Introduction
Endothelial cell apoptosis is an important mechanism
of endothelial dysfunction, during heart failure. Moreover,
activation of nerve endocrine system, inflammatory factors,
oxidative factors and apoptosis factor could significantly affect
the severity of heart failure (1,2).
Prostaglandin A1 (PGA1) and triptolide (TRI) could inhibit the
extensive replication of various DNA/RNA viruses and are related to
the transcription of apoptosis factors B-cell lymphoma 2 (Bcl-2)
(3–5). Upregulation of Bcl-2 expression is an
improvement in cell apoptosis.
In the present study, cardiac microvascular
endothelial cells (CMVECs) were isolated and cultured. After MTT
test, appropriate hypoxia and oxygen time points were selected.
PGA1 and TRI were then given intervention cells. Terminal
deoxynucleotidyl transferase (TUNEL) along with quantitative
polymerase chain reaction (qPCR) methods were used to study the
effects of PGA1 and TRI on CMVECs cells, in order to reveal the
molecular mechanism of endothelial cells. The present study
provides a new approach for the protection of endothelial cells and
the treatment of myocardial ischemia reperfusion injury.
Materials and methods
Experimental animals
Male Sprague-Dawley rats (5–7 days, weighing 200±20
g, purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd., Beijing, China) were selected, at room temperature
25±2°C, with free access to food and water. CMVECs cells were
extracted.
Main reagents
Cell culture medium, fetal bovine serum (FBS) and
trypsin powder were all purchased from Gibco (New York, NY, USA).
The CA Protein Quantification kit was obtained from Bi Yuntian
(Shanghai, China). Rabbit anti-rat CD31 monoclonal antibody was
obtained from CST (Boston, MA, USA). RNAiso Plus,
PrimeScript® RT reagent kit with gDNA Eraser (Perfect
Real-Time) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus)
both purchased from Bao Biological (Dalian, China). In situ
end-labeling assay for cell apoptosis was obtained from Roche
(Basel, Switzerland). Anti Bcl-2 rabbit anti-rat monoclonal
antibodies and immunofluorescence secondary antibody both from
CST.
Culture of rat CMVECs
The rats were anesthetized by ether inhalation.
After a further bath disinfection, the chest of rats was opened for
the excision of hearts. The hearts were first placed in 4°C
pre-cooling phosphate-buffered saline (PBS) liquid for cleaning.
This was followed by cutting of the heart tissues into pieces. The
bottom wall of the culture dish was dampened by 1 ml FBS and the
tissue block was evenly distributed in the culture dish. The
culture dish was placed in an incubator at 37°C. The culture medium
8 ml was added to the culture dish and was cultured for 24 h.
Endothelial cell culture medium was changed after cells showed
basic form. Cell culture was continued until culture grown to
confluence state with 0.25% trypsin +EDTA digestion passage.
This study was approved by the Animal Ethics
Committee of China-Japan Friendship Hospital Animal Center.
Identification of rat CMVECs
CMVECs specific antigen CD31 gene was used to
identify CMVECs by immunofluorescence. After trypsin digestion of
the primary CMVECs, the cells were placed into a 25 mm Petri dish
with pretreated coverslip on the bottom. After 2 days, cell growth
basically covered the bottom plate. After washing with PBS,
formaldehyde for the fixation was used for 15 min. BSA (5%) was
added and the incubation was performed for 30 min at room
temperature. PE anti-human CD31 (dilution, 1:70; cat. no. 320806;
Biolegend, Shanghai, China) was added for incubation at 4°C
overnight with PBS washing three times. The samples were observed
under fluorescence microscope (IX70; Olympus, Tokyo, Japan) after
PBS cleaning.
Selection of oxygen supply time for
cell hypoxia
Fused cells were randomly divided into the control
group (normal oxygen), hypoxia 12 h/reoxygenation 3, 6 and 9 h
group. First, the endothelial cells were placed in an anoxic
culture box with a gas composition of 95% N2 + 5%
CO2. After hypoxia for 12 h, the reoxygenation MTT
experiment, the optimal hypoxia and reoxygenation intervention time
point (hypoxia for 12 h/reoxygenation for 6 h was the best) was
selected. The growth of good primary rat CMVECs was selected,
according to the results of MTT. Cells were then randomly divided
into the normoxic control group (C, normal oxygen), hypoxia
reoxygenation group (H/R, hypoxia 12 h/reoxygenation 6 h), PGA1
group (H/R+PGA1), and TRI group (H/R+TRI).
