Introduction
Functional endoscopic sinus surgery (FESS) is a
minimally invasive technique used to restore nasal cavity
ventilation and sinus function (1).
Bleeding is an important challenge for clinicians to overcome in
FESS. Due to the enriched blood vessels in the nasal cavity and
hemostasis difficulty, bleeding not only hinders the surgical field
of FESS, but also prolongs the duration of surgery time and
increases the risk of complications (2).
Various methods are used to reduce bleeding during
FESS surgery, but controlled hypotension remains an important
factor (3). In the past, vasoactive
drugs were used for the control of blood pressure; however, these
agents are prone to causing hemodynamic instability and, in severe
cases, even organ ischemia leading to significant organ dysfunction
(4). Currently, anesthesia
techniques and drugs are more commonly used for controlling blood
pressure and heart rate (HR), in order to reduce FESS bleeding
(5). Previous studies have reported
that the use of propofol and remifentanil for total intravenous
anesthesia has a notable advantage in reducing bleeding compared
with inhalation anesthesia (6–8), which
has made propofol and remifentanil for total intravenous anesthesia
the preferred method for FESS surgery anesthesia. However, there
may still be room for improvement in reducing intraoperative
bleeding and improving the surgical field. At present, there is
little research in this area.
Dexmedetomidine is a highly selective α2
adrenoceptor agonist which inhibits activity of the sympathetic
nervous system, reduces the release of catecholamines, lowers blood
pressure and slows the HR (9).
Previous studies have indicated that dexmedetomidine, like
remifentanil, is effective in controlling blood pressure and
maintaining hemodynamic stability, so as to achieve the same
surgical field as remifentanil (10,11).
However, it is not yet known what the effects would be if these two
drugs were used in combination.
The primary objective of this prospective,
randomized, controlled study was to evaluate the effect of
dexmedetomidine combined with target-controlled infusion (TCI) of
propofol and remifentanil on FESS bleeding and the surgical
field.
Materials and methods
Patients and characteristics
The present study was approved by the Ethics
Committee of Shenzhen People's Hospital (Shenzhen, China). Written
informed consent was obtained from all participants. A total of 62
patients undergoing elective FESS surgery, American Society of
Anesthesiologists (ASA) class I–II, aged 19–53 years old,
participated in the present study between September 2013 and
January 2016. Exclusion criteria were as follows: body mass index
≥25, HR <50 times per min, heart block, hypertension, coronary
heart disease, diabetes, history of asthma, chronic obstructive
emphysema, treatments with antibiotics or anticoagulants,
coagulation abnormalities, failure of cooperation because of mental
disorders, and use of sedative and analgesic drugs within 48 h
before surgery. An anesthesiologist, who participated in the design
of the grouping but not in anesthesia and data collection, assigned
dexmedetomidine. A different anesthesiologist performed clinical
anesthesia and data collection. Surgeons and patients were blinded
to the groupings.
Patient grouping
Patients were randomly divided into an experimental
group and a control group with 31 cases in each group. In the
experimental group, the procedure was as following: Before the
induction of anesthesia, infusion of 0.5 µg kg−1
dexmedetomidine was performed within 15 min, followed by 0.5 µg
kg−1 h−1 dexmedetomidine via intravenous pump
continuous injection until the end of the surgery. The control
group were administered with an intravenous pump injection normal
saline equivalent to the volume of dexmedetomidine administered in
the experimental group. All patients entered the operating room
without pre-medication and having fasted for 8–12 h. An anesthesia
monitor was used (Compact Anesthesia Monitor; GE Healthcare,
Chicago, IL, USA) and routine electrocardiogram monitoring was
carried out. Saturation of peripheral oxygen (SpO2) and
end tidal carbon dioxide (PetCO2) were monitored. Depth
of anesthesia was measured using a bispectral index (BIS) system
(BIS Vista Monitoring System, Aspect Medical Systems; Covidien,
Dublin, Ireland). All patients were subjected to tracheal
intubation and general anaesthesia with TCI of propofol and
remifentanil to maintain the effect-site concentration. Oxygen
ventilation of 5 l·min−1, was administered 5 min before
using commercial TCI system (Base Observational, Fresenius Kabi AG,
Bad Homburg, Germany). TCI consisted of 4 µg ml−1
propofol (Schnider model) and 3 ng ml−1 remifentanil
(Minto model) for anesthesia induction of (12). Tracheal intubation was performed 120
sec after administration of intravenous injection of 0.2 mg
kg−1 cisatracurium ammonium and mask-assisted
ventilation following TCI (endotracheal tube diameter, 7.0 mm for
females and 7.5 mm for males). Subsequently, endotracheal tube was
connected with the anesthesia machine (Primus; Draeger Medical Ag
& Co. KGaA, Luebeck, Germany) to control the breathing, and
respiratory parameters were adjusted to the following: Tidal
volume, 6–10 ml kg−1; respiratory rate, 12–16 times/min;
inspiration/expiration ratio, 1:2; PetCO2 maintained at
35–45 mmHg; and airway pressure maintained at 10–20
mmH2O. TCI of 2–8 µg ml−1 propofol and 2–6 ng
ml−1 remifentanil was applied to maintain anesthesia.
