Introduction
Systemic lupus erythematosus (SLE) is a complex
autoimmune disease with diverse clinical manifestations, including
constitutional symptoms, rash, mucosal ulcers, inflammatory
polyarthritis, photosensitivity, serositis and certain
life-threatening manifestations, such as lupus nephritis (LN)
(1). The etiology of SLE involves
multiple factors including genes, infections, sex hormones and
environmental factors (2). Of these,
genes serve the most important role. Numerous types of genetic
variations contribute to the diversity of the human genome such as
chromosomal translocations, variable number tandem repeats,
inversions, single nucleotide polymorphism, copy number variations
(CNVs) and insertions and deletions. During the past decade, CNVs
have received substantial attention as a major performance of
genetic diversity, covering ~12% of the human genome (3). CNVs may arise either when a complete
gene or gene segment had been duplicated or when a gene is
abnormally absent. The CNVs of toll-like receptor 7, C-C chemokine
ligand 3-like 1, complement component C4 and immunoglobulin (Ig)G
Fcγ receptor 3B (FCGR3B) have been most intensively studied in
association with various autoimmune disorders (4–8). Fcγ
receptors (FcγRs) mediate a variety of immune functions that are
critical in immune responses, including immune complex clearance,
phagocytosis, antigen presentation, antibody-dependent cellular
cytotoxicity and cytokine production (9). In humans, five different FcγRs have
been identified: Three FCGR2 genes (FCGR2A, FCGR2B and FCGR2C) and
two FCGR3 genes (FCGR3A and FCGR3B) (10). Amongst these, an association between
FCGR3B and a risk of autoimmunity has been the most intensively
investigated to date. FCGR3B copy number deficiency is associated
with a number of different autoimmune diseases, including SLE
(11–15), Sjogren's syndrome (16) and systemic sclerosis (17). Although a low FCGR3B copy number is
reportedly associated with SLE susceptibility in Afro-Caribbean and
Caucasian populations, to the best of our knowledge, no information
is available regarding the Henan population in China. The
relationship of FCGR3B copy number with LN, the most common
life-threatening manifestation of SLE (18), has also not been studied in this
population. To further study the pathogenesis and genetic basis of
LN in the Henan population, the present study assessed the FCGR3B
copy number and investigated whether FCGR3B CNVs were associated
with susceptibility to LN.
Patients and methods
Patients
The current study comprised 328 healthy control
individuals recruited from the Physical Examination Center in the
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) between January 2012 and December 2013. The age of the
healthy control individuals was 31.96±11.69 years (mean ± standard
deviation) and the group consisted of 298 females and 30 males
(9.93:1). The weight of healthy controls was 45.5–75.0 kg. A total
of 142 Henan patients with LN who fulfilled the 1997 revised
criteria for the classification of SLE (19) were recruited at the First Affiliated
Hospital of Zhengzhou University between January 2010 and March
2013. The age of patients with LN was 29.34±10.72 years (mean ±
standard deviation) and the group consisted of 130 females and 12
males (10.83:1). The weight of LN patients was 44.0–78.5 kg.
Diagnosis of LN was established according to the American College
of Rheumatology criteria: 24-h proteinuria >0.5 g or a spot
urine protein/creatinine ratio of >0.5; proteinuria >3;
cellular casts including red blood cells, hemoglobin, granular,
tubular or mixed (20,21). All patients with LN were confirmed by
renal biopsy in the First Affiliated Hospital of Zhengzhou
University between January 2010 and March 2013. Cases and controls
were matched for age and gender. The current study was approved by
the Ethics Committee of the First Affiliated Hospital of Zhengzhou
University, and all participants provided written informed
consent.
Data collection
Information pertaining to demographic
characteristics, clinical and laboratory data were collected
retrospectively from medical records. Pathological phenotype was
assessed according to the revised International Society of
Nephrology/Renal Pathology Society (22). Activity index (AI) and chronic index
(CI) score were calculated by two renal pathologists (23). The systemic lupus erythematosis
disease activity index (SLEDAI) score for each patient was
calculated at the time of renal biopsy (24). The laboratory data, including blood,
urine, antibody and complement test results, were routinely
assessed in the Department of Laboratory Medicine of the First
Affiliated Hospital of Zhengzhou University.