Quantitative PCR analysis
The collected CMVECs were transferred into the 1.5
ml reagent containing TRIzol and were placed at room temperature
for 5 min. After centrifugation at 4°C for 5 min at 12,000 × g, the
supernatant was carefully placed into a new Eppendorf (EP) tube.
Chloroform (0.2 ml) was added to the supernatant, mixed evenly, and
placed at room temperature for 5 min. Centrifugation was performed
again for 15 min at 12,000 × g, at 4°C. Supernatant was absorbed
into another new EP tube. The same volume of isopropanol was added
after reversing several times with full mixing, and placed at room
temperature for 10 min. Centrifugation was again performed for 10
min. The supernatant was discarded, for precipitation, 1 ml 75%
ethanol was added, and mixed evenly, followed by 12,000 × g
centrifugation at 4°C for 5 min. Supernatant was discarded and the
process repeated. RNA concentration was then measured. The total
RNA solution was diluted with RNase-free H2O into 1
µg/µl, according to PrimeScript® RT reagent kit with
gDNA Eraser reagent kit instructions. Prepared reverse
transcription reaction liquid was added the corresponding RNA
sample and the reverse transcription was performed to obtain cDNA.
The cDNA was stored at −20°C. According to SYBR® Premix
Ex Taq™ II (Tli RNaseH Plus) reagent kit instructions, mRNA levels
were determined. The corresponding RNA primer sequences are shown
in Table I.
 | Table I.RT-PCR primer sequences of Bcl-2 and
β-actin mRNA. |
Table I.
RT-PCR primer sequences of Bcl-2 and
β-actin mRNA.
| Gene | Primer sequence
(5′-3′) |
|---|
| Bcl-2 | Forward:
GAGGATTGTGGCCTTCTTTG |
|
| Reverse:
GTTCCACAAAGGCATCCCAG |
| β-actin | Forward:
TCAGGTCATCACTATCGGCAAT |
|
| Reverse:
AAAGAAAGGGTGTAAAACGCA |
Detection of apoptosis in CMVECs
The cells with 1×105/ml (1.5 ml)
concentration were inoculated in 24-well plates, cultured at 37°C
medium, for grouping model. Cells of the normoxic control group (C,
normal oxygen), hypoxia reoxygenation group (H/R, hypoxia 12
h/reoxygenation 60 h), PGA1 group (H/R+PGA 180 nmol/l) and TRI
group (H/R+TRI 10 µg/l) were collected, respectively. The
supernatant was removed and washed with PBS (PGA1 and TRI were used
for the solution with Me2SO and normal saline was
diluted to a final concentration of 5%, with microporous membrane
for sterilization). Fixation was performed for 10 min with 10%
paraformaldehyde in the 37°C incubator which was followed by
washing with PBS. Permeabilization was then performed with a 0.1%
Triton X-100 in the 37°C incubator for 10 min and washed with PBS.
Using 5% skim milk for 1 h, TUNEL detection liquid was added.
Incubation was then performed at 37°C for 1 h. After PBS washing,
the nuclei were stained with DAPI (1:100) and observed under an
inverted fluorescence microscope.
Statistical analysis
The experimental results were analyzed using SPSS
17.0 statistical software (Chicago, IL, USA). The Student's t-test,
and one-way ANOVA were used for comparisons between groups.
P<0.05, was considered to indicate a statistically significant
difference.