Through adjusting the dosage of the propofol and remifentanil, BIS
value was maintained at 45–55 and mean arterial pressure (MAP) was
maintained at 70–80 mmHg. Intravenous injection of 0.25 mg atropine
was administered if HR was <50 times/min, which was considered
as bradycardia.
Surgery
All patients were in the supine position for
surgery. Prior to the start of surgery, four pieces of tampon,
soaked in adrenaline and lidocaine mixture solution (0.1%
epinephrine: 2% lidocaine, 1:1) were used to shrink the blood
vessels in the nasal mucosa. All surgical procedures were performed
by the same surgeon using standard procedures.
At the end of surgery, propofol and remifentanil
were replaced with intravenous injection of 40 mg parecoxib sodium
for postoperative analgesia. Patients were moved into
Postanesthesia Care Unit (PACU), where another anesthesiologist,
responsible for recovery from anesthesia, finished extubation after
patients were awake. 1 µg kg−1 fentanyl was administered
if visual analog scale (VAS) score was >5 in the PACU.
Intra-operative variables and recovery
profiles for patients
In the operating room, MAP and HR were recorded at
baseline (T0), before anesthesia induction (T1), before tracheal
intubation (T2), at the moment of tracheal intubation (T3), before
the start of surgery (T4), 15 min after the start of surgery (T5),
at the end of surgery (T6) and at the moment of extubation
(T7).
The following parameters were recorded: Duration of
surgery, duration of anesthesia (from anesthesia induction to
anesthetic drug withdrawal), consumption of propofol and
remifentanil, incidence of bradycardia and blood loss. Following
completion of the surgery, surgeons evaluated the surgical field
using the Numeric Rating Scale (NRS), from 0 (worst) to 10 (best)
(10). Extubation time (from
anesthetic drug withdrawal to extubation and respiratory rate after
extubation were recorded. The Observer's Assessment of
Alertness/Sedation (OAA/S) scale (13) was used to assess sedation 5 min after
extubation and pain severity was recorded using VAS (11) before the patient left the recovery
room. The use of fentanyl was observed in recovery room. Hypoxemia
after extubation (SpO2 <90%) and postoperative nausea
and vomiting (PONV) were also recorded.
Statistical analysis
Statistical analysis of the data was performed using
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). Data were presented as
the mean ± standard deviation and analyzed with a Student's t-test
while count data were presented as percentage (%) or median
(interquartile range) and analyzed using the χ2 test,
respectively. MAP and HR were analyzed using one-way analysis of
variance (ANAOVA) followed by Tukey's HSD post hoc test. Grade
data, including NRS, OAA/S and VAS, were analyzed using the Mann
Whitney-U test. P<0.05 was considered as the level of
significant difference.
Results
Demographic characteristics of
patients
A total of 64 patients participated in the present
study. Data from 62 patients were successfully collected and
analyzed. The patients' characteristics did not differ between the
two groups (Table I).
 | Table I.Demographic characteristics of
patients. |
Table I.