Genotyping
Peripheral blood was collected at the time of renal
biopsy. Genomic DNA was extracted from peripheral blood using a
Gentra Puregene Blood Core Kit C (Qiagen, Inc., Valencia, CA, USA)
following the manufacturer's protocol. The primers for target
segments were obtained from the GenBank database (http://www.ncbi.nlm.nih.gov/genbank) (Table I). The copy numbers of FCGR3B were
measured by a custom-by-design Multiplex AccuCopy kit (Genesky
Biotech Co., Ltd., Shanghai, China) based on a multiplex
fluorescence competitive polymerase chain reaction (PCR) principle
as described previously (25). Data
was produced according to the manufacturer's protocol. Briefly, a
20 µl PCR reaction was prepared for each sample, containing: 1X
Multiplex PCR Master Mix (Genesky Biotech Co., Ltd.), 1X
Competitive DNA mix (Genesky Biotech Co., Ltd.), 1 µl Fluorescence
Primer Mix (Sangon Biotech Co., Ltd., Shanghai, China) and 10 ng
sample DNA. The PCR program was completed as follows: 95°C for 10
min; 11 cycles × (94°C for 20 sec; 65°C-0.5°C/cycle for 40 sec;
72°C for 1.5 min); 24 cycles x(94°C for 20 sec; 59°C for 30 sec;
72°C for 1.5 min); 60°C for 60 min and held at 4°C. PCR products
were diluted 20-fold prior to being run by capillary
electrophoresis using an ABI 3730XL genetic analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Raw
data were analyzed using GeneMapper, version 4.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and height/area data
for all specific peaks were exported into an excel file. The
sample/competitive (S/C) peak ratio was calculated for the two
target segments and three reference segments. The reference
segments were screened and selected at three loci of POLR2A, POP1,
and RPP14 as described previously (25). The S/C ratio for each target fragment
was first normalized based on three reference segments,
respectively. The three normalized S/C ratios were further
normalized to the median value in all samples for each reference
segment, respectively, and then the mean was calculated. If one of
the three normalized S/C ratios deviated >25% from the mean of
the other two, it was excluded from further analysis.
 | Table I.Primers for different segments of
FCGR3B. |
Table I.
Primers for different segments of
FCGR3B.
| Segments | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| FCGR3B-1 |
CCATTTCCCGACCATGACCTC |
CTACCAGTCCCGCCCTTCG |
| FCGR3B-2 |
GCCCAGAGATAAGGGTGTCTTCC |
AAGTACAGAACAAACCCTGTGTCACTG |
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The distribution of FCGR3B
copy number in subgroups was compared using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference. The association between the copy number of FCGR3B and
the risk of developing LN was investigated using logistic
regression analysis-effects on risk being estimated by odds ratios
(ORs) with 95% confidence intervals. Subsequently, the
χ2 test or Fisher's exact probabilities were used to
determine the association between FCGE3B copy number and the
clinical phenotypes of LN.
Results
Baseline characteristics
The LN cohort comprised 130 females and 12 males
with a female to male ratio of 10.83:1. The disease duration was
56.3±47.8 months. The 328 healthy subjects contained 298 females
and 30 males, and the female to male ratio was 9.93:1. Other
clinical characteristics of LN at the time of biopsy are presented
in Table II.
 | Table II.Clinical characteristics of lupus
nephritis. |
Table II.
Clinical characteristics of lupus
nephritis.