Results
Observation and identification of
CMVECs
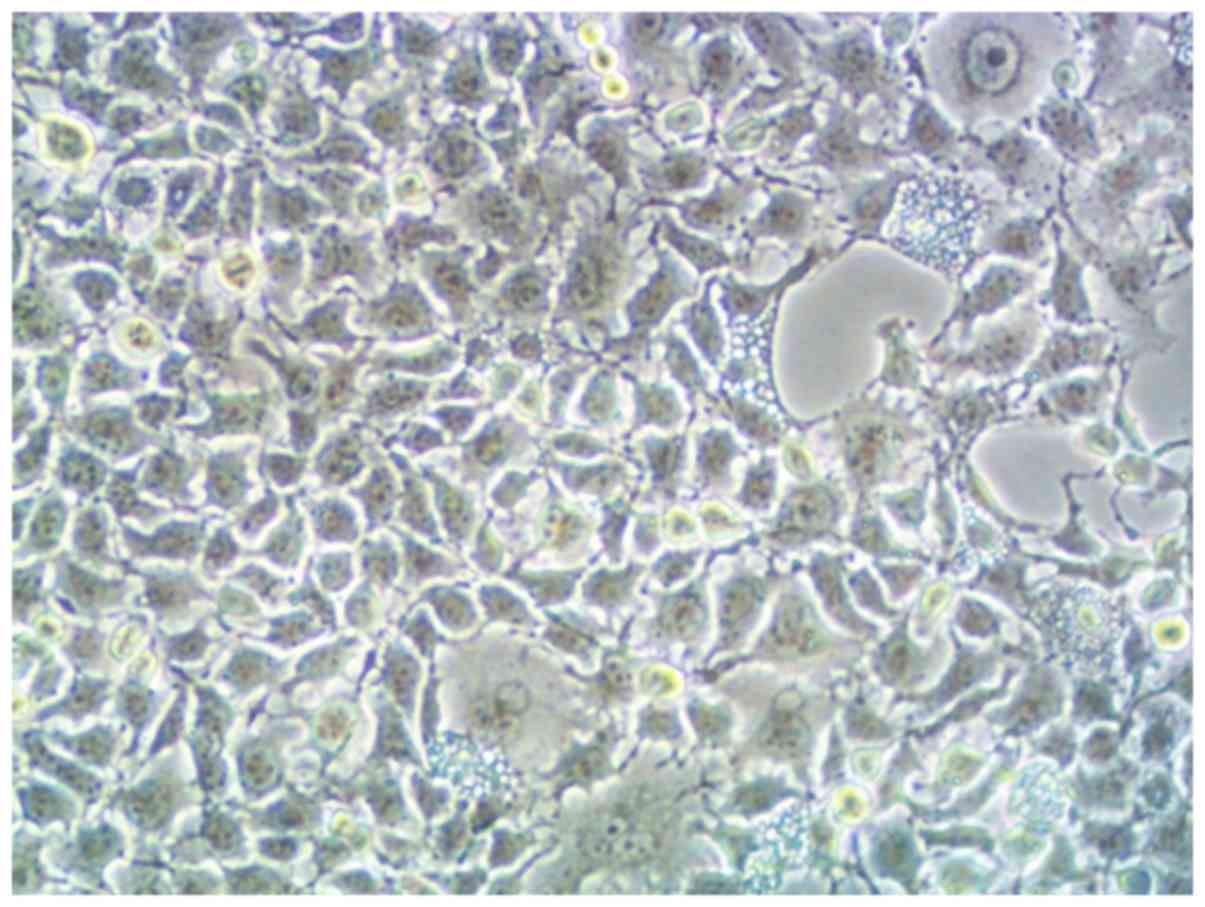
Since the third day of isolation and culture of
CMVECs, under the microscope, the cells were polygonal or
diamond-shaped. After replacing the cell culture medium, the cells
were cultured for 4 days and the cells were fused, and then
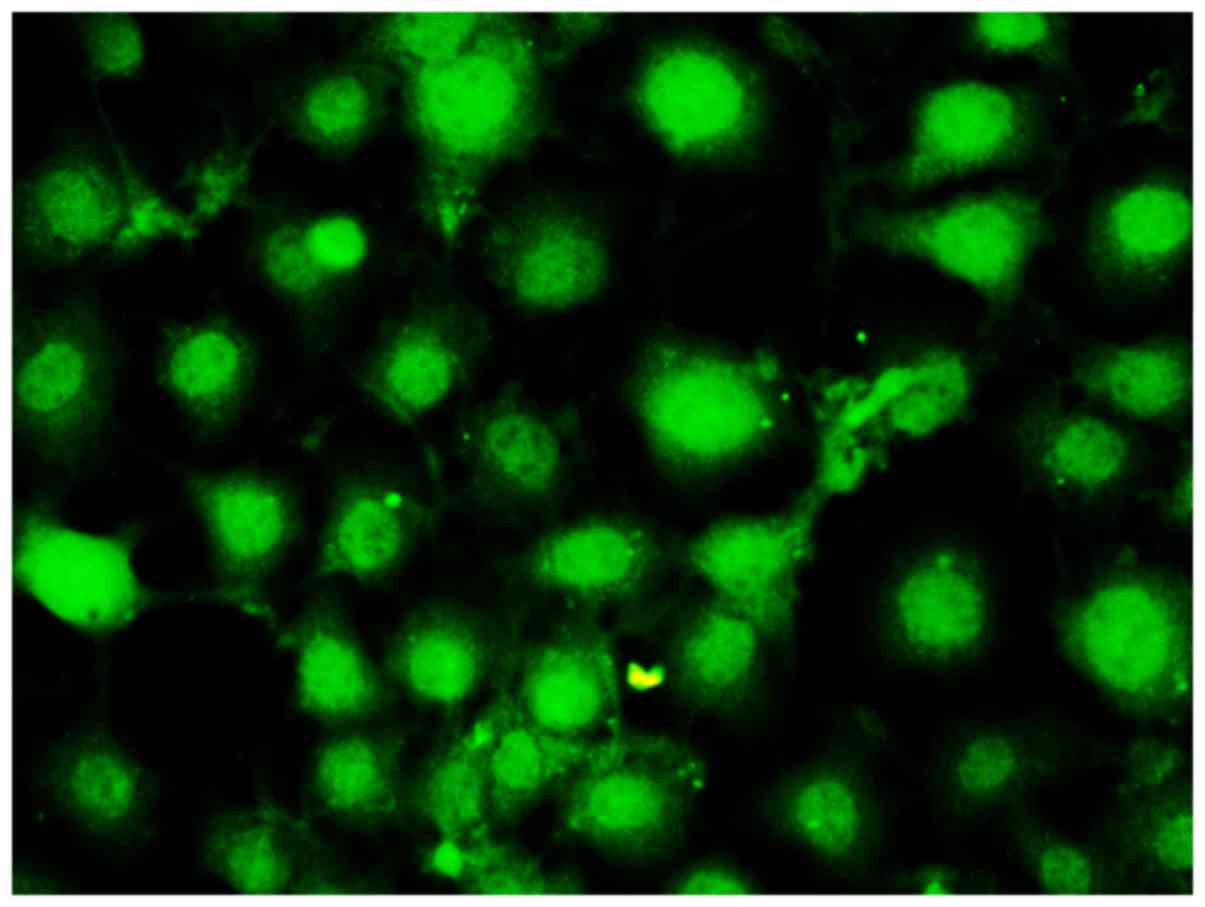
cultured for 5 days. Fully fused cells are shown in Fig. 1. Immunofluorescence CD31 staining was
performed for cell identification, and a large number of CD31
positively stained cells were observed (Fig. 2), suggesting that isolated cells were
endothelial cells with high purity. In this study, second
generations of stably growing cells were used for intervention.