Demographic characteristics of
patients.
| Characteristic | Experimental group
(n=31) | Control group
(n=31) | P-value |
|---|
| Age, years | 35.7±8.4 | 36.2±9.9 | 0.44 |
| Sex,
male/female | 21/10 | 17/14 | 0.43 |
| ASA classification,
I/II | 26/5 | 28/3 | 0.71 |
| Height, cm | 169.5±7.9 | 166.9±8.7 | 0.33 |
| Weight, kg | 65.3±10.1 | 61.9±9.2 | 0.67 |
| Body mass index,
kg/m2 | 22.6±1.8 | 22.1±1.4 | 0.10 |
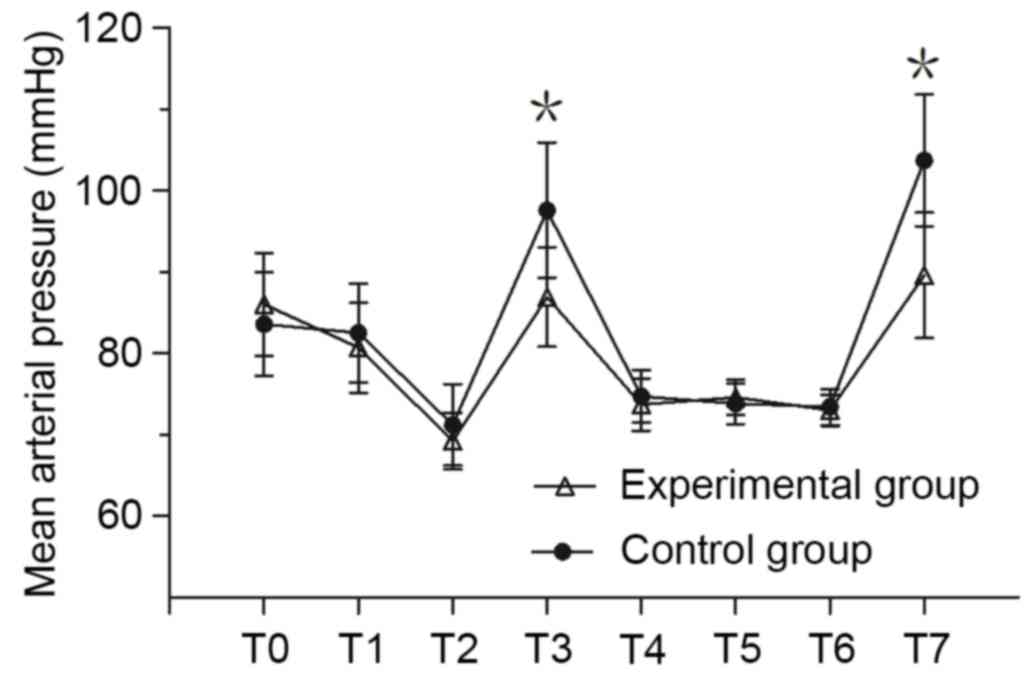
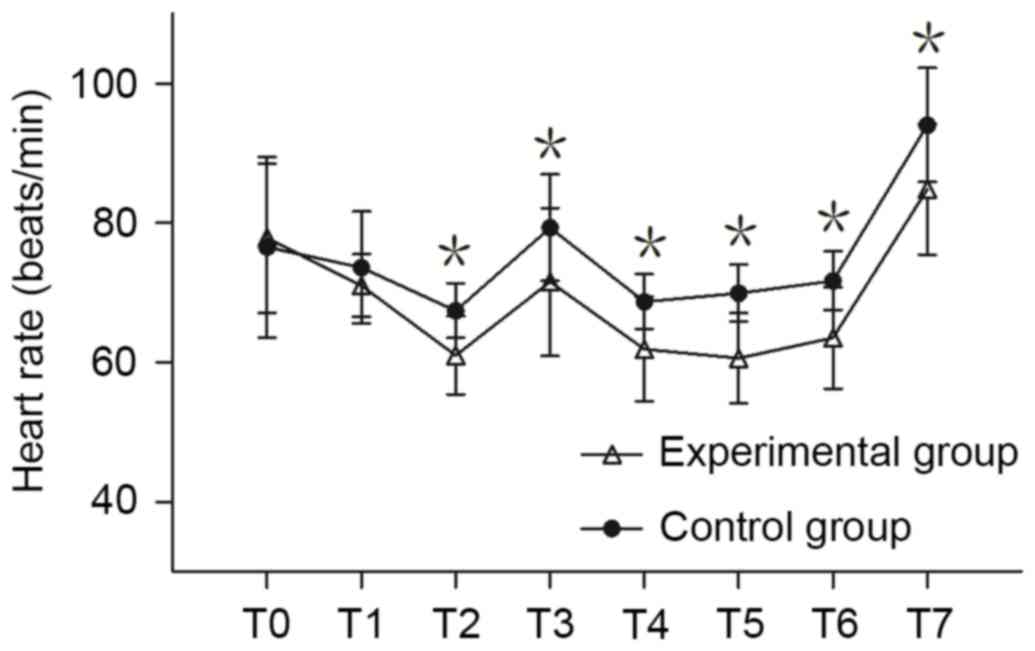
MAP and HR measurement
MAP and HR of the experimental group at T3 and T7
was significantly lower compared with the control group (P<0.05;
Fig. 1). There was no significant
difference in MAP between the two groups at any other time point.