| Characteristic | Result |
|---|
| Malar rash (%) | 46.5 |
| Photosensitivity
(%) | 16.3 |
| Oral ulcer (%) | 22.5 |
| Alopecia (%) | 28.2 |
| Fever (%) | 49.3 |
| Vasculitis (%) | 11.3 |
| Arthritis (%) | 45.1 |
| Serositis (%) | 21.8 |
| Antinuclear
antibodies (%) | 96.8 |
| Anti-dsDNA
antibodies (%) | 60.6 |
| Low complement
levels (%) | 67.6 |
| Proteinuria, mean ±
SD (g/24 h) | 4.37±4.37 |
| SLEDAI, mean ±
SD | 10.00±7.07 |
| AI, mean ± SD | 5.47±2.99 |
| CI, mean ± SD | 2.01±2.27 |
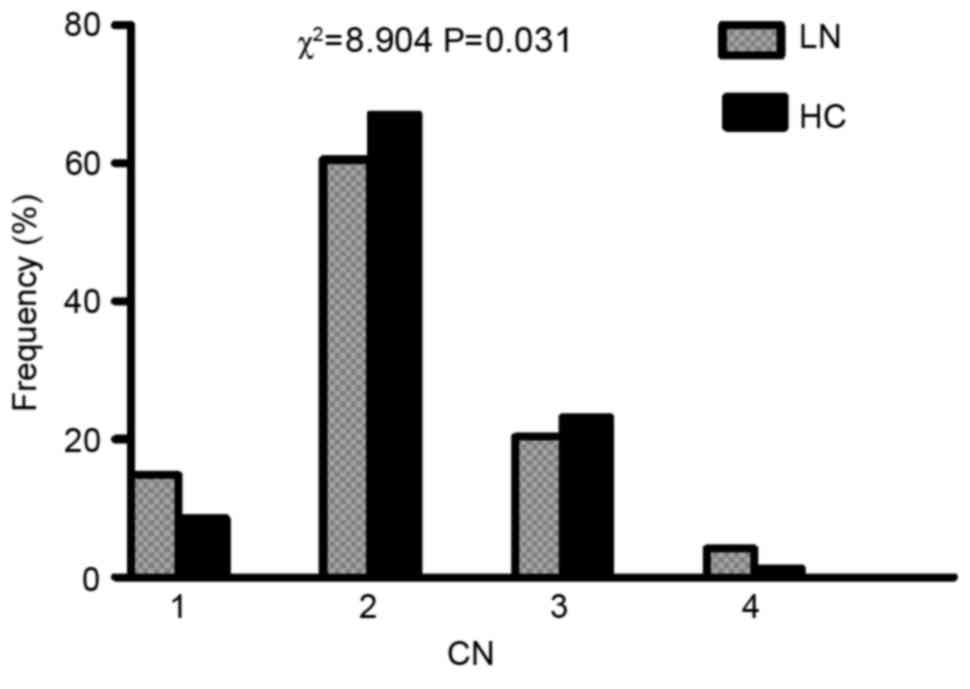
Distribution of FCGR3B copy
number
The present study successfully acquired the copy
numbers of 142 LN patients and 328 controls. The copy numbers of
FCGR3B in LN and healthy controls ranged from 1–4. The percentage
of Henan healthy subjects with FCGR3B low copy number (<2) was
8.5%, whereas the percentage of FCGR3B high copy number (>2) was
24.4%. The distribution of FCGR3B copy number was significantly
different between LN and healthy control subjects (P=0.031;
Fig. 1).
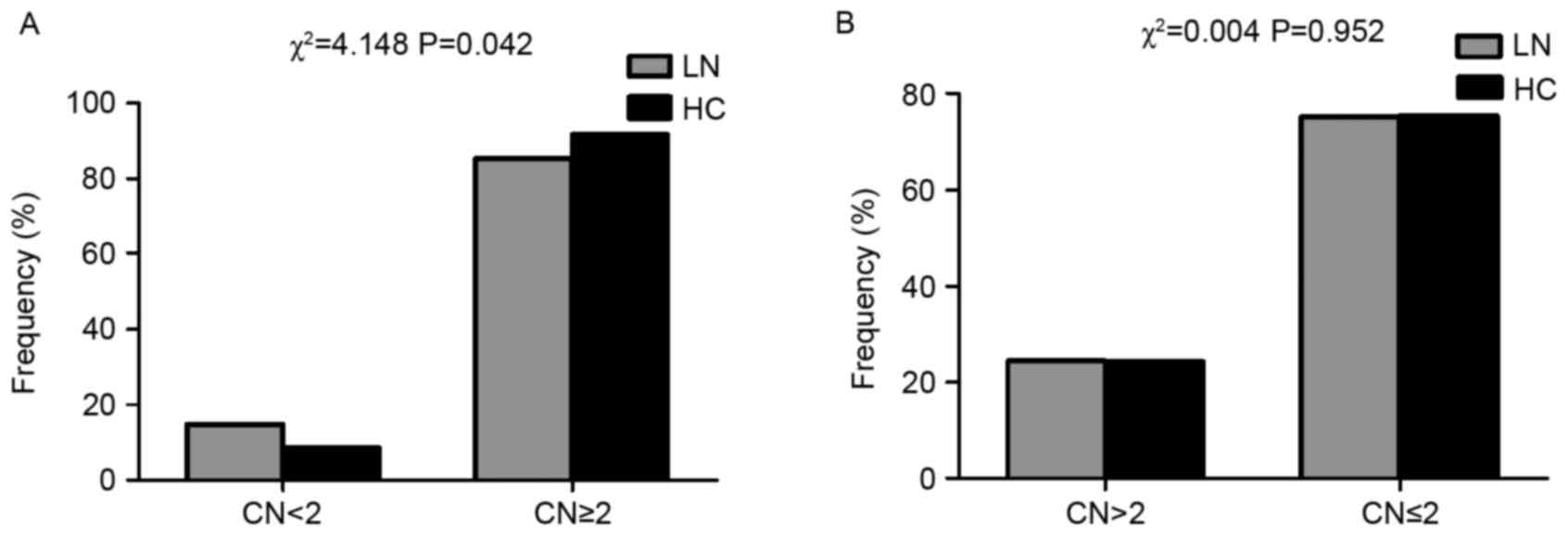
Association of LN susceptibility with
FCGR3B low copy number
The present study examined whether the presence of a
low copy number (<2) or high copy number (>2) was different
between LN and healthy control subjects. As presented in Fig. 2A, the low copy number of FCGR3B was
significantly enriched in LN (P=0.042). However, the high copy
number exhibited no significant difference between LN and healthy
control subjects (P>0.05; Fig.
