriculture
(USDA): Animal Welfare Act and Animal Welfare Regulations. USDA;
Washington, DC: 2013
|
|
15
|
Hoefer IE, van Royen N, Rectenwald JE,
Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ
and Buschmann IR: Arteriogenesis proceeds via ICAM-1/Mac-1-mediated
mechanisms. Circ Res. 94:1179–1185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chalothorn D, Zhang H, Clayton JA, Thomas
SA and Faber JE: Catecholamines augment collateral vessel growth
and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ
Physiol. 289:H947–H959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jakobsson A and Nilsson GE: Prediction of
sampling depth and photon pathlength in laser Doppler flowmetry.
Med Biol Eng Comput. 31:301–307. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chalothorn D, Clayton JA, Zhang H, Pomp D
and Faber JE: Collateral density, remodeling, and VEGF-A expression
differ widely between mouse strains. Physiol Genomics. 30:179–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helisch A, Wagner S, Khan N, Drinane M,
Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz
HM and Schaper W: Impact of mouse strain differences in innate
hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol.
26:520–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carmen, Schoninger Olaf, Wieser A.A.
Ghermanan, et al: Anatomy. 2th. New Emperor Publishing Ltd.;
Klagenfurt: pp. 122–125. 2001, (In German).
|
|
21
|
van Oostrom MC, van Oostrom O, Quax PH,
Verhaar MC and Hoefer IE: Insights into mechanisms behind
arteriogenesis: What does the future hold? J Leukoc Biol.
84:1379–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buschmann I and Schaper W: The
pathophysiology of the collareral circulation (arteriogenesis). J
Pathol. 190:338–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaper W: Collateral circulation: Past
and present. Basic Res Cardiol. 104:5–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heil M and Schaper W: Influence of
mechanical, cellular, and molecular factors on collateral artery
growth (arteriogenesis). Circ Res. 95:449–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heil M, Eitenmüller I, Schmitz-Rixen T and
Schaper W: Arteriogenesis versus angiogenesis: Similarities and
differences. J Cell Mol Med. 10:45–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buschmann I and Schaper W: Arteriogenesis
versus angiogenesis: Two mechanisms of vessel growth. News Physiol
Sci. 14:121–125. 1999.PubMed/NCBI
|
|
27
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmeliet P and Tessier-Lavigne M: Common
mechanisms of nerve and blood vessel wiring. Nature. 436:193–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pipp F, Boehm S, Cai WJ, Adili F, Ziegler
B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W and
Schmitz-Rixen T: Elevated fluid shear stress enhances postocclusive
collateral artery growth and gene expression in the pig hindlimb.
Arterioscler Thromb Vasc Biol. 24:1664–1668. 2004. View Article : Google Scholar : PubMed/NCBI
|