There was no significant difference in HR between the two groups at
T0 and T1; however, the HR of the experimental group was
significantly lower compared with the control group at T2-T7
(P<0.05; Fig. 2).
Intraoperative variables of
patients
Intraoperative observation results are shown in
Table II. There was no significant
difference between the two groups in terms of the surgical duration
or anesthesia duration. In the experimental group, the mean
infusion rates of propofol and remifentanil during surgery were
101.5±8.2 µg kg−1 min−1 and 6.1±0.2 µg
kg−1 h−1 respectively, which was
significantly lower than the control group, in which the infusion
rates were 117.9±4.3 µg kg−1 min−1 (P=0.001)
and 9.1±0.4 µg kg−1 h−1 (P=0.045),
respectively. The incidence rate of bradycardia was higher in the
experimental group compared with the control group (22.6 vs. 9.7%);
however, this difference was not statistically significant. Blood
loss in the experimental group was 195±52.5 ml, which was
significantly lower than the control group (260.7±71.6 ml;
P=0.007). Surgeon satisfaction scores were significantly higher in
the experimental group compared with the control group [median
(interquartile range): 8 (7) vs. 7
(6); P<0.01].
 | Table II.Intraoperative variables. |
Table II.
Intraoperative variables.
| Variable | Experimental group
(n=31) | Control group
(n=31) | P-value |
|---|
| Duration of
surgery, min | 70.3±6.2 | 83.8±8.8 | 0.149 |
| Duration of
anesthesia, min | 86.3±6.0 | 98.0±8.8 | 0.059 |
| Propofol
consumption, µg/kg/min | 101.5±8.2 | 117.9±4.3 | 0.001 |
| Remifentanil
consumption, µg/kg/h | 6.1±0.2 | 9.1±0.4 | 0.045 |
| Incidence of
bradycardiaa | 7 (22.6) | 3 (9.7) | 0.301 |
| Blood loss, ml | 195.0±52.5 | 260.7±71.6 | 0.007 |
| Satisfaction
scoreb | 8 (7) | 7 (6) | <0.01 |
Recovery outcomes
Postoperative recovery data of the patients were
shown in Table III. There was no
significant difference in extubation time between the experimental
group and the control group (17.1±3.2 vs. 14.8±2.8 min; P>0.05).
There was no significant difference in respiratory rate in PACU
between the experimental group and the control group (14.9±1.8 vs.
15.4±1.9 beats min−1). The VAS score of the experimental
group was significantly improved when compared with the control
group [median (interquartile range): 2 (1) vs. 3 (2);
P<0.01]. None of the patients were administered fentanyl for
analgesic recovery. There was no significant difference in
postoperative OAA/S sedation score between the two groups [median
(interquartile range): 1 (1) vs. 1
(1)]. There was no significant
difference in the incidence of PONV between the experimental group
and control group (6.5 vs. 12.9%). There was no case of hypoxia in
either group.
 | Table III.Recovery profiles of patients. |
Table III.
Recovery profiles of patients.
| Variable | Experimental group
(n=31) | Control group
(n=31) | P-value |
|---|
| Extubation time,
min | 17.1±3.2 | 14.8±2.8 | 0.422 |
| Respiratory rate in
PACU, breaths/min | 14.9±1.8 | 15.4±1.9 | 0.705 |
| Postoperative
paina | 2 (1) | 3 (2) | <0.010 |
| Postoperative
sedationa | 1 (1) | 1 (1) | 0.084 |
| Incidence of
PONVb | 2 (6.5) | 4 (12.9) | 0.671 |
Discussion
The results of the present study suggested that
target-controlled infusion of propofol and remifentanil combined
with dexmedetomidine for FESS was able to reduce the intraoperative
bleeding and improve the satisfaction score of the surgical field,
compared with target-controlled infusion of propofol and
remifentanil alone. Dexmedetomidine also reduced increases in MAP
and HR during tracheal intubation and extubation, and reduced
postoperative pain.