Hypoxia and reoxygenation time point
by MTT method
The CMVECs cells were inoculated into a 96-well
plate. After the normal adherent production of cells, the
endothelial cells were placed in the anoxic culture box with a gas
composition of 95% N2 + 5% CO2 and cultured
in hypoxia for 12 h. Hypoxia and reoxygenation experiment was
performed. The groups were: control group (normal oxygen, without
any stimulation), and hypoxia 12 h/reoxygenation 3, 6 and 9
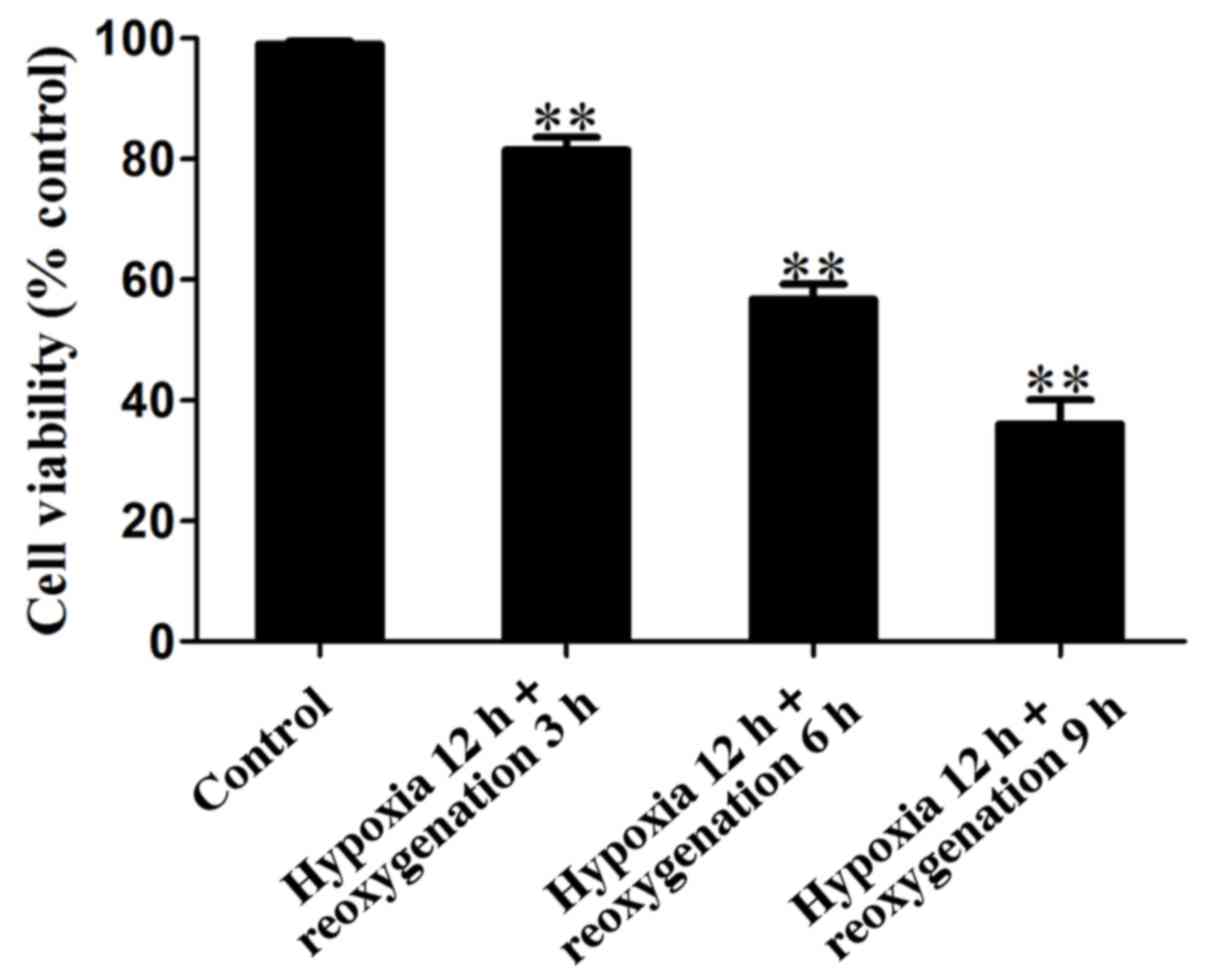
h/group. Fig. 3 shows that in
hypoxia for 12 h/reoxygenation for 3 h, the activity of the cells
was >80%, in hypoxia for 12 h/reoxygenation for 6 h, cell
viability decreased significantly, and the activity of cells was