2B).
Furthermore, the present study investigated whether
FCGR3B CNVs were associated with a susceptibility to LN using
logistic regression analysis. As presented in Table III, the high copy number (>2) of
FCGR3B was not associated with LN susceptibility (OR=1.152; 95%
confidence intervals, 0.711–1.866; P=0.565), however, the low copy
number (<2) was a risk factor for LN (OR=2.059; 95% confidence
intervals, 1.081–3.921; P=0.028).
 | Table III.Influence of FCGR3B gene CN on
susceptibility to LN. |
Table III.
Influence of FCGR3B gene CN on
susceptibility to LN.
| FCGR3B CN | LN, n (total) | HC, n (total) |
P-valuea | ORa | 95% CI |
|---|
| =2 | 86 (142) | 220 (328) | – | 1 | – |
| Low, <2 | 21 (142) | 28 (328) | 0.028b | 2.059 | 1.081–3.921 |
| High, >2 | 35 (142) | 80 (328) | 0.565 | 1.152 | 0.711–1.866 |
Association of FCGR3B CNVs and
clinical phenotypes of LN
Copy number frequencies were compared among LN
patients stratified by each clinical characteristic. Firstly, the
LN patients were classified to proliferative LN (types III and IV)
and non-proliferative LN (types II and V). However, no association
was observed between the FCGR3B CNVs and pathological type
(P=0.657; Table IV). In addition,
associations between SLEDAI, AI, CI and proteinuria and the number
of FCGR3B CNVs was assessed, however no significant difference was
observed (P>0.05; data not shown).
 | Table IV.Association of FCGR3B CN and
pathological types of LN. |
Table IV.
Association of FCGR3B CN and
pathological types of LN.
|
| FCGR3B CN |
|
|---|
|
|
|
|
|---|
| Pathological
type | 1 | 2 | 3 | 4 | Total |
|---|
| PLN, n | 13 | 49 | 17 | 2 | 81 |
| NPLN, n | 8 | 37 | 12 | 4 | 61 |
| Total | 21 | 86 | 29 | 6 | 142 |
Discussion
FCGR3B, which is associated with immune complex
clearance, is a member of the Fcγ receptor family, and is primarily
expressed on human neutrophils (26). Previous studies regarded FCGR3B CNV
as a risk factor for a range of autoimmune diseases, including
rheumatoid arthritis and SLE (11–15,27). A
copy number <2 of FCGR3B has emerged as a susceptibility factor
in Caucasian patients with SLE. However, the CNV of FCGR3B varied
between different geographic regions, making it difficult to
extrapolate findings from one center to another. Studies into the
copy number of the FCGR3B gene are scarce in China, specifically in
the Henan province located in central China. In the current study,
the association between CNVs of FCGR3B and diagnosis of LN in the
Henan population in China was assessed.
A previous study demonstrated that FCGR3B CNV
profiles were significantly different among ethnic groups (28). The frequencies of FCGR3B low copy
number (<2) and high copy number (>2) in the Henan population
are 8.5 and 24.4%, respectively, which are significantly higher
than that of Caucasian populations (3.8–7.4 and 8.8–12%,
respectively) (28,29). This suggests that there are ethnical
variations of FCGR3B CNV.