Surgical bleeding is a critical issue in FESS since
bleeding makes the anatomical structure difficult to identify,
leading to serious complications and affecting the outcomes of
surgery (5). Numerous studies have
indicated controlled hypotension with anesthetic or vasoactive
drugs may reduce bleeding and improve the quality of FESS (11,14–16).
However, vasoactive drugs cannot reduce the FESS bleeding when MAP
was controlled at level of 65–75 mmHg (17); conversely, vascular dilation caused
by the drug would increase surgical bleeding and has a negative
impact on the surgical field (14).
Previous studies have demonstrated a significant correlation
between the HR and surgical field bleeding during surgery (18–20).
Compared with nitroglycerin, esmolol reduces bleeding by slowing
down HR and offering an improved surgical approach with only
minimal reduction in MAP (18).
Therefore, FESS should be performed at a lower level of HR
(20) as controlling HR is more
effective on improving the surgical approach than lowering MAP.
Compared with inhalation anesthesia, including isoflurane,
sevoflurane, total intravenous anesthesia with propofol and
remifentanil is able quickly and effectively to lower HR and MAP,
reduce bleeding and provide an improved surgical approach (6–8,21,22). The
reason for this is that inhalation anesthetics dilate blood vessels
and cause reflex tachycardia (23).
Although inhalation anesthetics can reduce MAP, the surgical
approach was still not improved because of the increased HR and
dilated nasal mucosal vessels during FESS (24). Even if balanced anesthesia combined
with esmolol controls the HR, the surgical approach is not
ameliorated compared with total intravenous anesthesia (25).
Dexmedetomidine is a highly selective α2
adrenoceptor agonist that inhibits norepinephrine release, thereby
reducing HR and blood pressure (9).
This effect makes it similar to remifentanil (10,11) and
esmolol (26) in reducing bleeding
and improving the surgical approach. In the present study, total
intravenous anesthesia with propofol and remifentanil combined with
dexmedetomidine maintained HR at a lower level, reduced bleeding
and improved surgical satisfaction scores. In addition, unlike
anesthetic drugs which dilate blood vessels, dexmedetomidine
constricts peripheral arteries and veins (27). Although there is no clear clinical
evidence that dexmedetomidine can contract blood vessels in the
nasal mucosa, it is not ruled out that reducing bleeding may be
associated with vasoconstriction.
In order to reduce bleeding, clinical control of
hypotension typically controls MAP below the normal range in
clinical practice (3). But
hypotension reduces visceral blood flow, which causes important
organ dysfunction, particularly in elderly patients and patients
with organ damage (28). A previous
study indicated that it was not necessary to deliberately decrease
the MAP to a dangerous level for reaching a clear surgical field
(29). In the present study,
administration of dexmedetomidine provided good vision during the
surgery although MAP was maintained at relatively high level (70–80
mmHg), which increased patient safety.
During surgical procedures, the dosage of anesthetic
drugs, including propofol and remifentanil, was increased in order
to control blood pressure (10),
which resulted in deep anesthesia, thereby affecting the long-term
prognosis of patients (30). As an
anesthetic adjuvant, dexmedetomidine may reduce the dosage of
opioids and propofol (31). In the
present study, compared with propofol and remifentanil alone,
dexmedetomidine was able to significantly decrease the mean
infusion rate of propofol (101.5±8.2 vs. 117.9±4.3 µg
kg−1 min−1) and remifentanil (6.1±0.2 vs.
9.1±0.4 µg kg−1 h−1) and reduce the dosage at
the same depth of anesthesia. In cases where it is not possible to
monitor the depth of anesthesia, this effect may be able to avoid
the side effects caused by deep anesthesia.
Dexmedetomidine has also been reported to have
additional advantages, including decreasing stress response during
tracheal intubation (32) and
extubation (33) and improving
postoperative analgesia quality (32). In the present study, dexmedetomidine
reduced MAP and HR during intubation and extubation and reduced the
postoperative VAS value, which is consistent with previous studies
(32,33). Guven et al (34) reported that dexmedetomidine was able
to reduce the incidence of PONV. This conclusion was not reached in
the present study because Guven administered a higher initial dose
of dexmedetomidine compared with the present study (1.0 vs. 0.5 µg
kg−1 h−1).