approximately 50%, while in hypoxia 12 h/reoxygenation 9 h group,
cell activity was <30% and most of the cells died. Thus, the
selection of hypoxia for 12 h/reoxygenation for 12 h was the
optimal hypoxia and reoxygenation time point and the next
experimental study followed.
TUNEL detection of CMVEC apoptosis
ratio of different intervention groups
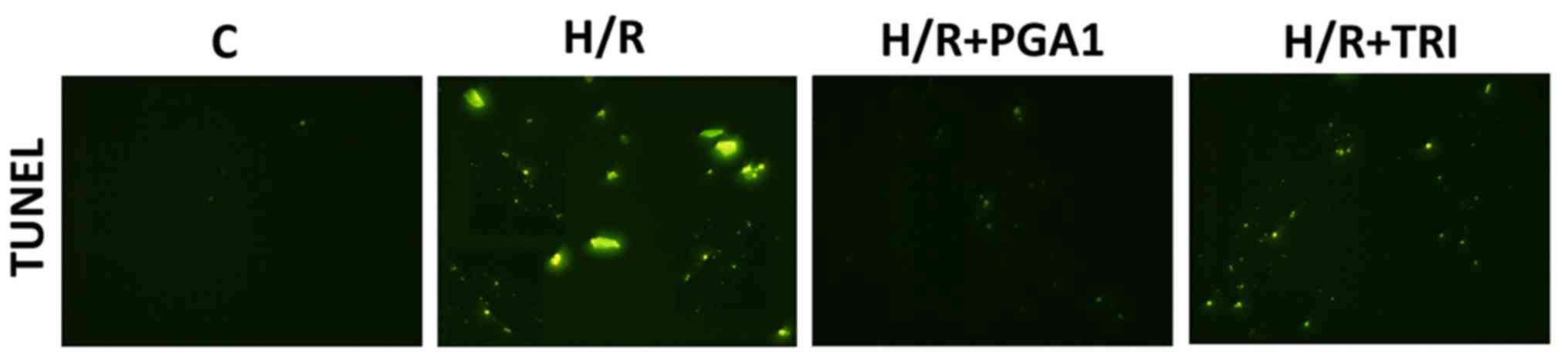
As shown in Fig. 4,
almost no TUNEL-labeled positive cells were found in the CMVECs
blank control group, suggesting that CMVECs normally had no
apoptosis. Compared with the control group, a large number of
TUNEL-labeled positive cells appeared in the CMVECs after hypoxia
and reoxygenation, suggesting that the apoptosis rate increased
significantly (Fig. 4, P<0.01).