The current study is, to the best of our knowledge,
the first to demonstrate that a low copy number of FCGR3B is a risk
factor for LN in the Henan population. The association between a
low copy number of FCGR3B and LN is in accordance with studies in
Afro-Caribbean and Caucasian populations (11–15) and
is consistent with Chen et al's (8) and Niederer et al's (28) studies of Chinese populations.
Furthermore, one previously published meta-analysis indicated that
a low copy number of FCGR3B was a risk factor for LN (30), suggesting that there is a positive
association between low copy number of FCGR3B and a risk of
developing LN. In contrast to the findings of the present study, no
association between LN with FCGR3B CNVs was observed in
southeastern and northern Chinese populations (11,31,32).
This discrepancy suggests that there may be regional variations.
However, the sample size, experimental methodology and disease
heterogeneity may also contribute to the difference, and the exact
pathogenesis of the effect of low copy number on LN remains
unknown. A previous study by Willcocks et al (11) demonstrated that in a family with
FCGR3B-deficiency and the normal population, FCGR3B CNVs exhibit a
gene dosage effect on protein levels of FCGR3B in serum and this is
associated with neutrophil uptake of immune complexes. Reduced
FCGR3B expression is thus, likely to contribute to the impaired
clearance of immune complexes, which is a feature of LN, explaining
the association between low FCGR3B CN and LN.
A study by Nossent et al (15) demonstrated that a low copy number of
FCGR3B was associated with SLEDAI, increased levels of anti-dsDNA
antibody and ribosomal P. Furthermore, Chen et al (8) revealed that FCGR3B low copy number
genotypes were significantly enriched in SLE patients with ulcer
and nephritis. However, the present study failed to find any
association between FCGR3B CNV and the clinical features of LN. It
has been demonstrated that patients with SLE from East Asia have
more severe clinical manifestations, such as proliferative
nephritis (33,34). Therefore, it is to be expected that
different ethnic groups may have different genetic susceptibility
to SLE. This has been supported by various previous studies
(28,35–37).
In conclusion, the present study was, to the best of
our knowledge, the first to demonstrate that a low copy number of
FCGR3B increases the risk of LN in the Henan population of China.
However, the sample size is relatively small and although the
AccuCopy method used in the current study has been validated by a
number of studies (25,38), accurately measuring CNV remains a
technical challenge, which requires further investigation.
Acknowledgements
The present study was supported by grants from
National Key Technology R&D Program (grant no. 2011BAI10B04)
and National Basic Research Program of China 973 Program (grant no.
2012CB517606). The authors of the current study would like to thank
Dr Lijuan Zhang, Dr Yongsheng Lei and Dr Weixia Liu for their help
in collecting the blood samples and clinical data of patients and
Dr Linlin Li from the Department of Biostatistics, College of
Public Health, Zhengzhou University for helping to analyze the
data.
References
|
1
|
Yu C, Gershwin ME and Chang C: Diagnostic
criteria for systemic lupus erythematosus: A critical review. J
Autoimmun. 48–49:10–13. 2014. View Article : Google Scholar
|
|
2
|
Choi J, Kim ST and Craft J: The
pathogenesis of systemic lupus erythematosus-an update. Curr Opin
Immunol. 24:651–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redon R, Ishikawa S, Fitch KR, Feuk L,
Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, et
al: Global variation in copy number in the human genome. Nature.
444:444–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pacheco GV, Cruz DC, Herrera González LJ,
Mendoza Pérez GJ, Amaro Adrián GI, Ueji Nakazawa YE and Ramirez
Angulo AV: Copy number variation of TLR-7 gene and its association
with the development of systemic lupus erythematosus in female
patients from Yucatan Mexico. Genet Epigenet. 6:31–36.
2014.PubMed/NCBI
|
|
5
|
Kim JH, Jung SH, Bae JS, Lee HS, Yim SH,
Park SY, Bang SY, Hu HJ, Shin HD, Bae SC and Chung YJ: Deletion
variants of RABGAP1L, 10q21.3, and C4 are associated with the risk
of systemic lupus erythematosus in Korean women. Arthritis Rheum.
65:1055–1063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Ortiz H, Velázquez-Cruz R,
Espinosa-Rosales F, Jiménez-Morales S, Baca V and Orozco L:
Association of TLR7 copy number variation with susceptibility to
childhood-onset systemic lupus erythematosus in Mexican population.