However, the present study had some limitations.
Firstly, the control group had a relatively high MAP and there was
no patient group with low MAP. Therefore, further analysis is
required to investigate whether dexmedetomidine can reduce FESS
hemorrhage and improve the surgical approach in cases of low MAP.
Secondly, the quality of the surgical approach was assessed by the
same surgeon in the present study, which may be subjective because
different surgeons may give different scores based on proficiency,
mental state and other factors. Therefore, large sample,
multi-center clinical trials are required to confirm the findings
of the present study.
In conclusion, the present study indicated that
propofol and remifentanil target-controlled infusion combined with
dexmedetomidine for FESS reduced intraoperative bleeding and
improved the quality of the surgical field in the case of
relatively high MAP. In addition, dexmedetomidine also reduced the
increase in MAP and HR during intubation and extubation, and
improved the quality of postoperative analgesia.
References
|
1
|
Slack R and Bates G: Functional endoscopic
sinus surgery. Am Fam Physician. 58:707–718. 1998.PubMed/NCBI
|
|
2
|
Cinčikas D, Ivaškevičius J, Martinkėnas JL
and Balseris S: A role of anesthesiologist in reducing surgical
bleeding in endoscopic sinus surgery. Medicina (Kaunas).
46:730–734. 2010.PubMed/NCBI
|
|
3
|
Degoute CS: Controlled hypotension: A
guide to drug choice. Drugs. 67:1053–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ge YL, Lv R, Zhou W, Ma XX, Zhong TD and
Duan ML: Brain damage following severe acute normovolemic
hemodilution in combination with controlled hypotension in rats.
Acta Anaesthesiol Scand. 51:1331–1337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amorocho MR and Sordillo A: Anesthesia for
functional endoscopic sinus surgery: A review. Anesthesiol Clin.
28:497–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirelli G, Bigarini S, Russolo M,
Lucangelo U and Gullo A: Total intravenous anaesthesia in
endoscopic sinus-nasal surgery. Acta Otorhinolaryngol Ital.
24:137–144. 2004.PubMed/NCBI
|
|
7
|
Miłoński J, Zielińska-Bliźniewska H,
Golusiński W, Urbaniak J, Sobański R and Olszewski J: Effects of
three different types of anaesthesia on perioperative bleeding
control in functional endoscopic sinus surgery. Eur Arch
Otorhinolaryngol. 270:2045–2050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marzban S, Haddadi S, Nia Mahmoudi H,
Heidarzadeh A, Nemati S and Nabi Naderi B: Comparison of surgical
conditions during propofol or isoflurane anesthesia for endoscopic
sinus surgery. Anesth Pain Med. 3:234–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerlach AT and Dasta JF: Dexmedetomidine:
An updated review. Ann Pharmacother. 41:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim H, Ha SH, Kim CH, Lee SH and Choi SH:
Efficacy of intraoperative dexmedetomidine infusion on
visualization of the surgical field in endoscopic sinus surgery.
Korean J Anesthesiol. 68:449–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Kim Y, Park C, Jeon Y, Kim D, Joo J
and Kang H: Comparison between dexmedetomidine and remifentanil for
controlled hypotension and recovery in endoscopic sinus surgery.
Ann Otol Rhinol Laryngol. 122:421–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeganeh N, Roshani B, Latifi H and Almasi
A: Comparison of target-controlled infusion of sufentanil and
remifentanil in blunting hemodynamic response to tracheal
intubation. J Inj Violence Res. 5:101–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hall DL, Weaver J, Ganzberg S, Rashid R
and Wilson S: Bispectral EEG index monitoring of high-dose nitrous
oxide and low-dose sevoflurane sedation. Anesth Prog. 49:56–62.
2002.PubMed/NCBI
|
|
14
|
Boezaart AP, van der Merwe J and Coetzee
A: Comparison of sodium nitroprusside- and esmolol-induced
controlled hypotension for functional endoscopic sinus surgery. Can
J Anaesth. 42:373–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crawley BK, Barkdull GC, Dent S, Bishop M
and Davidson TM: Relative hypotension and image guidance: Tools for
training in sinus surgery. Arch Otolaryngol Head Neck Surg.