The apoptosis rates of CMVECs decreased significantly after
intervention of PGA1 and TRI (Fig.
4, P<0.01). Thus, PGA1 and TRI significantly reduced cell
apoptosis.
Effects of PGA1 and TRI on the
expression of Bcl-2 mRNA
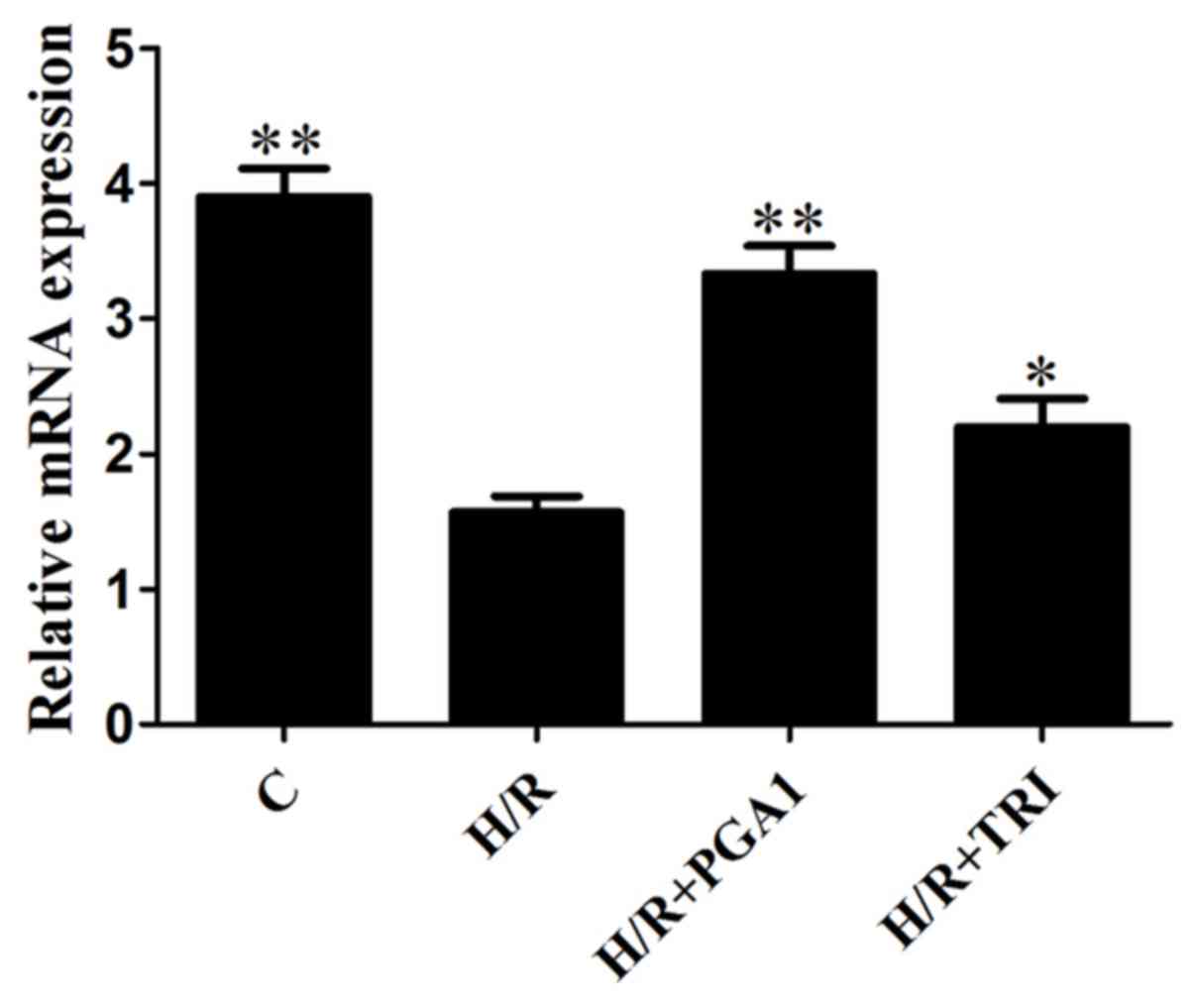
As shown in Fig. 5,
compared with the blank control group, Bcl-2 mRNA expression of
CMVECs significantly reduced after oxygen stimulation (P<0.05).