Ann Rheum Dis. 69:1861–1865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McKinney C, Merriman ME, Chapman PT, Gow
PJ, Harrison AA, Highton J, Jones PB, McLean L, O'Donnell JL,
Pokorny V, et al: Evidence for an influence of chemokine ligand
3-like 1 (CCL3L1) gene copy number on susceptibility to rheumatoid
arthritis. Ann Rheum Dis. 67:409–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JY, Wang CM, Chang SW, Cheng CH, Wu
YJ, Lin JC, Yang B, Ho HH and Wu J: Association of FCGR3A and
FCGR3B copy number variations with systemic lupus erythematosus and
rheumatoid arthritis in Taiwanese patients. Arthritis Rheumatol.
66:3113–3121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colombo FR, Torrente Y, Casati R, Benti R,
Corti S, Salani S, D'Angelo MG, DeLiso A, Scarlato G, Bresolin N
and Gerundini P: Biodistribution studies of 99mTc-labeled myoblasts
in a murine model of muscular dystrophy. Nucl Med Biol. 28:935–940.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nimmerjahn F and Ravetch JV: Fcgamma
receptors: Old friends and new family members. Immunity. 24:19–28.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willcocks LC, Lyons PA, Clatworthy MR,
Robinson JI, Yang W, Newland SA, Plagnol V, McGovern NN, Condliffe
AM, Chilvers ER, et al: Copy number of FCGR3B, which is associated
with systemic lupus erythematosus, correlates with protein
expression and immune complex uptake. J Exp Med. 205:1573–1582.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fanciulli M, Norsworthy PJ, Petretto E,
Dong R, Harper L, Kamesh L, Heward JM, Gough SC, de Smith A,
Blakemore AI, et al: FCGR3B copy number variation is associated
with susceptibility to systemic, but not organ-specific,
autoimmunity. Nat Genet. 39:721–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morris DL, Roberts AL, Witherden AS, Tarzi
R, Barros P, Whittaker JC, Cook TH, Aitman TJ and Vyse TJ: Evidence
for both copy number and allelic (NA1/NA2) risk at the FCGR3B locus
in systemic lupus erythematosus. Eur J Hum Genet. 18:1027–1031.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molokhia M, Fanciulli M, Petretto E,
Patrick AL, McKeigue P, Roberts AL, Vyse TJ and Aitman TJ: FCGR3B
copy number variation is associated with systemic lupus
erythematosus risk in Afro-Caribbeans. Rheumatology (Oxford).
50:1206–1210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nossent JC, Becker-Merok A, Rischmueller M
and Lester S: Susceptibility for lupus nephritis by low copy number
of the FCGR3B gene is linked to increased levels of pathogenic
autoantibodies. Autoimmune Dis. 2013:7508142013.PubMed/NCBI
|
|
16
|
Nossent JC, Rischmueller M and Lester S:
Low copy number of the Fc-γ receptor 3B gene FCGR3B is a risk
factor for primary Sjogren's syndrome. J Rheumatol. 39:2142–2147.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKinney C, Broen JC, Vonk MC, Beretta L,
Hesselstrand R, Hunzelmann N, Riemekasten G, Scorza R, Simeon CP,
Fonollosa V, et al: Evidence that deletion at FCGR3B is a risk
factor for systemic sclerosis. Genes Immun. 13:458–460. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bertsias GK, Tektonidou M, Amoura Z,
Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T,
Doria A, et al: Joint European league against rheumatism and
European renal Association-European dialysis and transplant
association (EULAR/ERA-EDTA) recommendations for the management of
adult and paediatric lupus nephritis. Ann Rheum Dis. 71:1771–1782.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan EM, Cohen AS, Fries JF, Masi AT,
McShane DJ, Rothfield NF, Schaller JG, Talal N and Winchester RJ:
The 1982 revised criteria for the classification of systemic lupus
erythematosus. Arthritis Rheum. 25:1271–1277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dooley MA, Aranow C and Ginzler EM: Review
of ACR renal criteria in systemic lupus erythematosus. Lupus.