135:994–999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardesín A, Pontes C, Rosell R, Escamilla
Y, Marco J, Escobar MJ and Bernal-Sprekelsen M: Hypotensive
anaesthesia and bleeding during endoscopic sinus surgery: An
observational study. Eur Arch Otorhinolaryngol. 271:1505–1511.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobi KE, Böhm BE, Rickauer AJ, Jacobi C
and Hemmerling TM: Moderate controlled hypotension with sodium
nitroprusside does not improve surgical conditions or decrease
blood loss in endoscopic sinus surgery. J Clin Anesth. 12:202–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srivastava U, Dupargude AB, Kumar D, Joshi
K and Gupta A: Controlled hypotension for functional endoscopic
sinus surgery: Comparison of esmolol and nitroglycerine. Indian J
Otolaryngol Head Neck Surg. 65:440–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wormald PJ, Athanasiadis T, Rees G and
Robinson S: An evaluation of effect of pterygopalatine fossa
injection with local anesthetic and adrenalin in the control of
nasal bleeding during endoscopic sinus surgery. Am J Rhinol.
19:288–292. 2005.PubMed/NCBI
|
|
20
|
Nair S, Collins M, Hung P, Rees G, Close D
and Wormald PJ: The effect of beta-blocker premedication on the
surgical field during endoscopic sinus surgery. Laryngoscope.
114:1042–1046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eberhart LH, Folz BJ, Wulf H and Geldner
G: Intravenous anesthesia provides optimal surgical conditions
during microscopic and endoscopic sinus surgery. Laryngoscope.
113:1369–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wormald PJ, van Renen G, Perks J, Jones JA
and Langton-Hewer CD: The effect of the total intravenous
anesthesia compared with inhalational anesthesia on the surgical
field during endoscopic sinus surgery. Am J Rhinol. 19:514–520.
2005.PubMed/NCBI
|
|
23
|
Toivonen J, Virtanen H and Kaukinen S:
Deliberate hypotension induced by labetalol with halothane,
enflurane or isoflurane for middle-ear surgery. Acta Anaesthesiol
Scand. 33:283–289. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Snidvongs K, Tingthanathikul W,
Aeumjaturapat S and Chusakul S: Dexmedetomidine improves the
quality of the operative field for functional endoscopic sinus
surgery: Systematic review. J Laryngol Otol. 129:S8–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ragab SM and Hassanin MZ: Optimizing the
surgical field in pediatric functional endoscopic sinus surgery: A
new evidence-based approach. Otolaryngol Head Neck Surg. 142:48–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shams T, El Bahnasawe NS, Abu-Samra M and
El-Masry R: Induced hypotension for functional endoscopic sinus
surgery: A comparative study of dexmedetomidine versus esmolol.
Saudi J Anaesth. 7:175–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Talke P, Lobo E and Brown R: Systemically
administered alpha2-agonist-induced peripheral vasoconstriction in
humans. Anesthesiology. 99:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Larsen R and Kleinschmidt S: Controlled
hypotension. Anaesthesist. 44:291–308. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sieśkiewicz A, Drozdowski A and Rogowski
M: The assessment of correlation between mean arterial pressure and
intraoperative bleeding during endoscopic sinus surgery in patients
with low heart rate. Otolaryngol Pol. 64:225–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monk TG, Saini V, Weldon BC and Sigl JC:
Anesthetic management and one-year mortality after noncardiac
surgery. Anesth Analg. 100:4–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arcangeli A, D'Alò C and Gaspari R:
Dexmedetomidine use in general anaesthesia. Curr Drug Targets.
10:687–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gunalan S, Venkatraman R, Sivarajan G and
Sunder P: Comparative evaluation of bolus administration of
dexmedetomidine and fentanyl for stress attenuation during
laryngoscopy and endotracheal intubation. J Clin Diagn Res.
9:UC06–UC09. 2015.PubMed/NCBI
|
|
33
|
Fan Q, Hu C, Ye M and Shen X:
Dexmedetomidine for tracheal extubation in deeply anesthetized
adult patients after otologic surgery: A comparison with
remifentanil. BMC Anesthesiol. 15:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guven DG, Demiraran Y, Sezen G, Kepek O
and Iskender A: Evaluation of outcomes in patients given
dexmedetomidine in functional endoscopic sinus surgery. Ann Otol
Rhinol Laryngol. 120:586–592. 2011. View Article : Google Scholar : PubMed/NCBI
|