The expression of Bcl-2 after PGA1 and TRI intervention
significantly increased. Moreover, the elevated effect of PGA1 was
better than that of TRI.
Discussion
In recent years, with the rapid development of
cardiac surgery, myocardial ischemia reperfusion injury has been
the focus of active study (6).
Previous studies have shown that by the inhibition of inflammation,
oxidative stress and apoptosis could significantly alleviate
myocardial ischemia-reperfusion injury (7). In this study, PGA1 and triptolide
alcohol in vivo (TRI) significantly inhibited microvascular
endothelial cell (CMVECs) apoptosis and significantly decreased the
expression of Bcl-2 mRNA, to inhibit apoptosis of CMVECs. This
suggested that PGA1 and TRI could improve the cell injury and play
a protective role of CMVEC apoptosis in rats.
Donor heart ischemia reperfusion injury after
cardiac transplantation is the process of affecting the survival of
transplanted hearts. Although many factors contribute to the cause
of ischemia reperfusion injury, the downregulation of the apoptosis
factor and the promotion of apoptosis factor expression play an
important role in reperfusion injury (8,9).
Previous studies have shown that in endothelial cell injury a large
number of apoptotic bodies are produced and the Bcl-2 plays a
crucial role (10). Bcl-2 is an
important inhibitor of apoptosis and could inhibit cell death
caused by various cytotoxic factors (11). Bcl-2 not only acts on cancer cells in
stage G2/M and participates in apoptosis, but also significantly
enhances the expression of mutant p53 and Bax proteins in cells
(12). The value-added process of
endothelial cells is determined by a series of rules of gene
regulation. Apoptosis is the only way to renew cells. Apoptosis of
endothelial cells after ischemia reperfusion injury may be due to
the severe downregulation of anti-apoptotic factor Bcl-2 (13,14). In
that study through the in vitro culture of CMVEC cells PGA1
and TRI intervention therapy was administered, suggesting that PGA1
and TRI significantly improved the decreased expression of Bcl-2
mRNA induced by myocardial ischemia reperfusion. The TUNEL
experimental results showed that the damage of DNA also had
significant improvement. This experiment proved the importance of
Bcl-2 in heart ischemia reperfusion, and laid the foundation for
further mechanism research.
PGA1 is a kind of cyclization of 20 carbon fatty
acids with many biological activities (15). Clinical application of PGA1 on
essential hypertension, renal hypertension, diabetic hypertension
and pheochromocytoma hypertension has significant antihypertensive
effect. It has been reported that PGA1 had potent anti-inflammatory
effects (16,17). TRI is two mushroom complex compound
containing epoxy isolated from Tripterygium wilfordii
(18). TRI has a strong
physiological activity, widely used in clinical research and
research shows that it has antioxidant, anti-rheumatoid,
anti-Alzheimer's disease, anticancer and other effects (19). It is reported that TRI has
anti-inflammatory, anti-oxidation and inhibitory effects on
apoptosis (20). However, the
relationship between PGA1, TRI and ischemia reperfusion is not
understood well. Through TUNEL apoptosis staining and qPCR
experiments, we found that PGA1 and TRI had a strong protective
effects on CMVECs cell injury induced by hypoxia reperfusion (the
protective effect of PGA1 was stronger than that of TRI). These
results provided a new direction of treatment to myocardial
ischemia reperfusion, and confirmed that both PGA1 as well as TRI
are potential drugs for the treatment of myocardial ischemia
reperfusion.
In the present study, PGA1 and TRI inhibited the
apoptosis of rat CMVECs by upregulation of the expression of Bcl-2
mRNA. PGA1 and TRI, not only alleviated the injury of vascular
endothelial cells in hypoxia and oxygen, but also protected the
function of endothelial cells. Thus, both have wide application
prospects in the treatment of myocardial ischemia reperfusion
injury.
References
|
1
|
Aird WC: Phenotypic heterogeneity of the
endothelium: II. Representative vascular beds. Circ Res.