13:857–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weening JJ, D'Agati VD, Schwartz MM,
Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T,
Ferrario F, et al: The classification of glomerulonephritis in
systemic lupus erythematosus revisited. Kidney Int. 65:521–530.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Austin HA III, Muenz LR, Joyce KM,
Antonovych TT and Balow JE: Diffuse proliferative lupus nephritis:
Identification of specific pathologic features affecting renal
outcome. Kidney Int. 25:689–695. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2002.PubMed/NCBI
|
|
25
|
Du R, Lu C, Jiang Z, Li S, Ma R, An H, Xu
M, An Y, Xia Y, Jin L, et al: Efficient typing of copy number
variations in a segmental duplication-mediated rearrangement
hotspot using multiplex competitive amplification. J Hum Genet.
57:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ravetch JV and Perussia B: Alternative
membrane forms of Fc gamma RIII(CD16) on human natural killer cells
and neutrophils. Cell type-specific expression of two genes that
differ in single nucleotide substitutions. J Exp Med. 170:481–497.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robinson JI, Carr IM, Cooper DL, Rashid
LH, Martin SG, Emery P, Isaacs JD, Barton A BRAGGSS, Wilson AG, et
al: Confirmation of association of FCGR3B but not FCGR3A copy
number with susceptibility to autoantibody positive rheumatoid
arthritis. Hum Mutat. 33:741–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niederer HA, Willcocks LC, Rayner TF, Yang
W, Lau YL, Williams TN, Scott JA, Urban BC, Peshu N, Dunstan SJ, et
al: Copy number, linkage disequilibrium and disease association in
the FCGR locus. Hum Mol Genet. 19:3282–3294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hollox EJ, Detering JC and Dehnugara T: An
integrated approach for measuring copy number variation at the
FCGR3 (CD16) locus. Hum Mutat. 30:477–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan J, Zhao D, Wu L, Xu X, Pang Y, Zhang
J, Ma Y, Liu J and Wang J: FCGR3B copy number loss rather than gain
is a risk factor for systemic lupus erythematous and lupus
nephritis: A meta-analysis. Int J Rheum Dis. 18:392–397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv J, Yang Y, Zhou X, Yu L, Li R, Hou P
and Zhang H: FCGR3B copy number variation is not associated with
lupus nephritis in a Chinese population. Lupus. 19:158–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang H, Zhou Q, Chen D, Jiang H, Mao Y and
Chen J: The single nucleotide polymorphisms gene but not the copy
number variation of Fcgr3B is associated with lupus nephritis in
Chinese people. Lupus. 19:662–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shayakul C, Ong-aj-yooth L, Chirawong P,
Nimmannit S, Parichatikanond P, Laohapand T, Vasuvattakul S,
Vareesangthip K, Vanichakarn S, Malasit P, et al: Lupus nephritis
in Thailand: Clinicopathologic findings and outcome in 569
patients. Am J Kidney Dis. 26:300–307. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sisó A, Ramos-Casals M, Bové A,
Brito-Zerón P, Soria N, Nardi N, Testi A, Perez-de-Lis M,
Díaz-Lagares C, Darnell A, et al: Outcomes in biopsy-proven lupus
nephritis: Evaluation of 190 white patients from a single center.
Medicine (Baltimore). 89:300–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mamtani M, Rovin B, Brey R, Camargo JF,
Kulkarni H, Herrera M, Correa P, Holliday S, Anaya JM and Ahuja SK:
CCL3L1 gene-containing segmental duplications and polymorphisms in
CCR5 affect risk of systemic lupus erythaematosus. Ann Rheum Dis.
67:1076–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kyogoku C, Dijstelbloem HM, Tsuchiya N,
Hatta Y, Kato H, Yamaguchi A, Fukazawa T, Jansen MD, Hashimoto H,
van de Winkel JG, et al: Fcgamma receptor gene polymorphisms in
Japanese patients with systemic lupus erythematosus: Contribution
of FCGR2B to genetic susceptibility. Arthritis Rheum. 46:1242–1254.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Wu J, Carter RH, Edberg JC, Su K,
Cooper GS and Kimberly RP: A novel polymorphism in the Fcgamma
receptor IIB (CD32B) transmembrane region alters receptor
signaling. Arthritis Rheum. 48:3242–3252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai G, Xia Q, Fan D, Li X, Ding N, Hu Y,
Yang X, Liu L, Xin L, Wang L, et al: Association between DEFB103
gene copy number variation and ankylosing spondylitis: A
case-control study. Tissue Antigens. 86:195–198. 2015. View Article : Google Scholar : PubMed/NCBI
|