100:174–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper DL, Carmical JA, Panus PC and
Harirforoosh S: Formulation andin vitro evaluation of niacin-loaded
nanoparticles to reduce prostaglandinmediated vasodilatory
flushing. Eur Rev Med Pharmacol Sci. 19:3977–3988. 2015.PubMed/NCBI
|
|
3
|
Boller YC, Brandes LM, Russell RL, Lin ZP,
Patierno SR and Kennedy KA: Prostaglandin A1 inhibits
stress-induced NF-kappaB activation and reverses resistance to
topoisomerase II inhibitors. Oncol Res. 12:383–395. 2000.PubMed/NCBI
|
|
4
|
Hu K, Liu Z and Liu D: Triptolide inhibits
vascular endothelial growth factor expression and production by
endothelial cells. Zhonghua Yi Xue Za Zhi. 81:1106–1109. 2001.(In
Chinese). PubMed/NCBI
|
|
5
|
Popovic JK, Grujic Z, Grujic I, Bogavac M,
Celic D, Popovic KJ, Jakovljevic A and Popovic DJ: Prostaglandin
E2, trace elements and levels of oxidative processes in spontaneous
miscarriages. Eur Rev Med Pharmacol Sci. 20:4786–4790.
2016.PubMed/NCBI
|
|
6
|
Grandmougin D, Vanhuyse F, Fiore A,
Delolme MC, Liu Y, Laurent N, Bertram M, Folliguet T, Tran N and
Maureira JP: Effects of the self-myocardial retroperfusion with
aortic-coronary sinus shunt on cardiac output and ischemic events
in high-risk patients undergoing OPCAB surgery. J Cardiovasc Surg
(Torino). 56:929–937. 2015.PubMed/NCBI
|
|
7
|
Li X, Liu M, Sun R, Zeng Y, Chen S and
Zhang P: Protective approaches against myocardial ischemia
reperfusion injury. Exp Ther Med. 12:3823–3829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klingel K, Rieger P, Mall G, Selinka HC,
Huber M and Kandolf R: Visualization of enteroviral replication in
myocardial tissue by ultrastructural in situ hybridization:
Identification of target cells and cytopathic effects. Lab Invest.
78:1227–1237. 1998.PubMed/NCBI
|
|
9
|
Youle RJ and Strasser A: The Bcl-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsieh PC, Davis ME, Lisowski LK and Lee
RT: Endothelial-cardiomyocyte interactions in cardiac development
and repair. Annu Rev Physiol. 68:51–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber SA, Haisch C and Lodge PA:
Functional diversity in vascular endothelial cells: Role in
coxsackievirus tropism. J Virol. 64:4516–4522. 1990.PubMed/NCBI
|
|
12
|
Salakou S, Kardamakis D, Tsamandas AC,
Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos
DS, Papapetropoulos T, Petsas T, et al: Increased Bax/Bcl-2 ratio
up-regulates caspase-3 and increases apoptosis in the thymus of
patients with myasthenia gravis. In Vivo. 21:123–132.
2007.PubMed/NCBI
|
|
13
|
Gioacchini FM, Alicandri-Ciufelli M,
Rubini C, Magliulo G and Re M: Prognostic value of Bcl-2 expression
in squamous cell carcinoma of the larynx: A systematic review. Int
J Biol Markers. 30:e155–e160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JE, Woo SM, Min KJ, Kang SH, Lee SJ
and Kwon TK: Combined treatment with ABT-737 and VX-680 induces
apoptosis in Bcl-2- and c-FLIP-overexpressing breast carcinoma
cells. Oncol Rep. 33:1395–1401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gharbi S, Garzón B, Gayarre J, Timms J and
Pérez-Sala D: Study of protein targets for covalent modification by
the antitumoral and anti-inflammatory prostaglandin PGA1: Focus on
vimentin. J Mass Spectrom. 42:1474–1484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gayarre J, Stamatakis K, Renedo M and
Pérez-Sala D: Differential selectivity of protein modification by
the cyclopentenone prostaglandins PGA1 and
15-deoxy-Delta12,14-PGJ2: Role of glutathione. FEBS Lett.
579:5803–5808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elia G, Polla B, Rossi A and Santoro MG:
Induction of ferritin and heat shock proteins by prostaglandin A1
in human monocytes. Evidence for transcriptional and
post-transcriptional regulation. Eur J Biochem. 264:736–745. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu D and Kao PN: Immunosuppressive and
anti-inflammatory mechanisms of triptolide, the principal active
diterpenoid from the Chinese medicinal herb Tripterygium
wilfordii Hook. f. Drugs R D. 4:1–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Liu ZH, Chen ZH, Yang JW and Li LS:
Triptolide: A potent inhibitor of NF-kappa B in T-lymphocytes. Acta
Pharmacol Sin. 21:782–786. 2000.PubMed/NCBI
|
|
20
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|